Comparative Analysis of HPV16 Variants in the Untranslated Regulatory Region, L1, and E6 Genes among Vaccinated and Unvaccinated Young Women: Assessing Vaccine Efficacy and Viral Diversity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population Characteristics
2.2. HPV Genotyping and Viral Load Quantification
2.3. DNA Depletion Shotgun Sequencing
2.4. Targeted Amplification of L1, URR and E6 Regions
2.5. Sequence Assembly
2.6. Phylogenetic Analyses
2.7. Variant Analyses
3. Results
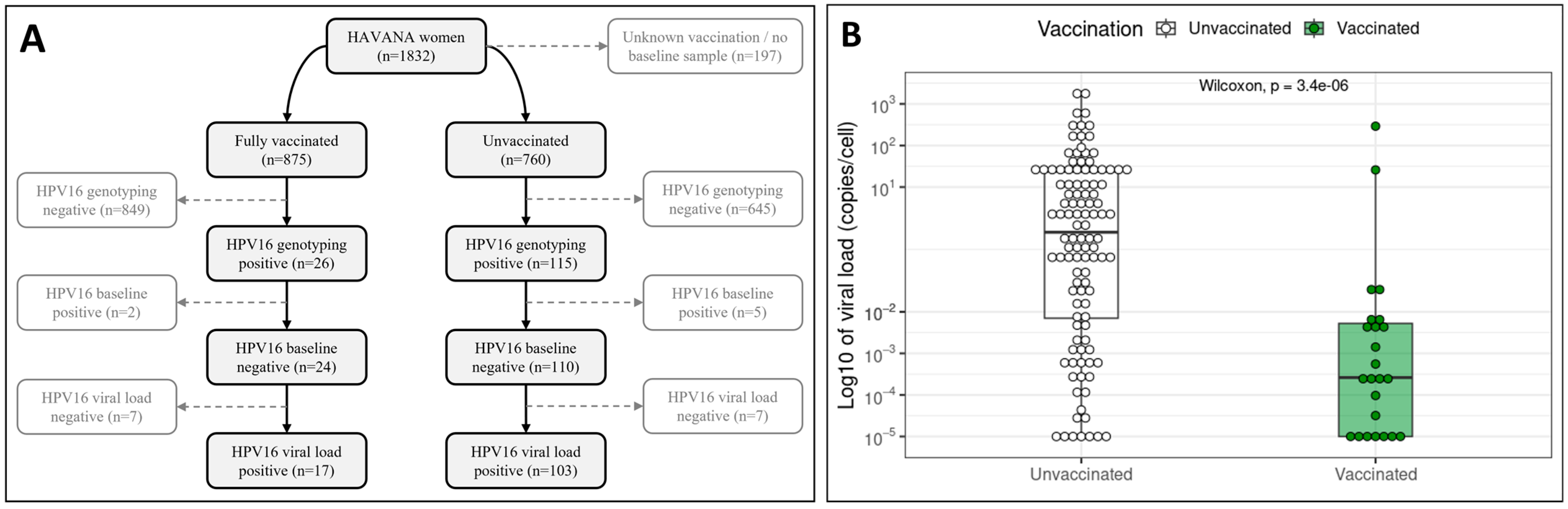
3.1. Population Characteristics and HPV16 Infections
3.2. L1–URR–E6 Sequence Quality
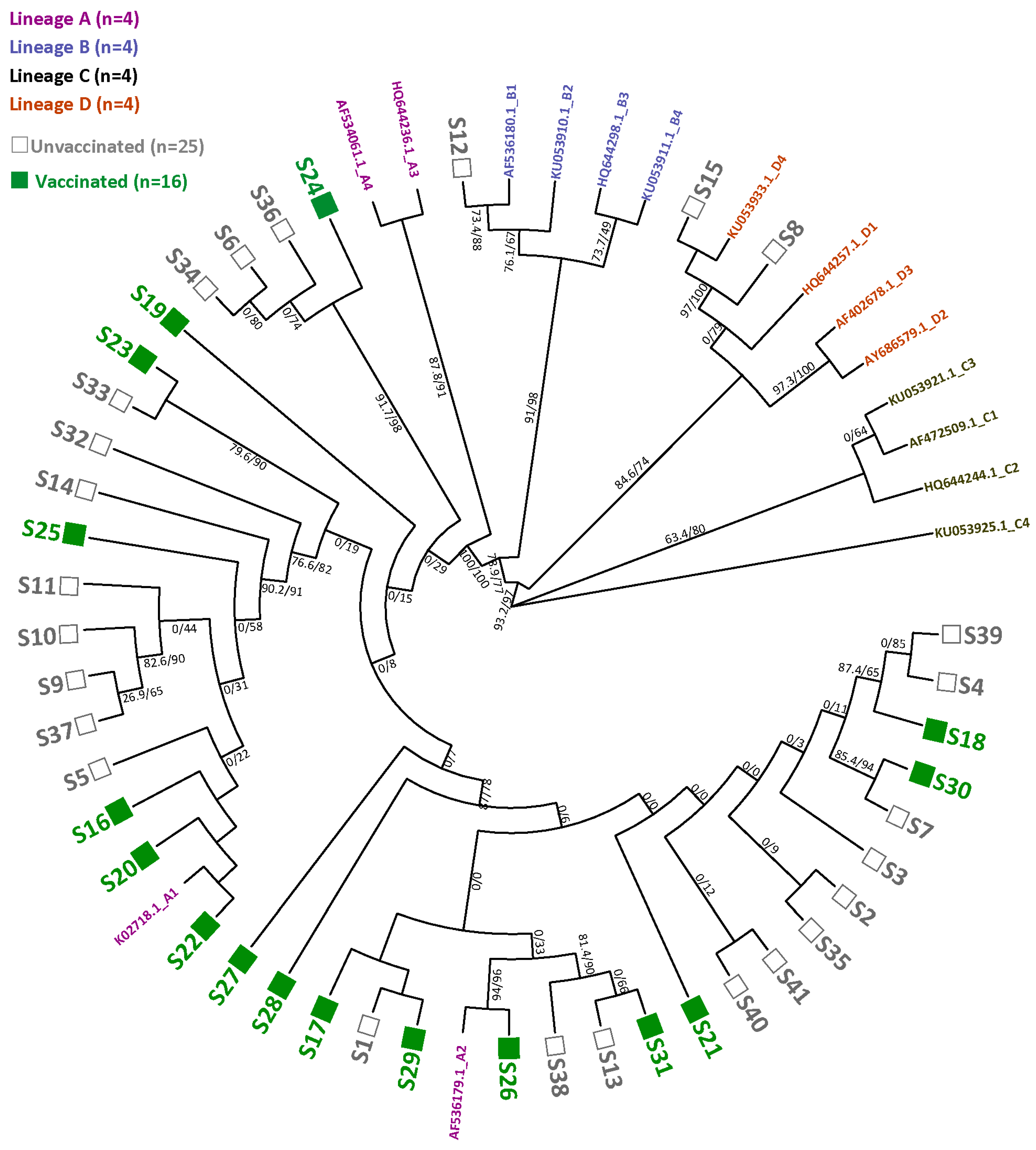
3.3. HPV16 Sequence Diversity and Phylogeny
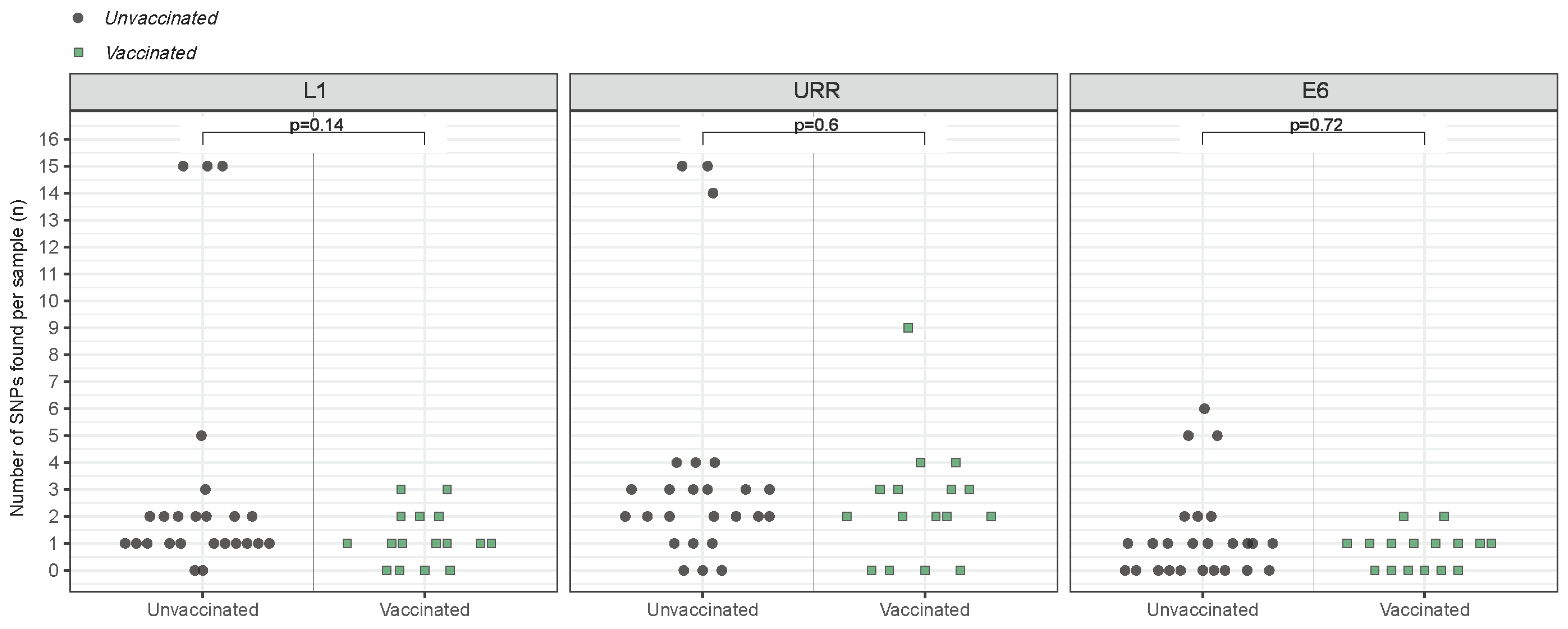
3.4. Variant-Specific SNP Count
