Changes in Water Utilization Characteristics of Trees in Forests across a Successional Gradient in Southern China
Abstract
:1. Introduction
2. Materials and Methods
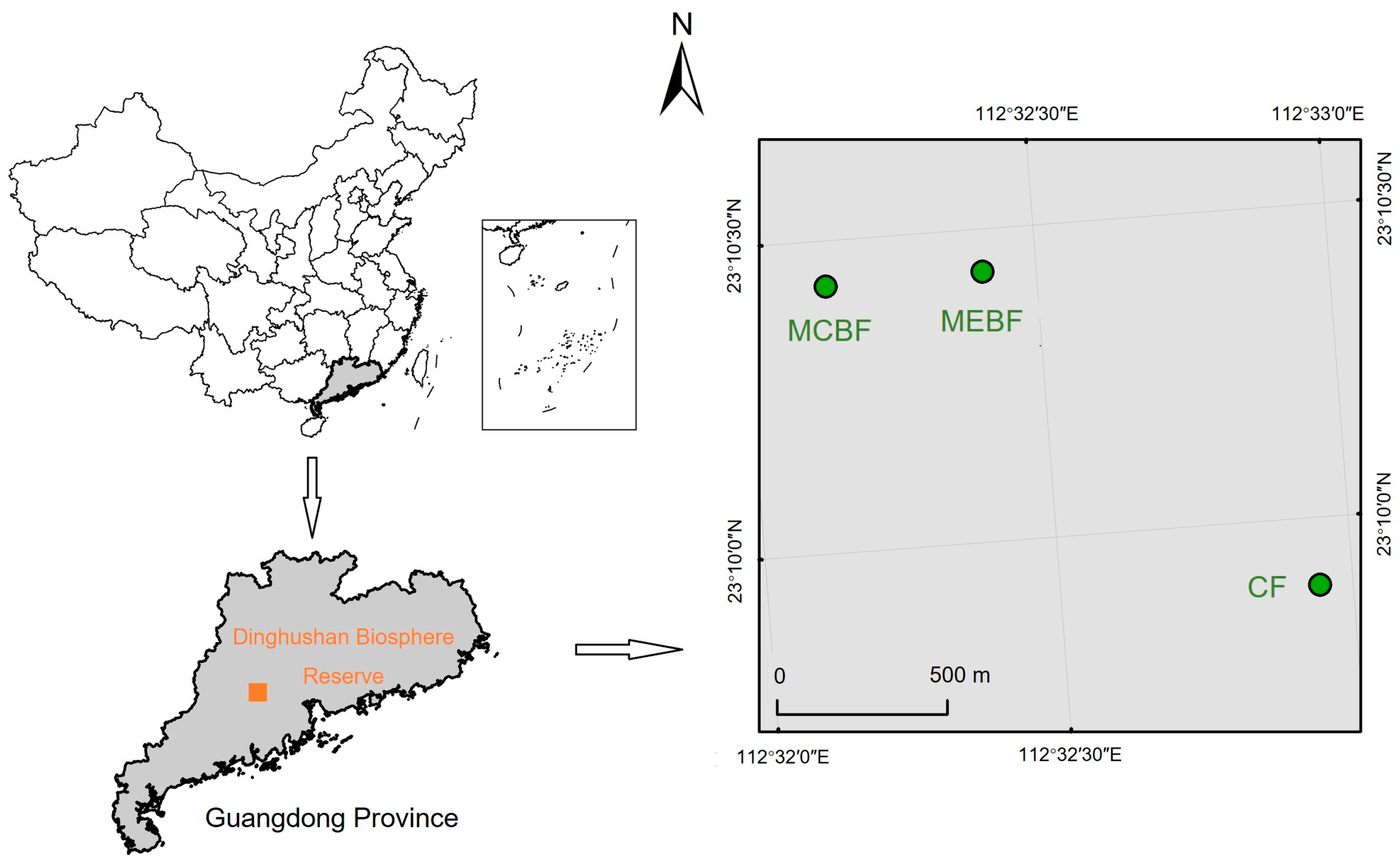
2.1. Study Area
2.2. Field Sampling
2.3. Sample Treatment and Isotope Analysis
2.4. Calculation of Water Utilization Characteristics of Trees
2.5. Examination of Soil and Plant Properties
2.6. Statistical Analyses
3. Results
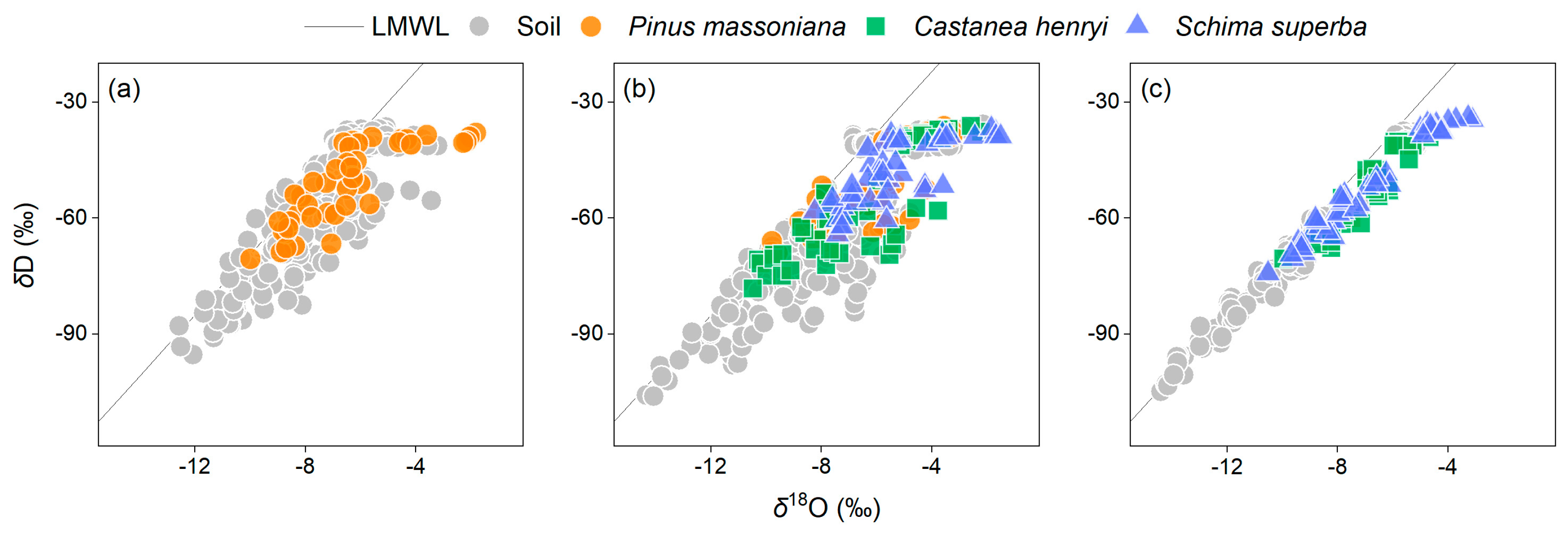
3.1. δ18O and δD of Soil and Xylem Water in Forests along Successional Gradients
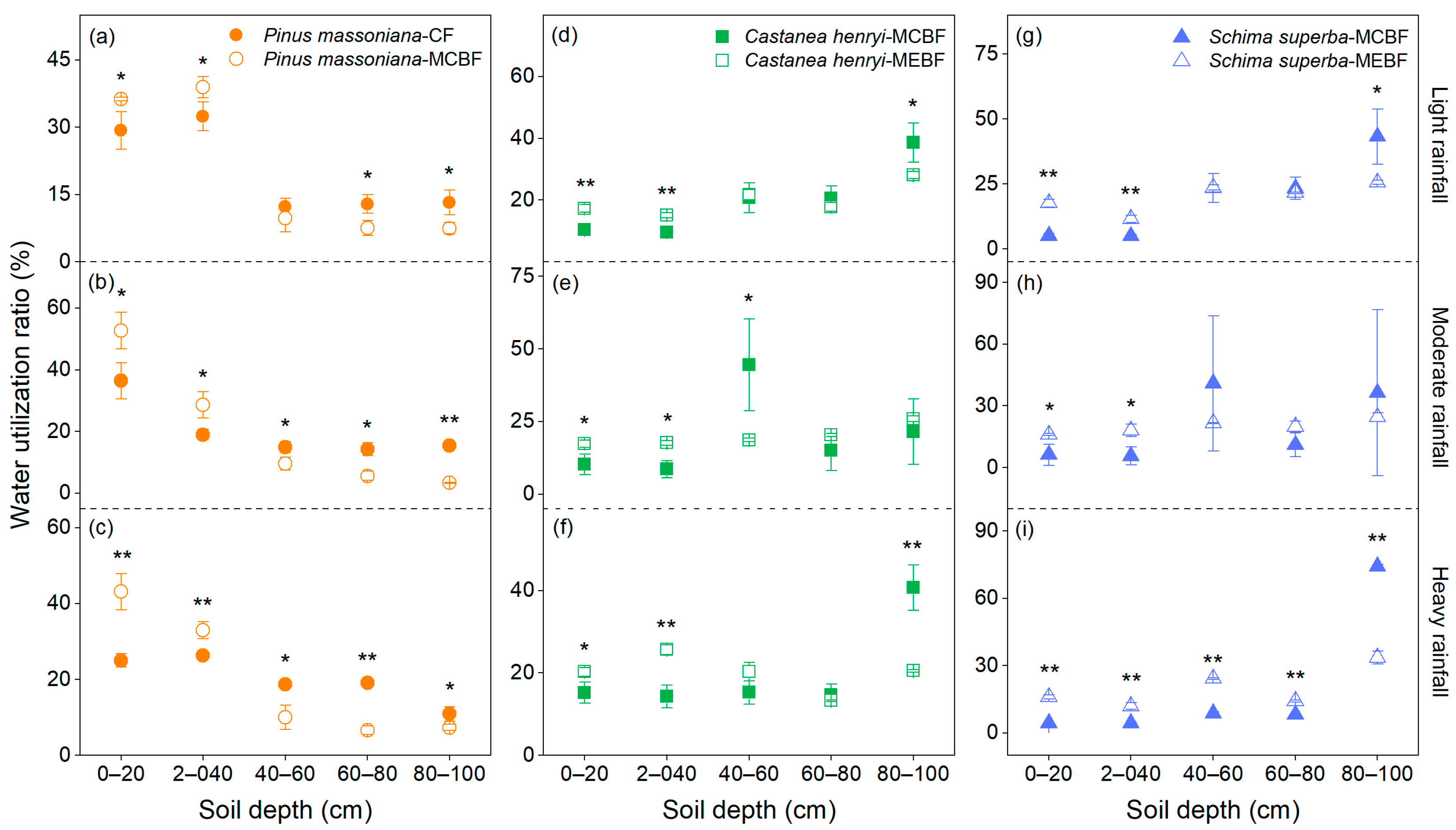
3.2. Water Utilization Characteristics of Dominant Trees in Forests at Different Successional Stages
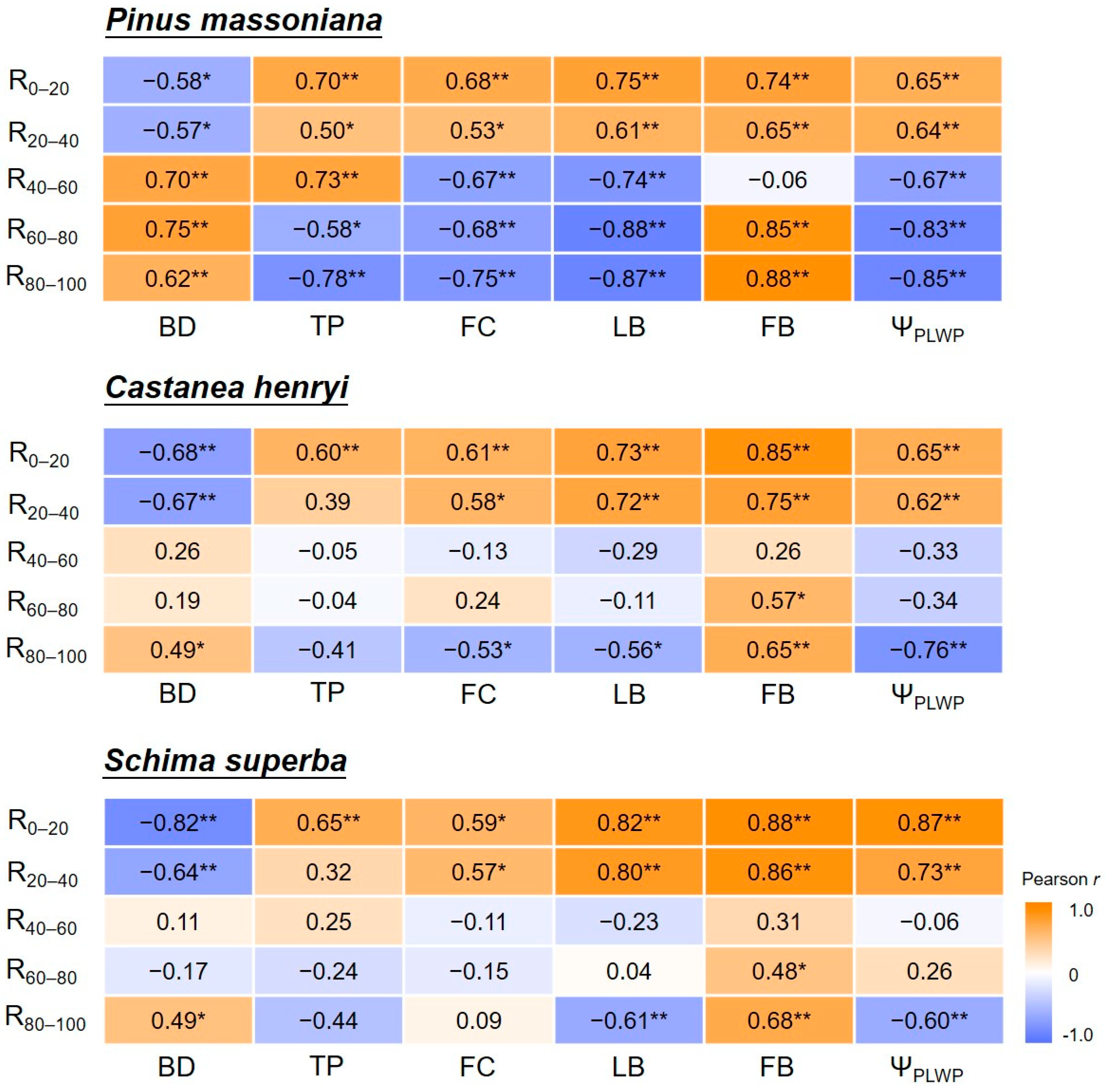
3.3. Factors Affecting Water Utilization Characteristics of Trees in Forests along Successional Gradients
3.4. Primary Drivers of Water Utilization of Trees in Different Successional Forests
4. Discussion
4.1. Differences in Water Utilization of Each Tree in Forests Along Successional Gradients
4.2. Primary Factors Affecting the Water Utilization Characteristics of Various Trees in Different Successional Forests
4.3. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, A.P.; Field, C.B. Forest fires and climate-induced tree range shifts in the western US. Nat. Commun. 2021, 12, 6583. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Semenchuk, P.; Essl, F.; Lenzner, B.; Moser, D.; Blackburn, T.M.; Cassey, P.; Biancolini, D.; Capinha, C.; Dawson, W.; et al. The impact of land use on non-native species incidence and number in local assemblages worldwide. Nat. Commun. 2023, 14, 2090. [Google Scholar] [CrossRef] [PubMed]
- Massey, R.; Rogers, B.M.; Berner, L.T.; Cooperdock, S.; Mack, M.C.; Walker, X.J.; Goetz, S.J. Forest composition change and biophysical climate feedbacks across boreal North America. Nat. Clim. Chang. 2023, 13, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Hornslein, N.J.; Siegert, C.; Renninger, H.J. Physiological response of mid-canopy sweetgum trees to overstory loblolly pine mortality. Trees 2019, 33, 139–151. [Google Scholar] [CrossRef]
