Full-Length ASFV B646L Gene Sequencing by Nanopore Offers a Simple and Rapid Approach for Identifying ASFV Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primer Design
2.2. ASF Strains Used to Evaluate the Set of Primers for B646L (p72)
2.3. Porcine Whole-Blood Samples for the Detection of the ASFV B646L Gene (p72)
2.4. ASFV Nucleic Acid Extraction
2.5. Detection of ASFV by Real-Time PCR
2.6. Full-Length Amplicon Generation of the B646L (p72 Protein)
2.7. Library Generation and Nanopore Sequencing
2.8. Bioinformatics Analysis Mapping to Best B646L (p72) Reference
2.9. B646L (p72) Alignment and Phylogenetic Tree Construction
3. Results and Discussion
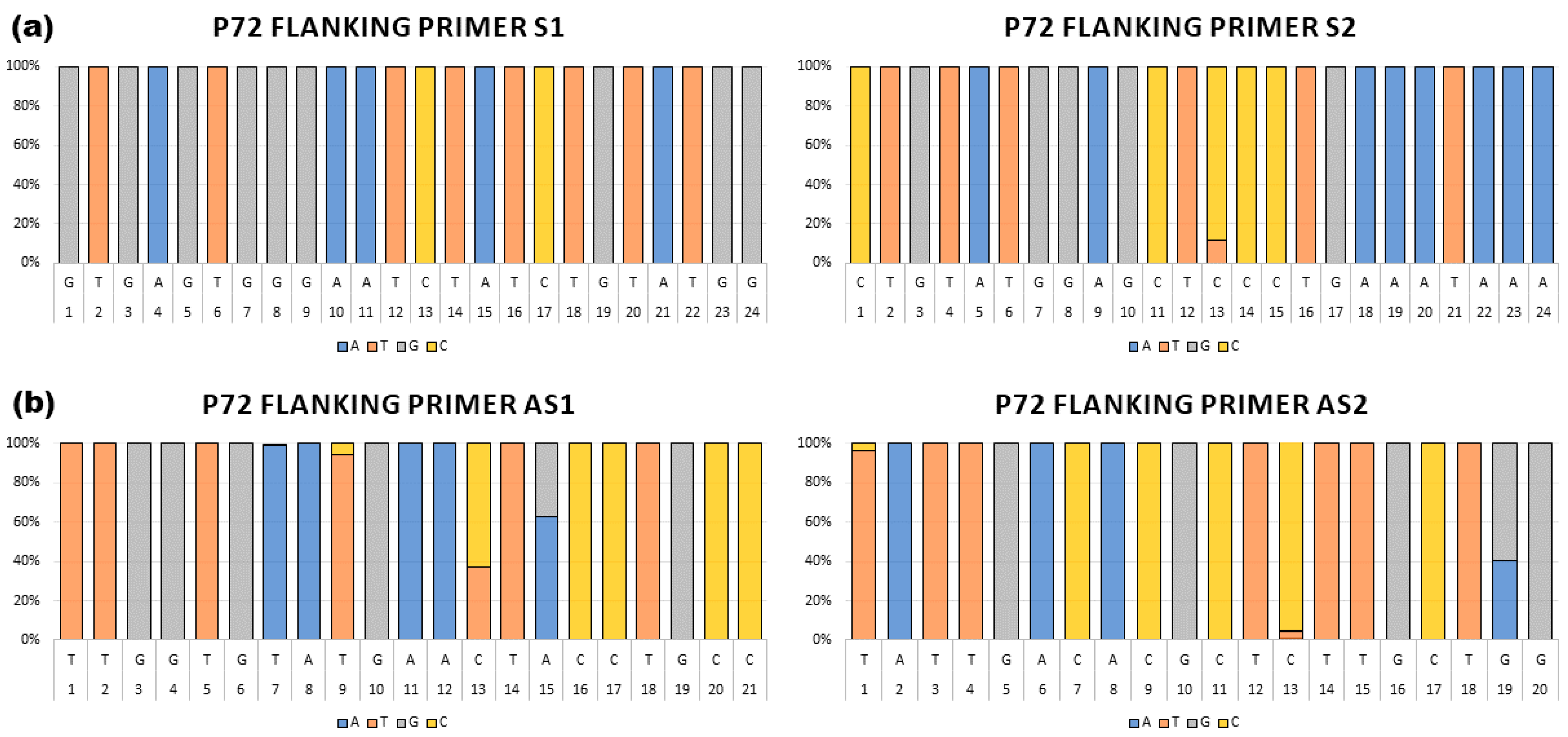
3.1. Primer Design and Selection
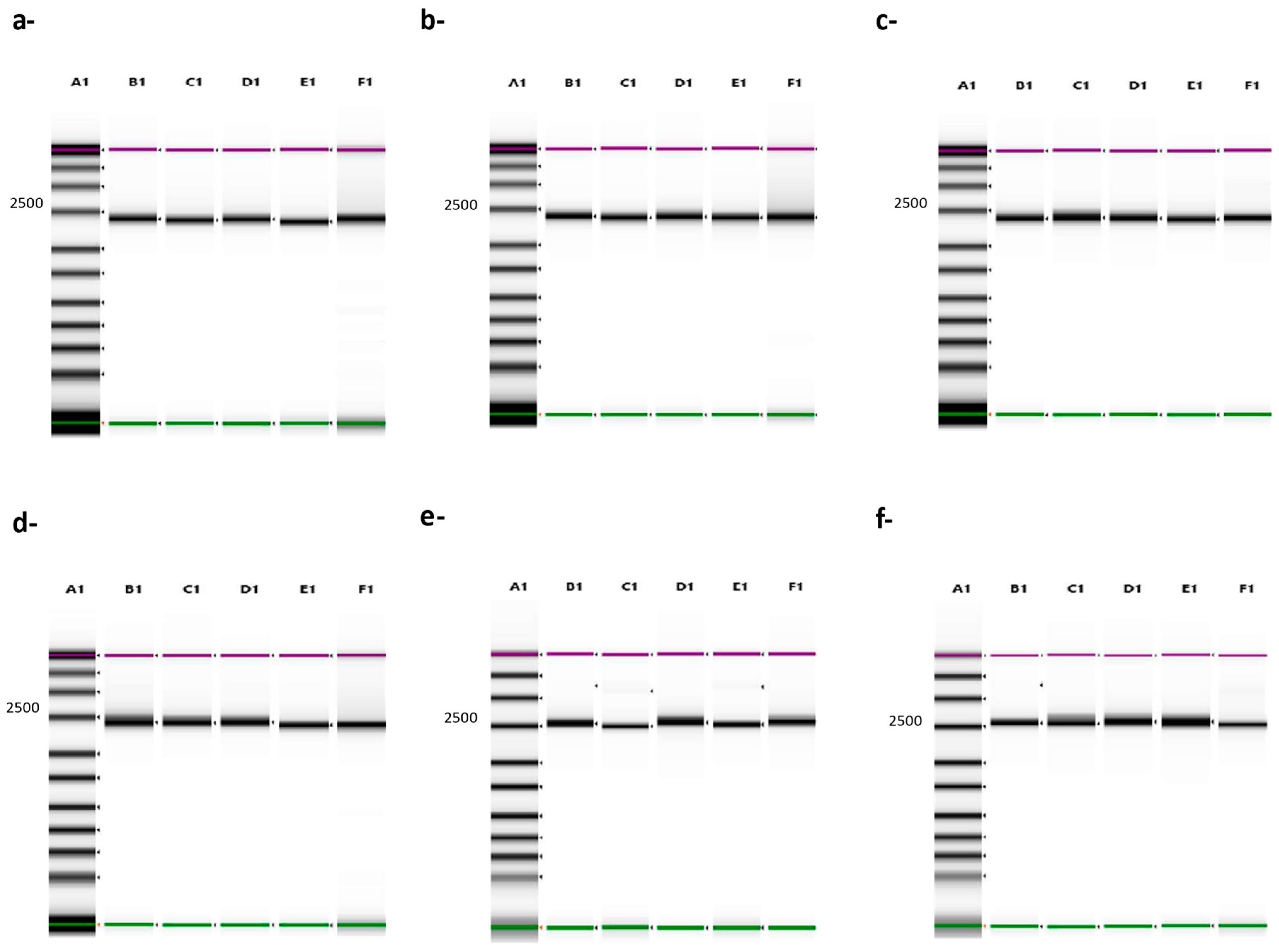
3.2. Evaluation of the Set of Primers to Generate Full-Length B646L (p72) Amplicons
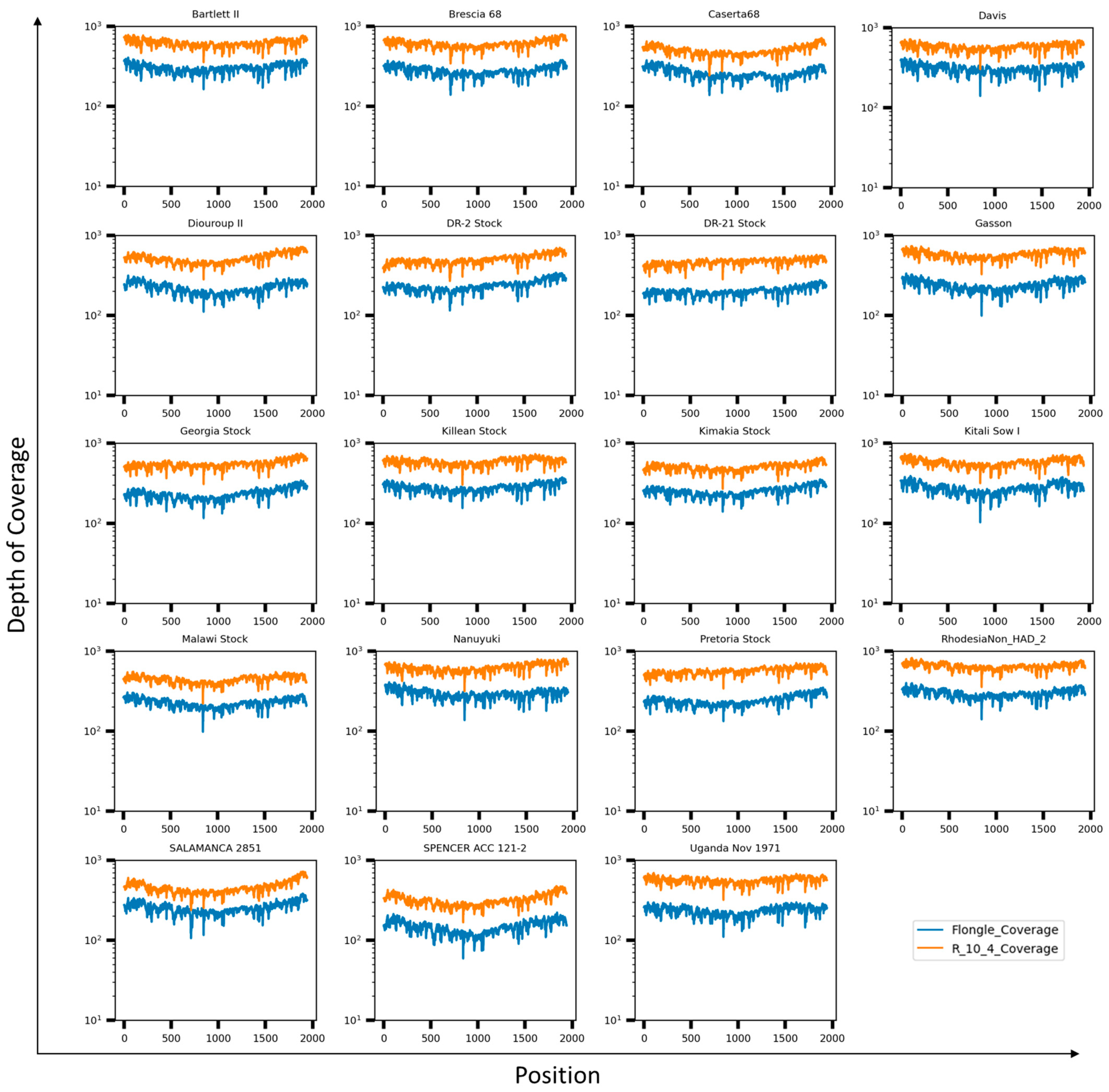
3.3. Genotyping ASFV by Using Full-Length Sequence of the B646L (p72) Gene
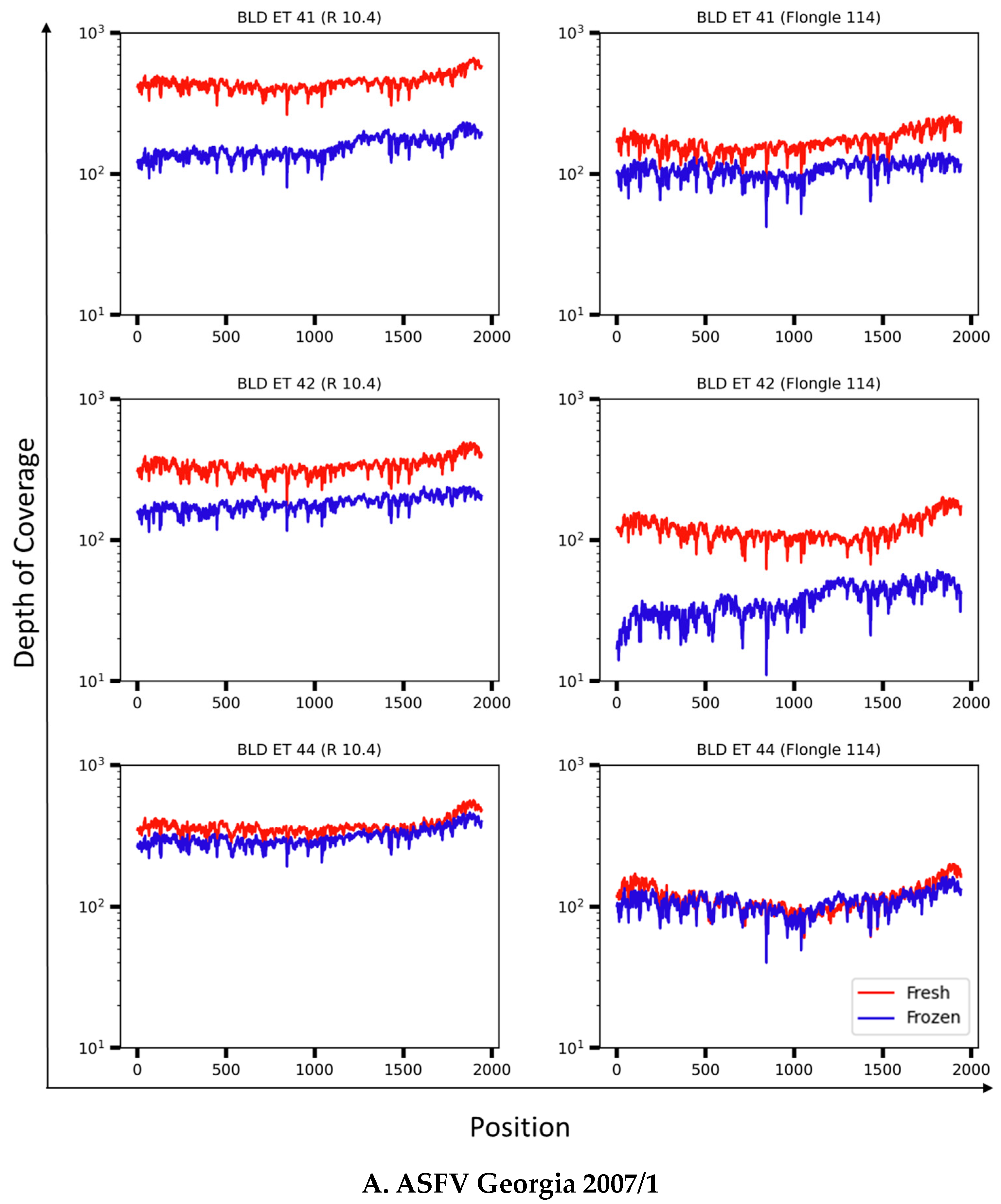
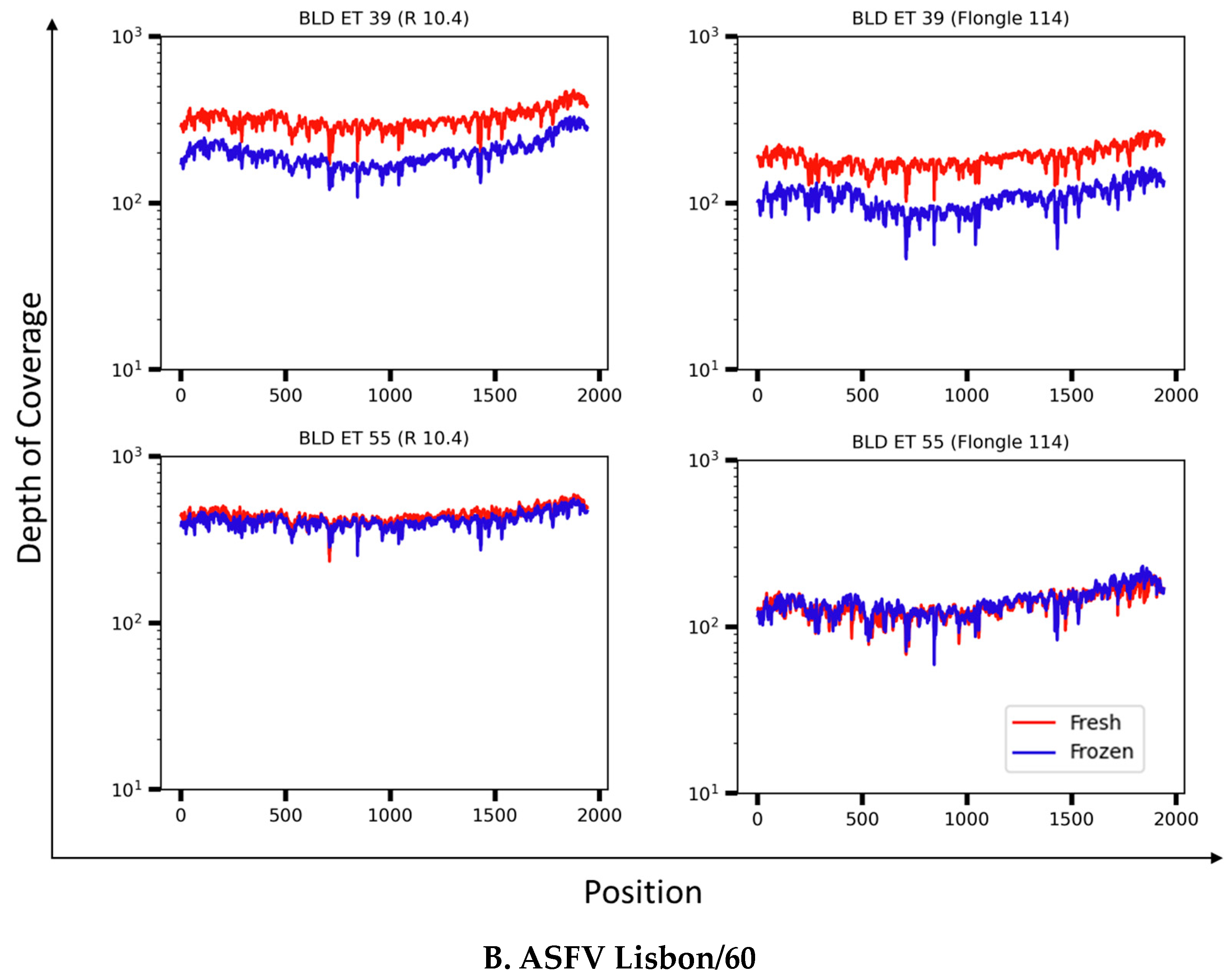
