Hypericin Inhibit Alpha-Coronavirus Replication by Targeting 3CL Protease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines, Virus, Reagents, and Antibodies
2.2. Cytotoxicity Assay
2.3. Antiviral Assays on PEDV and TGEV
2.4. Immunofluorescence Assay
2.5. Quantitative Real-Time PCR Assay
2.6. Western Blot Assay
2.7. TCID50 Assay
2.8. Molecular Docking
2.9. Protein Expression and Purification
2.10. PEDV 3CLpro Enzymatic Assay
2.11. Alignments of CoVs 3CLpro in Protein Sequence and Crystal Structure
2.12. Statistical Analysis
3. Results
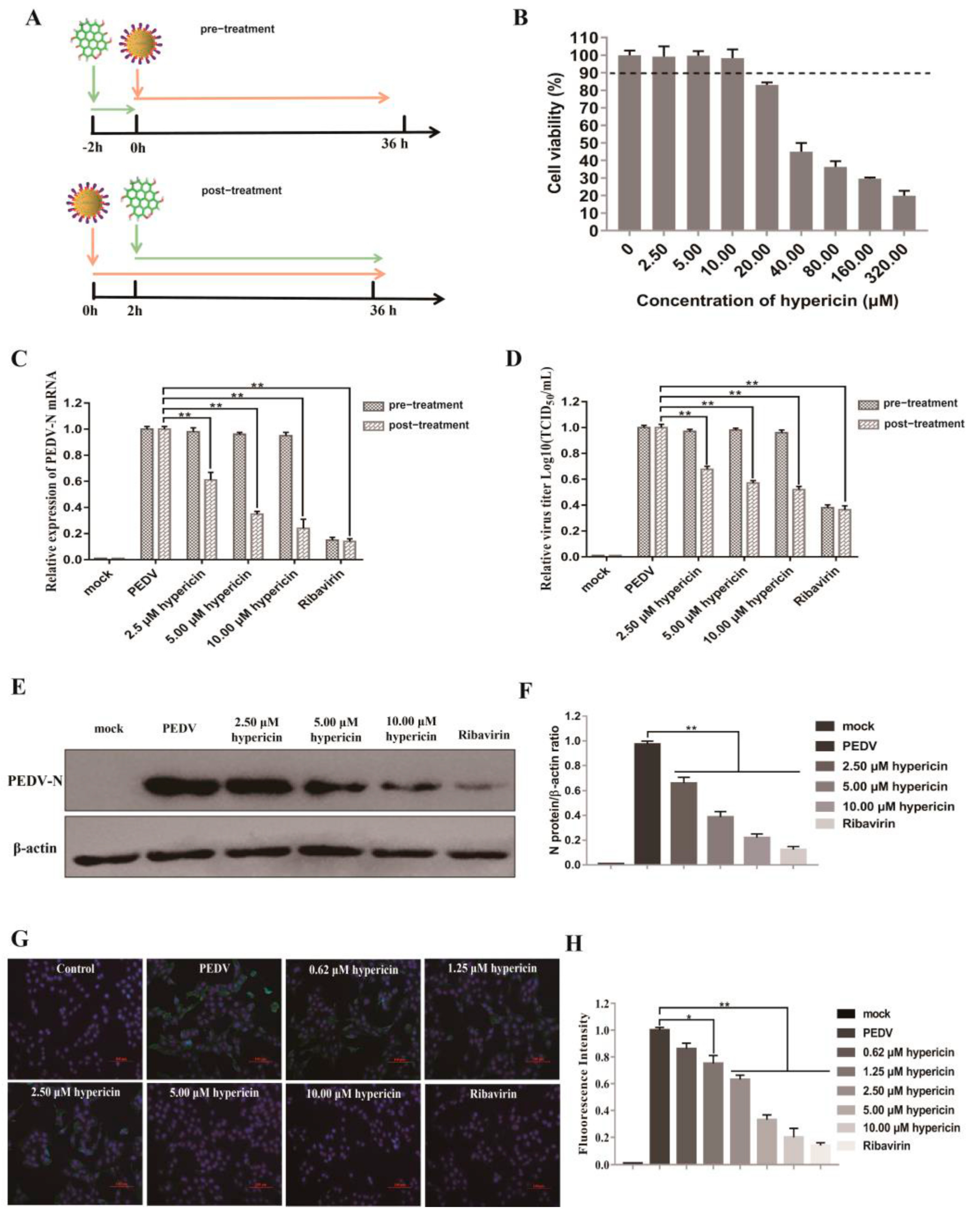
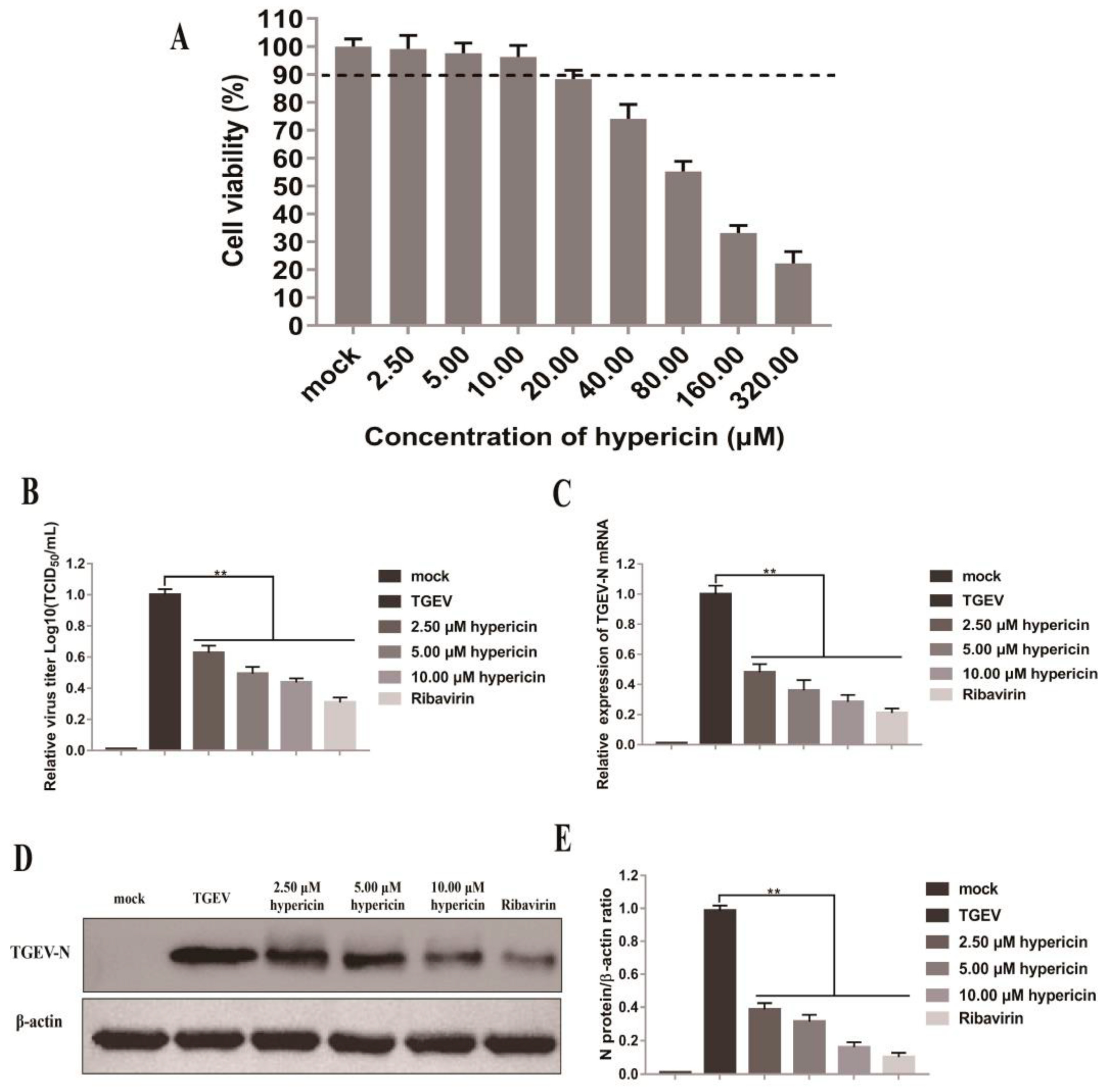
3.1. Hypericin in PEDV-Infected Cells
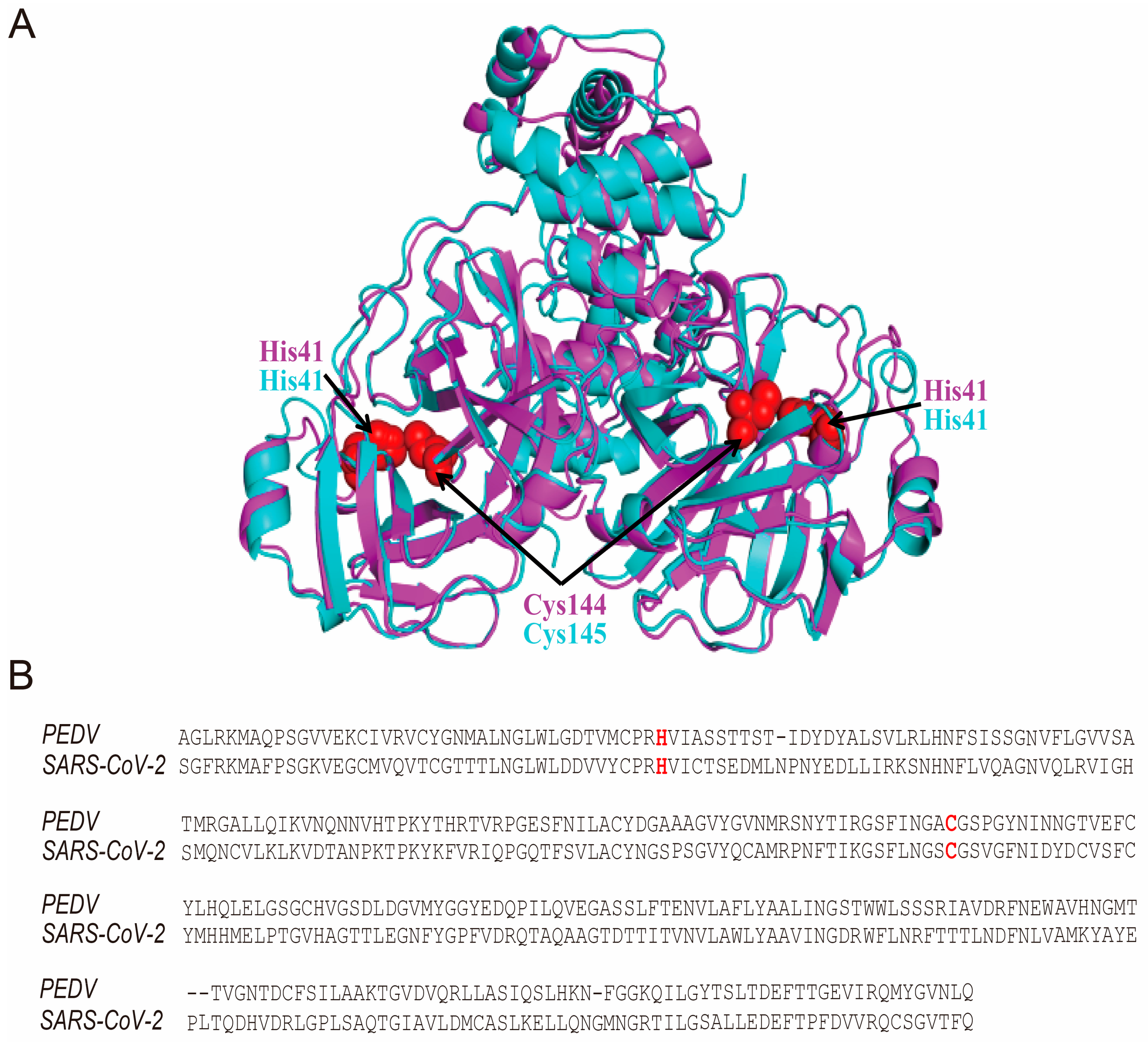