- Tor-ngern, P.; Chart-asa, C.; Chanthorn, W.; Rodtassana, C.; Yampum, S.; Unawong, W.; Nathalang, A.; Brockelman, W.; Srinoppawan, K.; Chen, Y.; et al. Variation of leaf-level gas exchange rates and leaf functional traits of dominant trees across three successional stages in a Southeast Asian tropical forest. Forest Ecol. Manag. 2021, 489, 119101. [Google Scholar] [CrossRef]
- Grossiord, C.; Sevanto, S.; Dawson, T.E.; Adams, H.D.; Collins, A.D.; Dickman, L.T.; Newman, B.D.; Stockton, E.A.; Mcdowell, N.G. Warming combined with more extreme precipitation regimes modifies the water sources used by trees. New Phytol. 2017, 213, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Li, P.; Li, Z.; Xiao, L.; Zhao, B.; Su, Y.; Feng, Z. Using water isotopes to analyze water uptake during vegetation succession on abandoned cropland on the Loess Plateau, China. Catena 2019, 181, 104095. [Google Scholar] [CrossRef]
- Karatassiou, M. Water use efficiency and net production of two semi arid grasslands in different successional stages. Appl. Ecol. Env. Res. 2016, 14, 41–53. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Hu, X.; Zheng, P.; Hirota, M.; Kamijo, T. Photosynthetic Properties of Co-Occurring Pioneer Species on Volcanically Devastated Sites in Miyake-jima Island, Japan. Plants 2021, 10, 2500. [Google Scholar] [CrossRef]
- Ávila-Lovera, E.; Urich, R.; Coronel, I.; Tezara, W. Ecophysiological traits change little along a successional gradient in a tropical dry deciduous woodland from Margarita Island, Venezuela. Front. For. Glob. Change 2023, 6, 1043574. [Google Scholar] [CrossRef]
- Yamanaka, T. Root functional change achieves water source separation under vegetation succession. Ecohydrology 2018, 11, e1985. [Google Scholar] [CrossRef]
- Ghimire, C.P.; van Meerveld, H.J.; Zwartendijk, B.W.; Bruijnzeel, L.A.; Ravelona, M.; Lahitiana, J.; Lubczynski, M.W. Vapour pressure deficit and solar radiation are the major drivers of transpiration in montane tropical secondary forests in eastern Madagascar. Agr. Forest Meteorol. 2022, 326, 109159. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Durfee, N.; Mata-Gonzalez, R.; Ochoa, C.G.; Noller, J.S. Water use and soil moisture relationships on western juniper trees at different growth stages. Water 2020, 12, 1596. [Google Scholar] [CrossRef]