3.5. SNP Diversity and Amino-Acid Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.F.; Peto, J.; Meijer, C.J.L.M.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Nelson, C.W.; Mirabello, L. Human papillomavirus genomics: Understanding carcinogenicity. Tumour. Virus Res. 2023, 15, 200258. [Google Scholar] [CrossRef]
- Arbyn, M.; Tommasino, M.; Depuydt, C.; Dillner, J. Are 20 human papillomavirus types causing cervical cancer? J. Pathol. 2014, 234, 431–435. [Google Scholar] [CrossRef] [PubMed]
- de Sanjosé, S.; Serrano, B.; Tous, S.; Alejo, M.; Lloveras, B.; Quirós, B.; Clavero, O.; Vidal, A.; Ferrándiz-Pulido, C.; Pavón, M.Á.; et al. Burden of Human Papillomavirus (HPV)-Related Cancers Attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI Cancer Spectr. 2019, 2, pky045. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, Z.; Aria, H.; Ghaedrahmati, F.; Bakhtiari, T.; Azizi, M.; Bastan, R.; Hosseini, R.; Eskandari, N. An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy. Front. Immunol. 2021, 12, 805695. [Google Scholar] [CrossRef]
- de Melker, H.E.; Conyn-van Spaendonck, M.A.; Boot, H.J.; Coutinho, R.A. Introductie van vaccinatie tegen baarmoederhalskanker [Introduction to vaccination against cervical cancer]. Ned. Tijdschr. Voor Geneeskd. 2009, 153, 658–661. [Google Scholar]
- European Medicines Agency. Cervarix: Human Papillomavirus Vaccine [Types 16, 18] (Recombinant, Adjuvanted, Adsorbed). 2016. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/cervarix (accessed on 22 February 2023).