3.4. Resolution of the B646L (p72) Gene in Whole-Blood Samples from ASFV Experimentally Infected Pigs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blome, S.; Franzke, K.; Beer, M. African swine fever—A review of current knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.E. On a form of swine fever occuring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef]
- Gonzales, W.; Moreno, C.; Duran, U.; Henao, N.; Bencosme, M.; Lora, P.; Reyes, R.; Nunez, R.; De Gracia, A.; Perez, A.M. African swine fever in the Dominican Republic. Transbound. Emerg. Dis. 2021, 68, 3018–3019. [Google Scholar] [CrossRef]
- Ramirez-Medina, E.; O’Donnell, V.; Silva, E.; Espinoza, N.; Velazquez-Salinas, L.; Moran, K.; Daite, D.A.; Barrette, R.; Faburay, B.; Holland, R.; et al. Experimental Infection of Domestic Pigs with an African Swine Fever Virus Field Strain Isolated in 2021 from the Dominican Republic. Viruses 2022, 14, 1090. [Google Scholar] [CrossRef]
- Spinard, E.; O’Donnell, V.; Vuono, E.; Rai, A.; Davis, C.; Ramirez-Medina, E.; Espinoza, N.; Valladares, A.; Borca, M.V.; Gladue, D.P. Full genome sequence for the African swine fever virus outbreak in the Dominican Republic in 1980. Sci. Rep. 2023, 13, 1024. [Google Scholar] [CrossRef]
- OIE. African Swine Fever (ASF)—Situation Report; OIE: Atlanta, GA, USA, 2021. [Google Scholar]