- Mata-González, R.; Abdallah, M.A.; Ochoa, C.G. Water use by mature and sapling western juniper (Juniperus occidentalis) trees. Rangel. Ecol. Manag. 2021, 74, 110–113. [Google Scholar] [CrossRef]
- Michael, R.N.; Yu, B.; Wintle, B.A.; Doronila, I.A.; Yuen, S.T.S. The effect of substrate compaction on plant water use and the implications for phytocap design specifications. Ecol. Eng. 2019, 127, 195–203. [Google Scholar] [CrossRef]
- Rasa, K.; Heikkinen, J.; Hannula, M.; Arstila, K.; Kulju, S.; Hyväluoma, J. How and why does willow biochar increase a clay soil water retention capacity? Biomass Bioenerg. 2018, 119, 346–353. [Google Scholar] [CrossRef]
- Zou, S.; Li, D.; Di, N.; Liu, J.; Li, L.; Liu, Y.; Xi, B.; Coleman, M. Stand development modifies effects of soil water availability on poplar fine-root traits: Evidence from a six-year experiment. Plant Soil 2022, 480, 165–184. [Google Scholar] [CrossRef]
- Ganesan, S.P.; Boldrin, D.; Leung, A.K. A closer look at root water potential: Experimental evidence based on drought stress of Chrysopogon zizanioides. Plant Soil 2024, 499, 569–585. [Google Scholar] [CrossRef]
- Du, H.; Fu, W.; Song, T.; Zeng, F.; Wang, K.; Chen, H.; Liu, M. Water-use efficiency in a humid karstic forest in southwestern China: Interactive responses to the environmental drivers. J. Hydrol. 2023, 617, 128973. [Google Scholar] [CrossRef]
- Kaneko, T.; Gould, N.; Campbell, D.; Snelgar, P.; Clearwater, M.J. The effect of soil type, fruit load and shaded area on hass avocado (Persea americana mill.) water use and crop coefficients. Agric. Water Manag. 2022, 264, 107519. [Google Scholar] [CrossRef]
- Song, X.; Lyu, S.; Wen, X. Limitation of soil moisture on the response of transpiration to vapor pressure deficit in a subtropical coniferous plantation subjected to seasonal drought. J. Hydrol. 2020, 591, 125301. [Google Scholar] [CrossRef]