- Dutch Health Board (Gezondheidsraad). Aanpassing Doses HPV-Vaccinatie. 2022. Available online: https://open.overheid.nl/documenten/ronl-0b7dac58a1fdda8250aeb17ad29891caeb9c4576/pdf (accessed on 21 June 2023).
- Artemchuk, H.; Eriksson, T.; Poljak, M.; Surcel, H.M.; Dillner, J.; Lehtinen, M.; Faust, H. Long-term Antibody Response to Human Papillomavirus Vaccines: Up to 12 Years of Follow-up in the Finnish Maternity Cohort. J. Infect. Dis. 2019, 219, 582–589. [Google Scholar] [CrossRef]
- Hoes, J.; Pasmans, H.; Knol, M.J.; Donken, R.; van Marm-Wattimena, N.; Schepp, R.M.; King, A.J.; van der Klis, F.R.M.; de Melker, H.E. Persisting Antibody Response 9 Years After Bivalent Human Papillomavirus (HPV) Vaccination in a Cohort of Dutch Women: Immune Response and the Relation to Genital HPV Infections. J. Infect. Dis. 2020, 221, 1884–1894. [Google Scholar] [CrossRef]
- Schwarz, T.F.; Galaj, A.; Spaczynski, M.; Wysocki, J.; Kaufmann, A.M.; Poncelet, S.; Suryakiran, P.V.; Folschweiller, N.; Thomas, F.; Lin, L.; et al. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15-55 years of age. Cancer Med. 2017, 6, 2723–2731. [Google Scholar] [CrossRef]
- Panwar, K.; Godi, A.; Cocuzza, C.E.; Andrews, N.; Southern, J.; Turner, P.; Miller, E.; Beddows, S. Binding antibody levels to vaccine (HPV6/11/16/18) and non-vaccine (HPV31/33/45/52/58) HPV antigens up to 7 years following immunization with either Cervarix(R) or Gardasil(R) vaccine. Vaccine 2022, 40, 1198–1202. [Google Scholar] [CrossRef]
- Donken, R.; King, A.J.; Bogaards, J.A.; Woestenberg, P.J.; Meijer, C.; de Melker, H.E. High Effectiveness of the Bivalent Human Papillomavirus (HPV) Vaccine Against Incident and Persistent HPV Infections up to 6 Years After Vaccination in Young Dutch Women. J. Infect. Dis. 2018, 217, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.; Pollock, K.G.; Cuschieri, K.; Palmer, T.; Cameron, R.L.; Watt, C.; Bhatia, R.; Moore, C.; Cubie, H.; Cruickshank, M.; et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: A 7-year cross-sectional study. Lancet Infect. Dis. 2017, 17, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Xu, L.; Simoens, C.; Martin-Hirsch, P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018, 5, CD009069. [Google Scholar] [CrossRef] [PubMed]
- Porras, C.; Tsang, S.H.; Herrero, R.; Guillén, D.; Darragh, T.M.; Stoler, M.H.; Hildesheim, A.; Wagner, S.; Boland, J.; Lowy, D.R.; et al. Efficacy of the bivalent HPV vaccine against HPV 16/18-associated precancer: Long-term follow-up results from the Costa Rica Vaccine Trial. Lancet Oncol. 2020, 21, 1643–1652. [Google Scholar] [CrossRef]
- Lehtinen, M.; Paavonen, J.; Wheeler, C.M.; Jaisamrarn, U.; Garland, S.M.; Castellsagué, X.; Skinner, S.R.; Apter, D.; Naud, P.; Salmerón, J.; et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012, 13, 89–99. [Google Scholar] [CrossRef]
- Harper, D.M. Impact of vaccination with Cervarix (trade mark) on subsequent HPV-16/18 infection and cervical disease in women 15-25 years of age. Gynecol. Oncol. 2008, 110 (Suppl. S1), S11–S17. [Google Scholar] [CrossRef]