- Bastos, A.D.; Penrith, M.L.; Cruciere, C.; Edrich, J.L.; Hutchings, G.; Roger, F.; Couacy-Hymann, E.; Thomson, G.R. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch. Virol. 2003, 148, 693–706. [Google Scholar] [CrossRef]
- Spinard, E.; Dinhobl, M.; Tesler, N.; Birtley, H.; Signore, A.V.; Ambagala, A.; Masembe, C.; Borca, M.V.; Gladue, D.P. A Re-Evaluation of African Swine Fever Genotypes Based on p72 Sequences Reveals the Existence of Only Six Distinct p72 Groups. Viruses 2023, 15, 2246. [Google Scholar] [CrossRef] [PubMed]
- Bold, D.; Souza-Neto, J.A.; Gombo-Ochir, D.; Gaudreault, N.N.; Meekins, D.A.; McDowell, C.D.; Zayat, B.; Richt, J.A. Rapid Identification of ASFV, CSFV and FMDV fromMongolian Outbreaks withMinION Short Amplicon Sequencing. Pathogens 2023, 12, 533. [Google Scholar] [CrossRef] [PubMed]
- Licheri, M.; Licheri, M.F.; Mehinagic, K.; Radulovic, E.; Ruggli, N.; Dijkman, R. Multiplex PCR Approach for Rapid African Swine Fever Virus Genotyping. Viruses 2024, 16, 1460. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, E.; Huang, L.; Ding, L.; Zhu, Y.; Zhang, J.; Shen, D.; Zhang, X.; Zhang, Z.; Ren, T.; et al. Highly lethal genotype I and II recombinant African swine fever viruses detected in pigs. Nat. Commun. 2023, 14, 3096. [Google Scholar] [CrossRef]
