Acute Chikungunya Virus Infection Triggers a Diverse Range of T Helper Lymphocyte Profiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement and Study Design
2.2. Blood Collection and Processing
2.3. Viral RNA Extraction and Detection of CHIKV by qRT-PCR
2.4. Total mRNA Extraction, cDNA Synthesis, and qRT-PCR
2.5. Statistical Analysis
3. Results
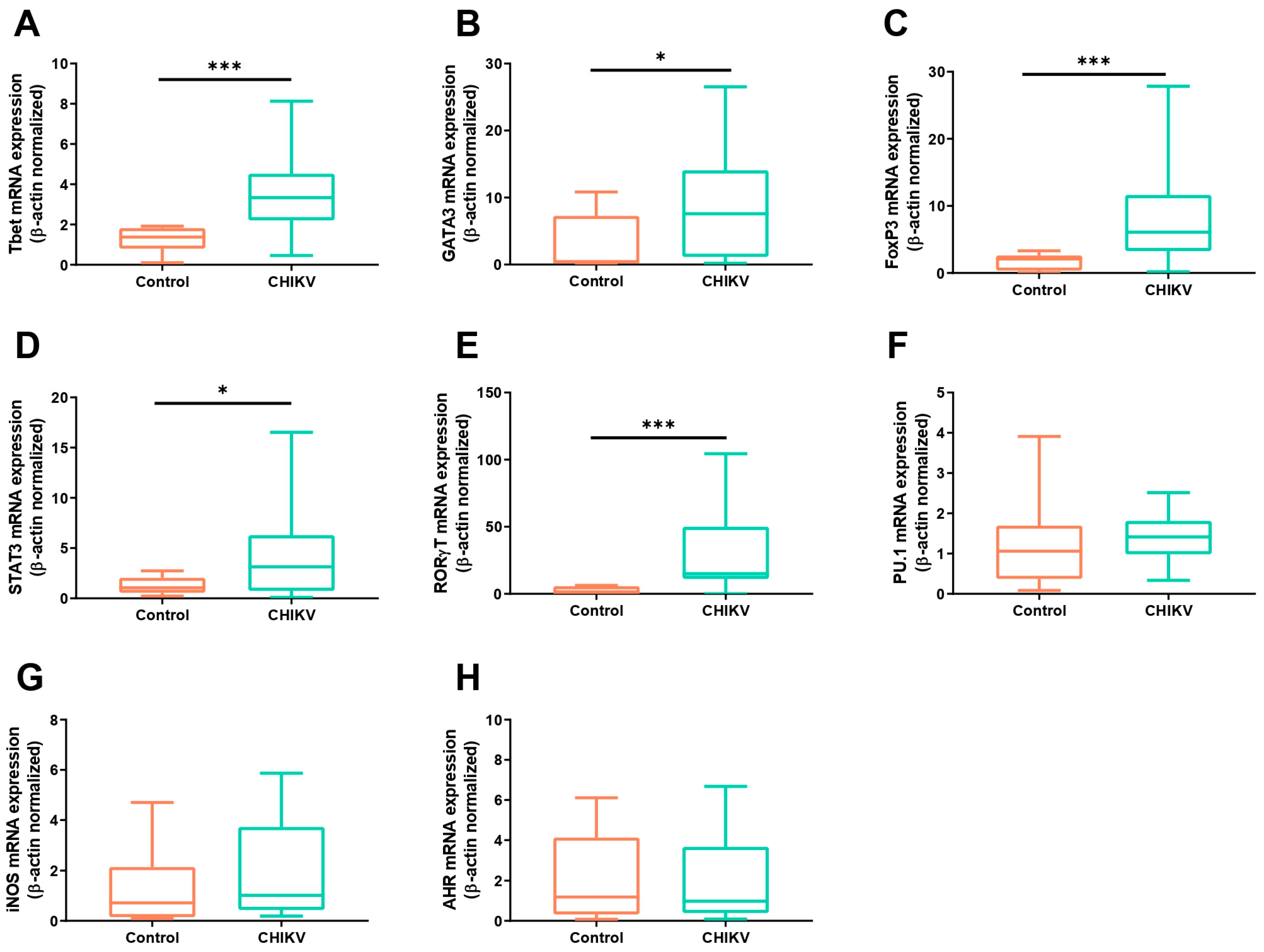