- Naud, P.S.; Roteli-Martins, C.M.; De Carvalho, N.S.; Teixeira, J.C.; de Borba, P.C.; Sanchez, N.; Zahaf, T.; Catteau, G.; Geeraerts, B.; Descamps, D. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: Final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum. Vaccin. Immunother. 2014, 10, 2147–2162. [Google Scholar] [CrossRef]
- Deschuyteneer, M.; Elouahabi, A.; Plainchamp, D.; Plisnier, M.; Soete, D.; Corazza, Y.; Lockman, L.; Giannini, S.; Deschamps, M. Molecular and structural characterization of the L1 virus-like particles that are used as vaccine antigens in Cervarix, the AS04-adjuvanted HPV-16 and -18 cervical cancer vaccine. Hum. Vaccin. 2010, 6, 407–419. [Google Scholar] [CrossRef]
- Burk, R.D.; Harari, A.; Chen, Z. Human papillomavirus genome variants. Virology 2013, 445, 232–243. [Google Scholar] [CrossRef]
- Mirabello, L.; Clarke, M.A.; Nelson, C.W.; Dean, M.; Wentzensen, N.; Yeager, M.; Cullen, M.; Boland, J.F.; Workshop, N.H.; Schiffman, M.; et al. The Intersection of HPV Epidemiology, Genomics and Mechanistic Studies of HPV-Mediated Carcinogenesis. Viruses 2018, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Cornet, I.; Gheit, T.; Franceschi, S.; Vignat, J.; Burk, R.D.; Sylla, B.S.; Tommasino, M.; Clifford, G.M.; Group, I.H.V.S. Human papillomavirus type 16 genetic variants: Phylogeny and classification based on E6 and LCR. J. Virol. 2012, 86, 6855–6861. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, L.; Zhang, A.; Zhou, A.; Yuan, J.; Wang, Y.; Sun, L.; Cao, H.; Zheng, W. Variant sublineages of human papillomavirus type 16 predispose women to persistent infection characterized by a sequence analysis of the E6, L1, and LCR regions. Int. J. Clin. Exp. Pathol. 2019, 12, 337–343. [Google Scholar] [PubMed]
- Yoshida, T.; Ogawa, T.; Nakanome, A.; Ohkoshi, A.; Ishii, R.; Higashi, K.; Ishikawa, T.; Katori, Y.; Furukawa, T. Investigation of the diversity of human papillomavirus 16 variants and L1 antigenic regions relevant for the prevention of human papillomavirus-related oropharyngeal cancer in Japan. Auris Nasus Larynx 2022, 49, 1033–1041. [Google Scholar] [CrossRef]
- El Aliani, A.; El Abid, H.; Kassal, Y.; Khyatti, M.; Attaleb, M.; Ennaji, M.M.; El Mzibri, M. HPV16 L1 diversity and its potential impact on the vaccination-induced immunity. Gene 2020, 747, 144682. [Google Scholar] [CrossRef]
- Iglesias, P.; Tendobi, C.; Carlos, S.; Lozano, M.D.; Barquin, D.; Chiva, L.; Reina, G. Characterization of Human Papillomavirus 16 from Kinshasa (Democratic Republic of the Congo)-Implications for Pathogenicity and Vaccine Effectiveness. Microorganisms 2022, 10, 2492. [Google Scholar] [CrossRef]
- Mollers, M.; Scherpenisse, M.; van der Klis, F.R.; King, A.J.; van Rossum, T.G.; van Logchem, E.M.; Feltkamp, M.C.; Meijer, C.J.; Snijders, P.J.; Boot, H.J.; et al. Prevalence of genital HPV infections and HPV serology in adolescent girls, prior to vaccination. Cancer Epidemiol. 2012, 36, 519–524. [Google Scholar] [CrossRef]
- van Lier, E.A.; Oomen, P.J.; Giesbers, H.; Drijfhout, I.H.; de Hoogh, P.A.A.M.; de Melker, H.E. Vaccinatiegraad Rijksvaccinatieprogramma Nederland: Verslagjaar 2011. p. 26. Available online: https://rivm.openrepository.com/handle/10029/259522 (accessed on 20 August 2024).