- Le, V.P.; Nguyen, V.T.; Le, T.B.; Mai, N.T.A.; Nguyen, V.D.; Than, T.T.; Lai, T.N.H.; Cho, K.H.; Hong, S.K.; Kim, Y.H.; et al. Detection of Recombinant African Swine Fever Virus Strains of p72 Genotypes I and II in Domestic Pigs, Vietnam, 2023. Emerg. Infect. Dis. 2024, 30, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Dinhobl, M.; Spinard, E.; Tesler, N.; Birtley, H.; Signore, A.; Ambagala, A.; Masembe, C.; Borca, M.V.; Gladue, D.P. Reclassification of ASFV into 7 Biotypes Using Unsupervised Machine Learning. Viruses 2023, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Zsak, L.; Borca, M.V.; Risatti, G.R.; Zsak, A.; French, R.A.; Lu, Z.; Kutish, G.F.; Neilan, J.G.; Callahan, J.D.; Nelson, W.M.; et al. Preclinical diagnosis of African swine fever in contact-exposed swine by a real-time PCR assay. J. Clin. Microbiol. 2005, 43, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Grau, F.R.; Schroeder, M.E.; Mulhern, E.L.; McIntosh, M.T.; Bounpheng, M.A. Detection of African swine fever, classical swine fever, and foot-and-mouth disease viruses in swine oral fluids by multiplex reverse transcription real-time polymerase chain reaction. J. Vet. Diagn. Investig. 