- Oliveira, C.E.; Zoz, T.; Castagnara, D.D.; Zoz, A.; Mortinho, E.S.; Fernandes, G.C.; Lustosa Sobrinho, R.; Faria, G.A. Growth of Crambe under Different Soil Bulk Densities and Water Restriction. Russ. J. Plant Physiol. 2023, 70, 63. [Google Scholar] [CrossRef]
- Melo, M.L.A.D.; Lier, Q.D.J. FluxPAW: A standalone software to calculate flux-based plant available water. Softw. Impacts 2023, 15, 100464. [Google Scholar] [CrossRef]
- Voltas, J.; Lucabaugh, D.; Chambel, M.R.; Ferrio, J.P. Intraspecific Variation in the Use of Water Sources by the Circum-Mediterranean Conifer Pinus halepensis. New Phytol. 2015, 208, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Choo, H.; Ji, K. Effect of temperature on electrical conductivity of soils—Role of surface conduction. Eng. Geol. 2023, 321, 107147. [Google Scholar] [CrossRef]
- Wang, D.; Fu, B.; Zhao, W.; Hu, H.; Wang, Y. Multifractal Characteristics of Soil Particle Size Distribution under Different Land-Use Types on the Loess Plateau, China. CATENA 2008, 72, 29–36. [Google Scholar] [CrossRef]
- Ankenbauer, K.J.; Loheide, S.P.I. The effects of soil organic matter on soil water retention and plant water use in a meadow of the Sierra Nevada, CA. Hydrol. Process. 2017, 31, 891–901. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; Qian, S.; He, M.; Cai, X.; Ding, J. Root characteristics explain greater water use efficiency and drought tolerance in invasive Compositae plants. Plant Soil 2023, 483, 209–223. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, M.; Dao, J.; Lin, L.; Chen, C.; Chen, Y.; Wang, Z. Fine-root morphology of woody and herbaceous plants responds differently to altered precipitation: A meta-analysis. Forest Ecol. Manag. 2024, 552, 121570. [Google Scholar] [CrossRef]
- Sun, G.; Alstad, K.; Chen, J.; Chen, S.; Ford, C.R.; Lin, G.; Liu, C.; Lu, N.; McNulty, S.G.; Miao, H.; et al. A general predictive model for estimating monthly ecosystem evapotranspiration. Ecohydrology 2011, 4, 245–255. [Google Scholar] [CrossRef]