- Kleter, B.; van Doorn, L.J.; Schrauwen, L.; Molijn, A.; Sastrowijoto, S.; ter Schegget, J.; Lindeman, J.; ter Harmsel, B.; Burger, M.; Quint, W. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J. Clin. Microbiol. 1999, 37, 2508–2517. [Google Scholar] [CrossRef]
- van der Weele, P.; van Logchem, E.; Wolffs, P.; van den Broek, I.; Feltkamp, M.; de Melker, H.; Meijer, C.J.; Boot, H.; King, A.J. Correlation between viral load, multiplicity of infection, and persistence of HPV16 and HPV18 infection in a Dutch cohort of young women. J. Clin. Virol. 2016, 83, 6–11. [Google Scholar] [CrossRef]
- Benschop, K.S.M.; Broberg, E.K.; Hodcroft, E.; Schmitz, D.; Albert, J.; Baicus, A.; Bailly, J.L.; Baldvinsdottir, G.; Berginc, N.; Blomqvist, S.; et al. Molecular Epidemiology and Evolutionary Trajectory of Emerging Echovirus 30, Europe. Emerg. Infect. Dis. 2021, 27, 1616–1626. [Google Scholar] [CrossRef]
- Hasan, M.R.; Rawat, A.; Tang, P.; Jithesh, P.V.; Thomas, E.; Tan, R.; Tilley, P. Depletion of Human DNA in Spiked Clinical Specimens for Improvement of Sensitivity of Pathogen Detection by Next-Generation Sequencing. J. Clin. Microbiol. 2016, 54, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Zwagemaker, F.; Hajji, K.; Schmitz, D.; Kroneman, A. The RIVM-IDS bioinformatics team. ViroConstrictor [Computer software]. Zendodo 2023. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Graubard, B.I.; Pickard, R.K.; Xiao, W.; Gillison, M.L. High-risk oral human papillomavirus load in the US population, National Health and Nutrition Examination Survey 2009-2010. J. Infect. Dis. 2014, 210, 441–447. [Google Scholar] [CrossRef]
- Brown, D.R.; Joura, E.A.; Yen, G.P.; Kothari, S.; Luxembourg, A.; Saah, A.; Walia, A.; Perez, G.; Khoury, H.; Badgley, D.; et al. Systematic literature review of cross-protective effect of HPV vaccines based on data from randomized clinical trials and real-world evidence. Vaccine 2021, 39, 2224–2236. [Google Scholar] [CrossRef]
- van Eer, K.; Middeldorp, M.; Dzebisasjvili, T.; Lamkaraf, N.; de Melker, H.; Steenbergen, R.D.M.; King, A.J. Effects of two and three vaccinations with the bivalent HPV vaccine on the prevalence and load of HPV in clearing and persistent infections in young women. J. Infect. Dis. 2023, 228, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Hoes, J.; Woestenberg, P.J.; Bogaards, J.A.; King, A.J.; de Melker, H.E.; Berkhof, J.; Hoebe, C.; van der Sande, M.A.B.; van Benthem, B.H.B.; Medical Microbiological, L.; et al. Population Impact of Girls-Only Human Papillomavirus 16/18 Vaccination in The Netherlands: Cross-Protective and Second-Order Herd Effects. Clin. Infect Dis. 2021, 72, e103–e111. [Google Scholar] [CrossRef]
- van der Weele, P.; Meijer, C.; King, A.J. Whole-Genome Sequencing and Variant Analysis of Human Papillomavirus 16 Infections. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Yeager, M.; Yu, K.; Clifford, G.M.; Xiao, Y.; Zhu, B.; Cullen, M.; Boland, J.F.; Wentzensen, N.; Nelson, C.W.; et al. HPV16 E7 Genetic Conservation Is Critical to Carcinogenesis. Cell 2017, 170, 1164–1174. [Google Scholar] [CrossRef]