2015, 27, 140–149. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Cock, P.J.; Antao, T.; Chang, J.T.; Chapman, B.A.; Cox, C.J.; Dalke, A.; Friedberg, I.; Hamelryck, T.; Kauff, F.; Wilczynski, B.; et al. Biopython: Freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 2009, 25, 1422–1423. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, W.; Zhang, Y.; Xu, Y. High speed BLASTN: An accelerated MegaBLAST search tool. Nucleic Acids Res. 2015, 43, 7762–7768. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Li, H. New strategies to improve minimap2 alignment accuracy. Bioinformatics 2021, 37, 4572–4574. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- O’Donnell, V.; Holinka, L.G.; Krug, P.W.; Gladue, D.P.; Carlson, J.; Sanford, B.; Alfano, M.; Kramer, E.; Lu, Z.; Arzt, J.; et al. African swine fever virus Georgia 2007 with a deletion of virulence-associated gene 9GL (B119L), when administered at low doses, leads to virus attenuation in swine and induces an effective protection against homologous challenge. J. Virol. 2015, 89, 8556–8566. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.K.; Grau, F.R.; Mayr, G.A.; Sturgill Samayoa, T.L.; Dodd, K.A.; Barrette, R.W. Rapid Sequence-Based Characterization of African Swine Fever Virus by Use of the Oxford Nanopore MinION Sequence Sensing Device and a Companion Analysis Software Tool. J. Clin. Microbiol. 2019, 58, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.; Pierce, J.L.; Osipenko, O.; Xu, L.; Berninger, A.; Lakin, S.M.; Barrette, R.W.; Gladue, D.P.; Faburay, B. Rapid Detection and Quick Characterization of African Swine Fever Virus Using the VolTRAX Automated Library Preparation Platform. Viruses 2024, 16, 731. [Google Scholar] [CrossRef]
- Kabuuka, T.; Mulindwa, H.; Bastos, A.D.S.; van Heerden, J.; Heath, L.; Fasina, F.O. Retrospective Multi-Locus Sequence Analysis of African Swine Fever Viruses by “PACT” Confirms Co-Circulation of Multiple Outbreak Strains in Uganda. Animals 2023, 14, 71. [Google Scholar] [CrossRef]



| Target B646L Gene | Sequence (5′–3′) | Position (bp) |
|---|---|---|
| Sense 1 | GTGAGTGGGAATCTATCTGTATGG | 150 nt upstream |
| Sense 2 | CTGTATGGAGCTCCCTGAAATAAA | 139 nt upstream |
| Antisense 1 | GGCAGGTAGTTCATACACCAA | 150 nt downstream |
| Antisense 2 | CCAGCAAGAGCGTGTCAATA | 113 nt downstream |