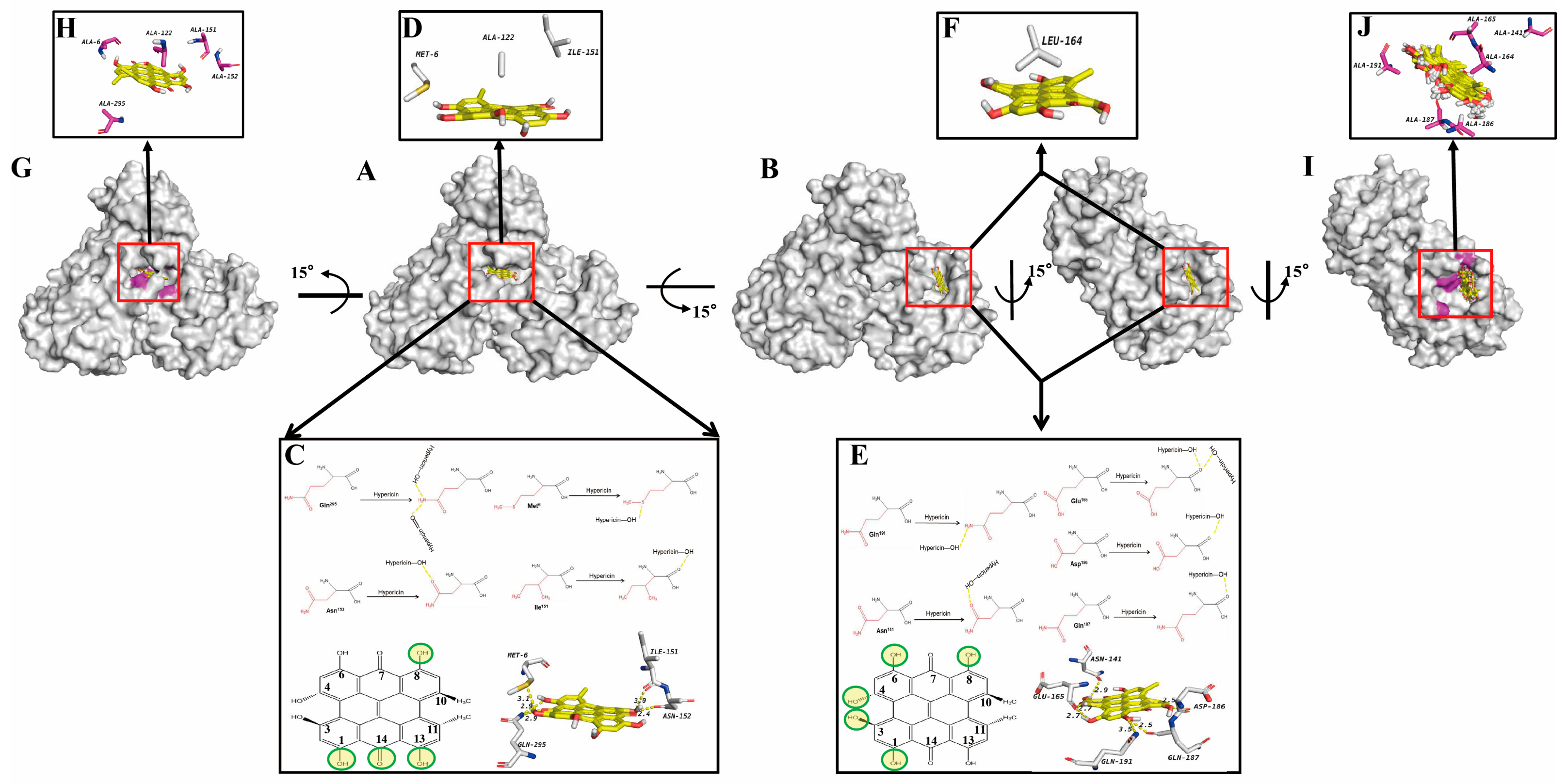
3.2. Molecular Docking of Hypericin onto PEDV 3CLpro
3.3. In Vitro 3CLpro Enzymatic Inhibition by Hypericin
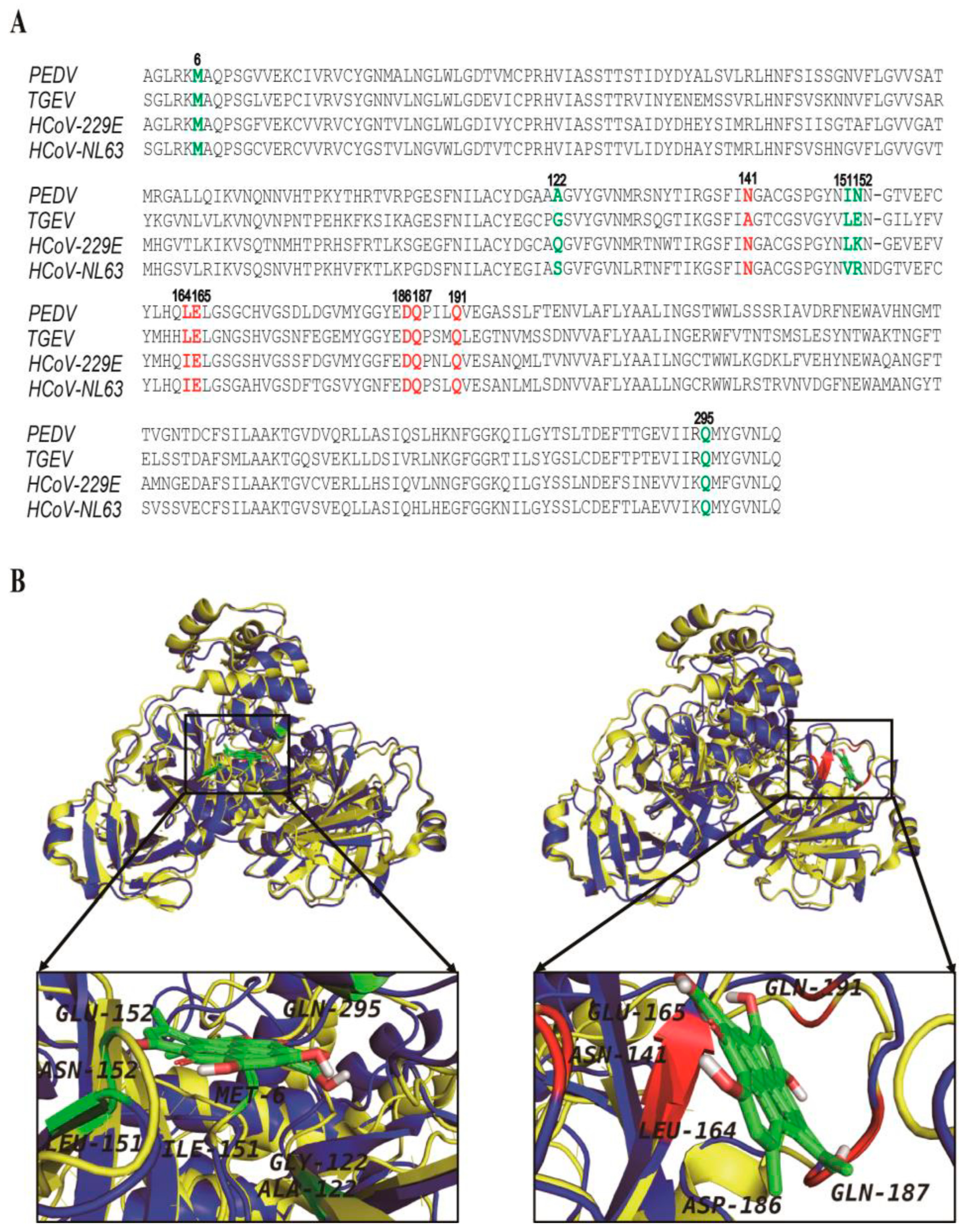
3.4. The Hypericin-3CLpro Binding Domains Are Highly Conserved in α-CoV
3.5. Antiviral Activity of Hypericin on TGEV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Turlewicz-Podbielska, H.; Pomorska-Mól, M. Porcine coronaviruses: Overview of the state of the art. Virol. Sin. 2021, 1–19. [Google Scholar] [CrossRef]
- Choudhury, B.; Dastjerdi, A.; Doyle, N.; Frossard, J.-P.; Steinbach, F. From the field to the lab—An European view on the global spread of PEDV. Virus Res. 2016, 226, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, C. Outbreak-related