- Debernardi, A.; Valot, B.; Almarcha, J.; Guenat, D.; Hocquet, D.; Algros, M.P.; Riethmuller, D.; Ramanah, R.; Mougin, C.; Pretet, J.L.; et al. Longitudinal follow-up of HPV16 sequence after cervical infection: Low intrahost variation and no correlation with clinical evolution. J. Med. Virol. 2022, 94, 5512–5518. [Google Scholar] [CrossRef]
- Clifford, G.M.; Tenet, V.; Georges, D.; Alemany, L.; Pavon, M.A.; Chen, Z.; Yeager, M.; Cullen, M.; Boland, J.F.; Bass, S.; et al. Human papillomavirus 16 sub-lineage dispersal and cervical cancer risk worldwide: Whole viral genome sequences from 7116 HPV16-positive women. Papillomavirus Res. 2019, 7, 67–74. [Google Scholar] [CrossRef]
- Pastrana, D.V.; Vass, W.C.; Lowy, D.R.; Schiller, J.T. NHPV16 VLP vaccine induces human antibodies that neutralize divergent variants of HPV16. Virology 2001, 279, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Harari, A.; Chen, Z.; Rodriguez, A.C.; Hildesheim, A.; Porras, C.; Herrero, R.; Wacholder, S.; Panagiotou, O.A.; Befano, B.; Burk, R.D.; et al. Cross-protection of the Bivalent Human Papillomavirus (HPV) Vaccine Against Variants of Genetically Related High-Risk HPV Infections. J. Infect. Dis. 2016, 213, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Evande, R.; Rana, A.; Biswas-Fiss, E.E.; Biswas, S.B. Protein-DNA Interactions Regulate Human Papillomavirus DNA Replication, Transcription, and Oncogenesis. Int. J. Mol. Sci. 2023, 24, 8493. [Google Scholar] [CrossRef]
- Christensen, N.D.; Dillner, J.; Eklund, C.; Carter, J.J.; Wipf, G.C.; Reed, C.A.; Cladel, N.M.; Galloway, D.A. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 1996, 223, 174–184. [Google Scholar] [CrossRef]
- Olcese, V.A.; Chen, Y.; Schlegel, R.; Yuan, H. Characterization of HPV16 L1 loop domains in the formation of a type-specific, conformational epitope. BMC Microbiol. 2004, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Oumeslakht, L.; Ababou, M.; Badaoui, B.; Qmichou, Z. Worldwide genetic variations in high-risk human papillomaviruses capsid L1 gene and their impact on vaccine efficiency. Gene 2021, 782, 145533. [Google Scholar] [CrossRef]
- Tsakogiannis, D.; Nikolaidis, M.; Zagouri, F.; Zografos, E.; Kottaridi, C.; Kyriakopoulou, Z.; Tzioga, L.; Markoulatos, P.; Amoutzias, G.D.; Bletsa, G. Mutation Profile of HPV16 L1 and L2 Genes in Different Geographic Areas. Viruses 2022, 15, 141. [Google Scholar] [CrossRef]
- Frati, E.; Bianchi, S.; Colzani, D.; Zappa, A.; Orlando, G.; Tanzi, E. Genetic variability in the major capsid L1 protein of human papillomavirus type 16 (HPV-16) and 18 (HPV-18). Infect. Genet. Evol. 2011, 11, 2119–2124. [Google Scholar] [CrossRef]
- Cao, M.; Chenzhang, Y.; Ding, X.; Zhang, Y.; Jing, Y.; Chen, Z. Genetic variability and lineage phylogeny of human papillomavirus type-16 and -53 based on the E6, E7, and L1 genes in Southwest China. Gene 2016, 592, 49–59. [Google Scholar] [CrossRef]