| ASF Strain | Country of Origin | Genotype (Historic) | Genotype | ASF rt-PCR (Ct) | GenBank Accession No. |
|---|---|---|---|---|---|
| Georgia/2007 | Georgia | II | 2 | 21.68 | FR682468 |
| DR-21 | Dominican Republic | II | 2 | 24.86 | n.a. |
| Killean III | Kenya | X | 9 | 20.02 | n.a. 1 |
| Kimakia-64 | Kenya | I | 1 | 20.29 | MN886925 |
| Malawi Lil-20/1 | Malawi | VIII | 8 | 18.17 | AY261361 |
| Pretoria-4/1 | South Africa | XX | 2 | 22.79 | AY578702 |
| Lisbon/60 | Portugal | I | 1 | 20.55 | n.a. 2 |
| Nanuyuki | Kenya | X | 9 | 23.9 | MN886933 |
| Kitali, Sow I | Kenya | IX | 9 | 22.73 | MN886937 |
| Gasson | Kenya | X | 9 | 21.9 | n.a. 3 |
| Spencer | South Africa | n.a | 1 | 17.28 | MN886930 |
| Salamanca | Spain | I | 1 | 19.67 | MN886929 |
| Davis | Kenya | X | 9 | 21.16 | MN886934 |
| Bartlett II | Kenya | X | 9 | 20.91 | MN886935 |
| Rhodesia non-HA | South Africa | VIII | 8 | 20.67 | n.a. |
| Caserta 68 | Italy | I | 1 | 22.3 | MN886926 |
| Brescia 68 | Italy | I | 1 | 23.79 | n.a. |
| Uganda 1971 | Uganda | X | 9 | 17.73 | n.a. |
| Diouroup II | Senegal | I | 1 | 22.36 | MN886936 |
| DR-2 | Dominican Republic | I | 1 | 23.03 | n.a. 4 |