- Hardanto, A.; Röll, A.; Hendrayanto, H.D. Tree soil water uptake and transpiration in mono-cultural and jungle rubber stands of Sumatra. Forest. Ecol. Manage. 2017, 397, 67–77. [Google Scholar] [CrossRef]
- Ratzmann, G.; Zakharova, L.; Tietjen, B. Optimal leaf water status regulation of plants in drylands. Sci. Rep. 2019, 9, 3768. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, G.; Yasutake, D.; Minami, K.; Kimura, K.; Marui, A.; Yueru, W.; Feng, J.; Wang, W.; Mori, M.; Kitano, M. Evaluation of the physiological significance of leaf wetting by dew as a supplemental water resource in semi-arid crop production. Agric. Water Manag. 2021, 255, 106964. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Q.; Zhang, B.; Gao, D.; Wang, T.; Xu, W.; Ren, R.; Wang, S. Contrasting Water-Use Patterns of Chinese Fir among Different Plantation Types in a Subtropical Region of China. Front. Plant Sci. 2022, 13, 946508. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.Y.; Huang, J.Q.; Zeng, X.P.; Zhang, Y.H.; Meng, Z.; Otieno, D.; Li, Y.L. Sap flow characteristics of the dominant tree species Pinus massoniana at the pioneer succession stage in the Dinghushan Mountain. Chin. J. Appl. Environ. Biol. 2022, 28, 1167–1174. [Google Scholar]
- Cheng, J.; Ouyang, X.; Huang, D.W.; Liu, S.Z.; Zhang, D.Q.; Li, Y.L. Sap flow characteristics of four dominant tree species in a mixed conifer-broadleaf forest in Dinghushan. Acta Ecol. Sin. 2015, 35, 4097–4104. [Google Scholar]
- Huang, Y.H.; Li, Y.L.; Xiao, Y.; Wenigmann, K.O.; Zhou, G.Y.; Zhang, D.Q.; Wenigmann, M.; Tang, X.L.; Liu, J.X. Controls of litter quality on the carbon sink in soils through partitioning the products of decomposing litter in a forest succession series in South China. Forest Ecol. Manag. 2011, 261, 1170–1177. [Google Scholar] [CrossRef]
- Li, Y.L.; Yang, F.F.; Ou, Y.X.; Zhang, D.Q.; Liu, J.X.; Chu, G.W.; Zhang, Y.R.; Otieno, D.; Zhou, G.Y. Changes in forest soil properties in different successional stages in lower tropical China. PLoS ONE 2013, 8, e81359. [Google Scholar] [CrossRef]