- Ou, Z.; Chen, Z.; Zhao, Y.; Lu, H.; Liu, W.; Li, W.; Ren, P.; Geng, C.; Xiao, M.; Hu, G.; et al. Genetic signatures for lineage/sublineage classification of HPV16, 18, 52 and 58 variants. Virology 2021, 553, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Yeager, M.; Cullen, M.; Boland, J.F.; Chen, Z.; Wentzensen, N.; Zhang, X.; Yu, K.; Yang, Q.; Mitchell, J.; et al. HPV16 Sublineage Associations With Histology-Specific Cancer Risk Using HPV Whole-Genome Sequences in 3200 Women. J Natl. Cancer Inst. 2016, 108, djw100. [Google Scholar] [CrossRef] [PubMed]
- Bletsa, G.; Zagouri, F.; Amoutzias, G.D.; Nikolaidis, M.; Zografos, E.; Markoulatos, P.; Tsakogiannis, D. Genetic variability of the HPV16 early genes and LCR. Present and future perspectives. Expert Rev. Mol. Med. 2021, 23, e19. [Google Scholar] [CrossRef] [PubMed]


| No Unique SNPs | ≥1 Unique SNPs | ≥2 Unique SNPs | |
|---|---|---|---|
| Unvaccinated (n = 25) | 15 (60) | 10 (40) | 4 (16) |
| Vaccinated (n = 16) | 10 (62.5) | 6 (37.5) | 2 (12.5) |
| Total (n = 41) | 25 (61) | 16 (39) | 6 (14.6) |
| Region | Position | Mutated Triplet | Amino Acid | Vaccinated [n = 16, (%)] | Unvaccinated [n = 25, (%)] | Fisher p-Value |
|---|---|---|---|---|---|---|
| L1 | C226T | CAT > TAT | H76Y | 0 (0) | 3 (12) | 0.27 |
| L1 (EF-loop) | C527A | ACC > AAC | T176N | 0 (0) | 3 (12) | 0.27 |
| L1 (FG-loop) | A796G | ACT > GCT | T266A | 12 (75) | 19 (76) | 1 |
| L1 (HI-loop) | A1057G | ACT > CCT | T353P | 0 (0) | 2 (8) | 0.51 |
| L1 | T1186C | TCC > CCC | S396P | 0 (0) | 1 (4) | 1 |
| L1 | A1290C | AAA > AAC | K430N | 1 (6.3) | 0 (0) | 0.39 |
| L1 | A1361G | AAG > AGG | K454R | 1 (6.3) | 1 (4) | 1 |
| L1 | T1422G | TTT > TTG | L474F | 0 (0) | 4 (16) | 0.14 |
| E6 | A28G | AGA > GGA | R10G | 1 (6.3) | 0 (0) | 0.39 |
| E6 | G29C | AGA > ACA | R10T | 0 (0) | 1 (4) | 1 |
| E6 | C40G, G42T | CAG > GAT | Q14D | 0 (0) | 1 (4) | 1 |
| E6 | G42T | CAG > CAT | Q14H | 0 (0) | 2 (8) | 0.51 |
| E6 | T148A | TTA > ATA | L50I | 1 (6.3) | 0 (0) | 0.39 |
| E6 | C232T | CAT > TAT | H78Y | 0 (0) | 3 (12) | 0.27 |
| E6 | T247G | TTG > GTG | L83V | 8 (50) | 13 (52) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Eer, K.; Dzebisasjvili, T.; Steenbergen, R.D.M.; King, A.J. Comparative Analysis of HPV16 Variants in the Untranslated Regulatory Region, L1, and E6 Genes among Vaccinated and Unvaccinated Young Women: Assessing Vaccine Efficacy and Viral Diversity. Viruses 2024, 16, 1381. https://doi.org/10.3390/v16091381
van Eer K, Dzebisasjvili T, Steenbergen RDM, King AJ. Comparative Analysis of HPV16 Variants in the Untranslated Regulatory Region, L1, and E6 Genes among Vaccinated and Unvaccinated Young Women: Assessing Vaccine Efficacy and Viral Diversity. Viruses. 2024; 16(9):1381. https://doi.org/10.3390/v16091381
Chicago/Turabian Stylevan Eer, Kahren, Tsira Dzebisasjvili, Renske D. M. Steenbergen, and Audrey J. King. 2024. "Comparative Analysis of HPV16 Variants in the Untranslated Regulatory Region, L1, and E6 Genes among Vaccinated and Unvaccinated Young Women: Assessing Vaccine Efficacy and Viral Diversity" Viruses 16, no. 9: 1381. https://doi.org/10.3390/v16091381