| ASFV Strain | ID | d.p.i. | qPCR (Ct) (Fresh) | qPCR (Ct) (Frozen) |
|---|---|---|---|---|
| Georgia 2007/1 | 41 | 6 | 20.3 | 21.15 |
| Georgia 2007/1 | 42 | 6 | 20.78 | 20.83 |
| Georgia 2007/1 | 44 | 6 | 18.8 | 18.81 |
| Lisbon/60 | 55 | 7 | 19.46 | 20.55 |
| Lisbon/60 | 39 | 9 | 20.18 | 20.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Donnell, V.; Spinard, E.; Xu, L.; Berninger, A.; Barrette, R.W.; Gladue, D.P.; Faburay, B. Full-Length ASFV B646L Gene Sequencing by Nanopore Offers a Simple and Rapid Approach for Identifying ASFV Genotypes. Viruses 2024, 16, 1522. https://doi.org/10.3390/v16101522
O’Donnell V, Spinard E, Xu L, Berninger A, Barrette RW, Gladue DP, Faburay B. Full-Length ASFV B646L Gene Sequencing by Nanopore Offers a Simple and Rapid Approach for Identifying ASFV Genotypes. Viruses. 2024; 16(10):1522. https://doi.org/10.3390/v16101522
Chicago/Turabian StyleO’Donnell, Vivian, Edward Spinard, Lizhe Xu, Amy Berninger, Roger W. Barrette, Douglas P. Gladue, and Bonto Faburay. 2024. "Full-Length ASFV B646L Gene Sequencing by Nanopore Offers a Simple and Rapid Approach for Identifying ASFV Genotypes" Viruses 16, no. 10: 1522. https://doi.org/10.3390/v16101522