porcine epidemic diarrhea virus strains similar to US Strains, South Korea, 2013. Emerg. Infect. Dis. 2014, 20, 1223–1226. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q.; Huang, L.; Yuan, C.; Wang, J.; Yang, Q. An alternative pathway of enteric PEDV dissemination from nasal cavity to intestinal mucosa in swine. Nat. Commun. 2018, 9, 3811. [Google Scholar] [CrossRef]
- Cong, Y.; Ulasli, M.; Schepers, H.; Mauthe, M.; V’Kovski, P.; Kriegenburg, F.; Thiel, V.; de Haan, C.; Reggiori, F. Nucleocapsid Protein Recruitment to Replication-Transcription Complexes Plays a Crucial Role in Coronaviral Life Cycle. J. Virol. 2020, 94, e01925-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, M.; Su, H.; Zhao, W.; Xie, H.; Shao, Q.; Xu, Y. What coronavirus 3C-like protease tells us: From structure, substrate selectivity, to inhibitor design. Med. Res. Rev. 2021, 41, 1965–1998. [Google Scholar] [CrossRef] [PubMed]
- Shivanika, C.; Deepak, K.S.; Venkataraghavan, R.; Pawan, T.; Sumitha, A.; Brindha, D.P. Molecular docking, validation, dynamics simulations, and pharmacokinetic prediction of natural compounds against the SARS-CoV-2 main-protease. J. Biomol. Struct. Dyn. 2020, 1, 1–27. [Google Scholar] [CrossRef]
- Islam, R.; Parves, R.; Paul, A.S.; Uddin, N.; Rahman, S.; Al Mamun, A.; Hossain, N.; Ali, A.; Halim, M.A. A molecular modeling approach to identify effective antiviral phytochemicals against the main protease of SARS-CoV-2. J. Biomol. Struct. Dyn. 2020, 39, 3213–3224. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, R.; Ruiz-Moreno, A.J.; Cong, Y.; Kumar, N.D.; Velasco-Velazquez, M.A.; Neochoritis, C.G.; Smith, J.; Reggiori, F.; Groves, M.R.; Dömling, A. Repurposing the HCV NS3–4A protease drug boceprevir as COVID-19 therapeutics. RSC Med. Chem. 2021, 12, 370–379. [Google Scholar] [CrossRef]
- China, Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Regulations on the Administration of Veterinary Drugs. Notice No. 250 of the Ministry of Agriculture and Villages of the People’s Republic of China; 2019. Available online: http://www.moa.gov.cn/gk/tzgg_1/gg/202001/t20200106_6334375.htm (accessed on 6 January 2020).