- Yan, J.H.; Wang, Y.P.; Zhou, G.Y.; Zhang, D.Q. Estimates of soil respiration and net primary production of three forests at different succession stages in South China. Global Change Biol. 2006, 12, 810–821. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Roden, J.; Dawson, T.E. Assessing ecosystem-level water relations through stable isotope ratio analyses. In Methods in Ecosystem Science; Sala, O.E., Jackson, R.B., Mooney, H.A., Howarth, R.W., Eds.; Springer: New York, NY, USA, 2000; pp. 181–198. [Google Scholar]
- Wang, J.; Fu, B.; Wang, L.; Lu, N.; Li, J. Water use characteristics of the common tree species in different plantation types in the Loess Plateau of China. Agr. Forest Meteorol. 2020, 288–289, 108020. [Google Scholar] [CrossRef]
- Stock, B.C.; Semmens, B.X. MixSIAR GUI User Manual, Version 3.1. 2013. Available online: https://www.imsbio.co.jp/RGM-files/R_CC/download/MixSIAR/inst/mixsiar_manual_3.1_small.pdf (accessed on 26 March 2024).
- Ozcan, M.; Gökbulak, F.; Hizal, A. Exclosure effects on recovery of selected soil properties in a mixed broadleaf forest recreation site. Land Degrad. Dev. 2011, 24, 266–276. [Google Scholar] [CrossRef]
- Xue, C.; Xu, Q.; Lin, L.; He, X.; Cao, L.; Li, H. Biomass Dynamic Predicting for Schima superba in Guangdong Based on Allometric and Theoretical Growth Equation. Sci. Silvae Sin. 2019, 55, 86–94. [Google Scholar]
- Zhang, Z.; Jin, G.Q.; Zhou, Z.C.; Sun, L.S. Biomass allocation differences with Pinus massoniana in Guangdong and Hubei provenances. J. Zhejiang A F Univ. 2019, 36, 271–278. [Google Scholar]
- Dhiman, I.; Bilheux, H.; DeCarlo, K.; Painter, S.L.; Santodonato, L.; Warren, J.M. Quantifying root water extraction after drought recovery using sub-mm in situ empirical data. Plant Soil 2018, 424, 73–89. [Google Scholar] [CrossRef]
- Mackay, D.S.; Savoy, P.R.; Grossiord, C.; Tai, X.; Pleban, J.R.; Wang, D.R.; McDowell, N.G.; Adams, H.D.; Sperry, J.S. Conifers depend on established roots during drought: Results from a coupled model of carbon allocation and hydraulics. New Phytol. 2020, 225, 679–692. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, G.; Bai, W.; Yuan, R.; Zhang, Y. Root Distribution and Water Uptake Applied by Hydrogen and Oxygen Stable Isotopes for Lianas in Northwest China. Forests 2024, 15, 626. [Google Scholar] [CrossRef]