- Ali, S.I.; Sheikh, W.M.; Rather, M.A.; Venkatesalu, V.; Bashir, S.M.; Nabi, S.U. Medicinal plants: Treasure for antiviral drug discovery. Phytother. Res. 2021, 35, 3447–3483. [Google Scholar] [CrossRef]
- Tahmasebi-Boldaji, R.; Hatamipour, M.-S.; Khanahmadi, M.; Sadeh, P.; Najafipour, I. Ultrasound-assisted packed-bed extraction of hypericin from Hypericum perforatum L. and optimization by response surface methodology. Ultrason. Sonochem. 2019, 57, 89–97. [Google Scholar] [CrossRef]
- Tang, J.; Colacino, J.M.; Larsen, S.H.; Spitzer, W. Virucidal activity of hypericin against enveloped and non-enveloped DNA and RNA viruses. Antivir. Res. 1990, 13, 313–325. [Google Scholar] [CrossRef]
- Du, X.; Xiao, R.; Fu, H.; Yuan, Z.; Zhang, W.; Yin, L.; He, C.; Li, C.; Zhou, J.; Liu, G.; et al. Hypericin-loaded graphene oxide protects ducks against a novel duck reovirus. Mater. Sci. Eng. C 2019, 105, 110052. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Cheng, W.; Wang, Y.; Yi, K.; Wang, Z.; Zhang, Y.; Shao, L.; Zhao, T. Hypericin-photodynamic therapy inhibits the growth of adult T-cell leukemia cells through induction of apoptosis and suppression of viral transcription. Retrovirology 2019, 16, 5. [Google Scholar] [CrossRef]
- Damke, G.M.Z.F.; Damke, E.; Bonfim-Mendonça, P.D.S.; Ratti, B.A.; Meirelles, L.E.D.F.; da Silva, V.R.S.; Gonçalves, R.S.; César, G.B.; Silva, S.D.O.; Caetano, W.; et al. Selective photodynamic effects on cervical cancer cells provided by P123 Pluronic®-based nanoparticles modulating hypericin delivery. Life Sci. 2020, 255, 117858. [Google Scholar] [CrossRef]
- Sardoiwala, M.N.; Kushwaha, A.C.; Dev, A.; Shrimali, N.; Guchhait, P.; Karmakar, S.; Choudhury, S.R. Hypericin-loaded transferrin nanoparticles induce PP2A-regulated BMI1 degradation in colorectal cancer-specific chemo-photodynamic therapy. ACS Biomater. Sci. Eng. 2020, 6, 3139–3153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, Y.; Li, B.; Petijová, L.; Hu, S.; Zhang, Q.; Niu, J.; Wang, D.; Wang, S.; Dong, Y.; et al. Whole-genome sequence data of Hypericum perforatum and functional characterization of melatonin biosynthesis by N-acetylserotonin O-methyltransferase. J. Pineal Res. 2020, 70, e12709. [Google Scholar] [CrossRef] [PubMed]
- Zirak, N.; Shafiee, M.; Soltani, G.; Mirzaei, M.; Sahebkar, A. Hypericum perforatum in the treatment of psychiatric and neurodegenerative disorders: Current evidence and potential mechanisms of action. J. Cell. Physiol. 2019, 234, 8496–8508. [Google Scholar] [CrossRef]
- Pal, D.; Mitra, A.K. MDR- and CYP3A4-mediated drug–herbal interactions. Life Sci. 2006, 78, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-M.; Wu, C.-H.; Wu, W.-J.; Hsiao, Y.-M.; Ko, J.-L. Hypericin inhibits hepatitis C virus replication via deacetylation and down-regulation of heme oxygenase-1. Phytomedicine 2018, 46, 193–198. [Google Scholar] [CrossRef]
- Chen, H.; Feng, R.; Muhammad, I.; Abbas, G.; Zhang, Y.; Ren, Y.; Huang, X.; Zhang, R.; Diao, L.; Wang, X.; et al. Protective effects of hypericin against infectious bronchitis virus induced apoptosis and reactive oxygen species in chicken embryo kidney cells. Poult. Sci. 2019, 98, 6367–6377. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, K.M.; Zhang, H.; Senthil, R.; Vijayakumar, K.K.; Sounderrajan, V.; Wei, Y.; Shakila, H. Structural basis for the inhibition of SARS-CoV2 main protease by Indian medicinal plant-derived antiviral compounds. J. Biomol. Struct. Dyn. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Li, X.; Bai, Y.; Lv, X.; Herrler, G.; Enjuanes, L.; Zhou, X.; Qu, B.; Meng, F.; Cong, C.; et al. Porcine aminopeptidase N mediated polarized infection by porcine epidemic diarrhea virus in target cells. Virology 2015, 478, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, X.; Wang, P.; Bai, R.; Cong, Y.; Suo, S.; Ren, X.; Chen, C. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials 2014, 35, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, C. Ribavirin efficiently suppresses porcine nidovirus replication. Virus Res. 2013, 171, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Gao, Y.; Ren, X.; Ren, Y.; Ge, X.; Li, G. Porcine epidemic diarrhea virus infection induces NF-κB activation through the TLR2, TLR3 and TLR9 pathways in porcine intestinal epithelial cells. J. Gen. Virol. 2015, 96, 1757–1767. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Reed, L.; Muench, H. A Simple method of estimating fifty per cent endpoints12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Goodsell, D.S.; Sanner, M.F.; Olson, A.J.; Forli, S. The AutoDock suite at 30. Protein Sci. 2021, 30, 31–43. [Google Scholar] [CrossRef]
- Tanchuk, V.Y.; Tanin, V.O.; Vovk, A.I.; Poda, G. A New, Improved Hybrid Scoring Function for Molecular Docking and Scoring Based on AutoDock and AutoDock Vina. Chem. Biol. Drug Des. 2016, 87, 618–625. [Google Scholar] [CrossRef]
- Ye, G.; Deng, F.; Shen, Z.; Luo, R.; Zhao, L.; Xiao, S.; Fu, Z.F.; Peng, G. Structural basis for the dimerization and substrate recognition specificity of porcine epidemic diarrhea virus 3C-like protease. Virology 2016, 494, 225–235. [Google Scholar] [CrossRef]
- Liang, Y.-Y.; Zhang, J.; Cui, H.; Shao, Z.-S.; Cheng, C.; Wang, Y.-B.; Wang, H.-S. Fluorescence resonance energy transfer (FRET)-based nanoarchitecture for monitoring deubiquitinating enzyme activity. Chem. Commun. 2020, 56, 3183–3186. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-Y.; Li, C.; Fischer, M.; Cairo, C.W.; Feng, Y.; Withers, S.G. A FRET Probe for Cell-Based Imaging of Ganglioside-Processing Enzyme Activity and High-Throughput Screening. Angew. Chem. Int. Ed. Engl. 2015, 54, 5389–5393. [Google Scholar] [CrossRef] [PubMed]
- Theerawatanasirikul, S.; Kuo, C.J.; Phetcharat, N.; Lekcharoensuk, P. In silico and in vitro analysis of small molecules and natural compounds targeting the 3CL protease of feline infectious peritonitis virus. Antivir. Res. 2020, 174, 104697. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, F.; Yu, Y.; Wang, Y. Application of FRET Biosensors in Mechanobiology and Mechanopharmacological Screening. Front. Bioeng. Biotechnol. 2020, 8, 595497. [Google Scholar] [CrossRef] [PubMed]
- Lu, M. Single-molecule FRET imaging of virus spike-host interactions. Viruses 2021, 13, 332. [Google Scholar] [CrossRef]
- Suh, J.-S.; Kim, H.-S.; Kim, T.-J. Development of a SARS-CoV-2-derived receptor-binding domain-based ACE2 biosensor. Sens. Actuators B Chem. 2021, 334, 129663. [Google Scholar] [CrossRef]
- Luo, L.; Chen, J.; Li, X.; Qiao, D.; Wang, Z.; Wu, X.; Du, Q.; Tong, D.; Huang, Y. Establishment of method for dual simultaneous detection of PEDV and TGEV by combination of magnetic micro-particles and nanoparticles. J. Infect. Chemother. 2020, 26, 523–526. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Liu, Y.; Luo, X.; Lei, W.; Xie, L. Antiviral and virucidal effects of curcumin on transmissible gastroenteritis virus in vitro. J. Gen. Virol. 2020, 101, 1079–1084. [Google Scholar] [CrossRef]
- Taher, M.M.; Lammering, G.M.; Hershey, C.M.; Valerie, K.C. Mood-Enhancing Antidepressant St. John’s Wort Inhibits the Activation of Human Immunodeficiency Virus Gene Expression by Ultraviolet Light. IUBMB Life 2002, 54, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lu, C. Raman spectroscopic study on structure of human immunodeficiency virus (HIV) and hypericin-induced photosensitive damage of HIV. Sci. China Ser. C Life Sci. 2005, 48, 117–132. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Q. Emerging Highly Virulent Porcine Epidemic Diarrhea Virus: Molecular Mechanisms of Attenuation and Rational Design of Live Attenuated Vaccines. Int. J. Mol. Sci. 2019, 20, 5478. [Google Scholar] [CrossRef] [Green Version]