- Vanhellemont, M.; Sousa-Silva, R.; Maes, S.L.; Van den Bulcke, J.; Hertzog, L.; DeGroote, S.R.E.; Van Acker, J.; Bonte, D.; Martel, A.; Lens, L.; et al. Distinct growth responses to drought for oak and beech in temperate mixed forests. Sci. Total Environ. 2019, 650, 3017–3026. [Google Scholar] [CrossRef]
- Kühnhammer, K.; van Haren, J.; Kübert, A.; Bailey, K.; Dubbert, M.; Hu, J.; Ladd, N.; Meredith, L.K.; Werner, C.; Beyer, M. Deep roots mitigate drought impacts on tropical trees despite limited quantitative contribution to transpiration. Sci. Total Environ. 2023, 893, 164763. [Google Scholar] [CrossRef]
- Dybzinski, R.; Taylor, N.; Prosser, M.; Niosi, O.; Demo, M.; Kilbane, E. Nitrogen, water, and phosphorus uptake as functions of fine-root mass in greenhouse microcosms of Poa pratensis. Plant Ecol. 2021, 222, 977–991. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, D.; Di, N.; Li, D.; Zhou, O.; Sun, Y.; Jia, L.; Ding, C.; Xi, B. Matching root water uptake patterns to fine root and soil water distributions. Plant Soil 2024, 495, 499–516. [Google Scholar] [CrossRef]
- Xi, B.; Wang, Y.; Jia, L.; Bloomberg, M.; Li, G.; Di, N. Characteristics of fine root system and water uptake in a triploid Populus tomentosa plantation in the North China Plain: Implications for irrigation water management. Agric. Water Manag. 2013, 117, 83–92. [Google Scholar] [CrossRef]
- Masumoto, T.; Ito, T.; Akatsuki, M.; Makita, N. Fine root hydraulic conductivity relates to root functional traits in four coniferous species. Rhizosphere 2022, 21, 100489. [Google Scholar] [CrossRef]
- Fort, F.; Volaire, F.; Guilioni, L.; Barkaoui, K.; Navas, M.L.; Roumet, C. Root traits are related to plant water-use among rangeland Mediterranean species. Funct. Ecol. 2017, 31, 1700–1709. [Google Scholar] [CrossRef]
- Thomas, A.; Yadav, B.K.; Šimůnek, J. Root water uptake under heterogeneous soil moisture conditions: An experimental study for unraveling compensatory root water uptake and hydraulic redistribution. Plant Soil 2020, 457, 421–435. [Google Scholar] [CrossRef]