- Thangavel, N.; Al Bratty, M.; Al Hazmi, H.A.; Najmi, A.; Alaqi, R.O.A. Molecular Docking and Molecular Dynamics Aided Virtual Search of OliveNet™ Directory for Secoiridoids to Combat SARS-CoV-2 Infection and Associated Hyperinflammatory Responses. Front. Mol. Biosci. 2020, 7, 627767. [Google Scholar] [CrossRef]
- Yagiz, G.; Noma, S.A.A.; Altundas, A.; Al-Khafaji, K.; Taskin-Tok, T.; Ates, B. Synthesis, inhibition properties against xanthine oxidase and molecular docking studies of dimethyl N-benzyl-1H-1,2,3-triazole-4,5-dicarboxylate and (N-benzyl-1H-1,2,3-triazole-4,5-diyl)dimethanol derivatives. Bioorg. Chem. 2021, 108, 104654. [Google Scholar] [CrossRef]
- Chamizo-González, F.; Gordillo, B.; Heredia, F.J. Elucidation of the 3D structure of grape seed 7S globulin and its interaction with malvidin 3-glucoside: A molecular modeling approach. Food Chem. 2021, 347, 129014. [Google Scholar] [CrossRef]
- Yang, H.; Xie, W.; Xue, X.; Yang, K.; Ma, J.; Liang, W.; Zhao, Q.; Zhou, Z.; Pei, D.; Ziebuhr, J.; et al. Design of Wide-Spectrum Inhibitors Targeting Coronavirus Main Proteases. PLoS Biol. 2005, 3, e324. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Jeong, H.J.; Kim, J.H.; Kim, Y.M.; Park, J.-Y.; Kim, D.; Naguyen, T.T.H.; Park, S.-J.; Chang, J.S.; Park, K.H. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg. Med. Chem. 2010, 18, 7940–7947. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.H.; Woo, H.-J.; Kang, H.-K.; Nguyen, V.D.; Kim, Y.-M.; Kim, D.-W.; Ahn, S.-A.; Xia, Y.; Kim, D. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol. Lett. 2012, 34, 831–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abian, O.; Ortega-Alarcon, D.; Jimenez-Alesanco, A.; Ceballos-Laita, L.; Vega, S.; Reyburn, H.T.; Rizzuti, B.; Velazquez-Campoy, A. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int. J. Biol. Macromol. 2020, 164, 1693–1703. [Google Scholar] [CrossRef]
- Vincent, S.; Arokiyaraj, S.; Saravanan, M.; Dhanraj, M. Molecular Docking Studies on the Anti-viral Effects of Compounds from Kabasura Kudineer on SARS-CoV-2 3CLpro. Front. Mol. Biosci. 2020, 7, 613401. [Google Scholar] [CrossRef] [PubMed]
- Jade, D.; Ayyamperumal, S.; Tallapaneni, V.; Nanjan, C.M.J.; Barge, S.; Mohan, S.; Nanjan, M.J. Virtual high throughput screening: Potential inhibitors for SARS-CoV-2 PLPRO and 3CLPRO proteases. Eur. J. Pharmacol. 2021, 901, 174082. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cao, H.; Cheng, Y.; Zhang, X.; Zeng, W.; Sun, Y.; Chen, S.; He, Q.; Han, H. Inhibition of Porcine Epidemic Diarrhea Virus Replication and Viral 3C-Like Protease by Quercetin. Int. J. Mol. Sci. 2020, 21, 8095. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Palm, G.J.; Mesters, J.R.; Siddell, S.G.; Ziebuhr, J.; Hilgenfeld, R. Structure of coronavirus main proteinase reveals combination of a chymotrypsin fold with an extra alpha-helical domain. EMBO J. 2002, 21, 3213–3224. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, H.; Zou, M.; Oerlemans, R.; Shao, C.; Ren, Y.; Zhang, R.; Huang, X.; Li, G.; Cong, Y. Hypericin Inhibit Alpha-Coronavirus Replication by Targeting 3CL Protease. Viruses 2021, 13, 1825. https://doi.org/10.3390/v13091825
Zhang Y, Chen H, Zou M, Oerlemans R, Shao C, Ren Y, Zhang R, Huang X, Li G, Cong Y. Hypericin Inhibit Alpha-Coronavirus Replication by Targeting 3CL Protease. Viruses. 2021; 13(9):1825. https://doi.org/10.3390/v13091825
Chicago/Turabian StyleZhang, Yue, Huijie Chen, Mengmeng Zou, Rick Oerlemans, Changhao Shao, Yudong Ren, Ruili Zhang, Xiaodan Huang, Guangxing Li, and Yingying Cong. 2021. "Hypericin Inhibit Alpha-Coronavirus Replication by Targeting 3CL Protease" Viruses 13, no. 9: 1825. https://doi.org/10.3390/v13091825
APA StyleZhang, Y., Chen, H., Zou, M., Oerlemans, R., Shao, C., Ren, Y., Zhang, R., Huang, X., Li, G., & Cong, Y. (2021). Hypericin Inhibit Alpha-Coronavirus Replication by Targeting 3CL Protease. Viruses, 13(9), 1825. https://doi.org/10.3390/v13091825