- Vanderborght, J.; Couvreur, V.; Javaux, M.; Leitner, D.; Schnepf, A.; Vereecken, H. Mechanistically derived macroscopic root water uptake functions: The α and ω of root water uptake functions. Vadose Zone J. 2024, 23, e20333. [Google Scholar] [CrossRef]
- Ding, Y.; Nie, Y.; Chen, H.; Wang, K.; Querejeta, J.I. Water uptake depth is coordinated with leaf water potential, water-use efficiency and drought vulnerability in karst vegetation. New Phytol. 2021, 229, 1339–1353. [Google Scholar] [CrossRef]
- Altieri, G.; Wiman, N.G.; Santoro, F.; Amato, M.; Celano, G. Assessment of leaf water potential and stomatal conductance as early signs of stress in young hazelnut tree in Willamette valley. Sci. Hortic. 2024, 327, 112817. [Google Scholar] [CrossRef]
- Bello, J.; Hasselquist, N.J.; Vallet, P.; Kahmen, A.; Perot, T.; Korboulewsky, N. Complementary water uptake depth of Quercus petraea and Pinus sylvestris in mixed stands during an extreme drought. Plant Soil 2019, 437, 93–115. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, G.; Li, X.; Wang, Y.; He, B.; Jiang, Z.; Zhang, S.; Sun, W. Identifying water sources used by alpine riparian plants in a restoration zone on the Qinghai-Tibet Plateau: Evidence from stable isotopes. Sci. Total Environ. 2019, 697, 134092. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, D.; Xu, Q.; Zuo, H.; Xu, W.; Diao, K.; Zhang, B. Changes in Water Utilization Characteristics of Trees in Forests across a Successional Gradient in Southern China. Forests 2024, 15, 1329. https://doi.org/10.3390/f15081329
Gao D, Xu Q, Zuo H, Xu W, Diao K, Zhang B. Changes in Water Utilization Characteristics of Trees in Forests across a Successional Gradient in Southern China. Forests. 2024; 15(8):1329. https://doi.org/10.3390/f15081329
Chicago/Turabian StyleGao, Deqiang, Qing Xu, Haijun Zuo, Wenbin Xu, Ke Diao, and Beibei Zhang. 2024. "Changes in Water Utilization Characteristics of Trees in Forests across a Successional Gradient in Southern China" Forests 15, no. 8: 1329. https://doi.org/10.3390/f15081329