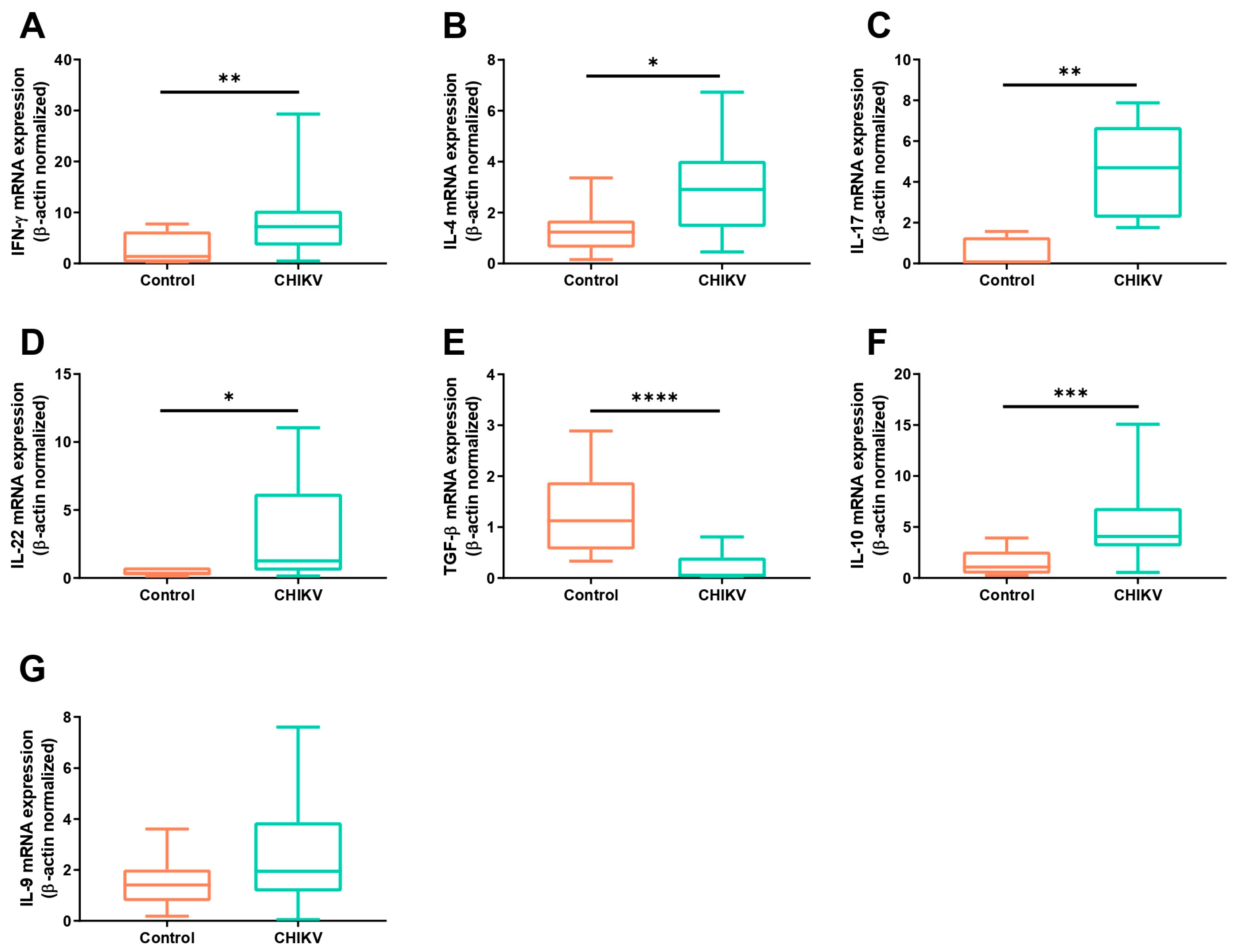
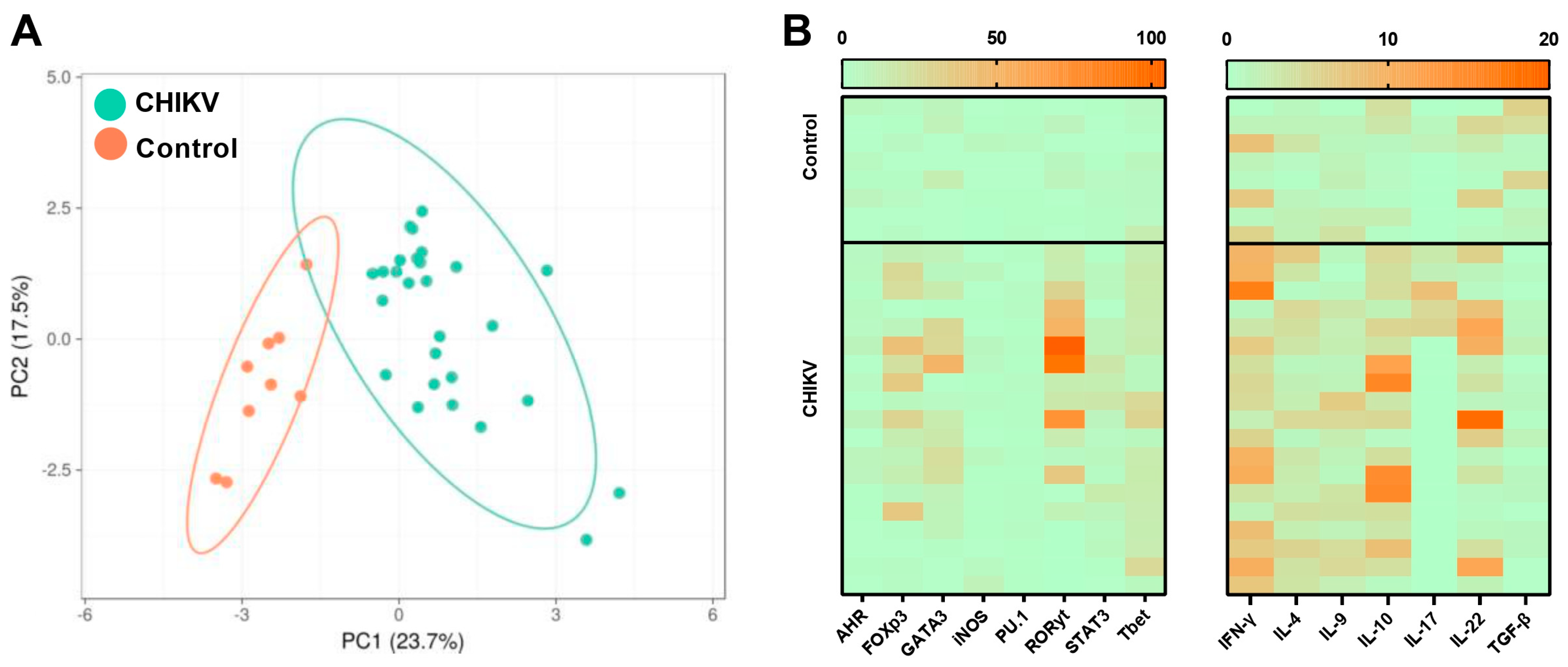
3.1. Symptomatic Acute Infection by CHIKV Induces a Mixture of T Helper Responses
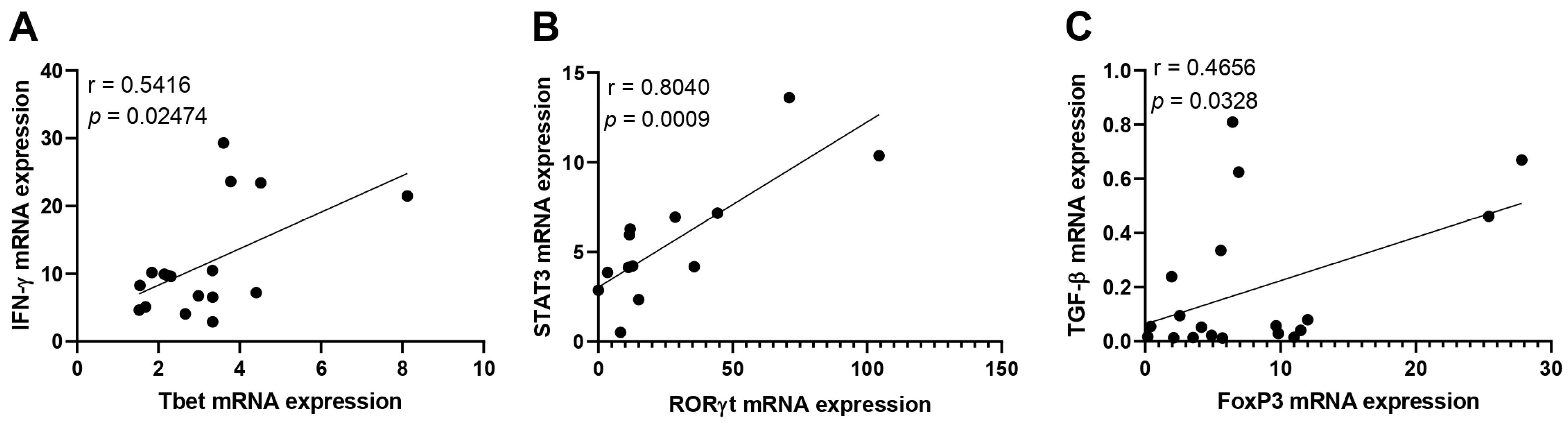
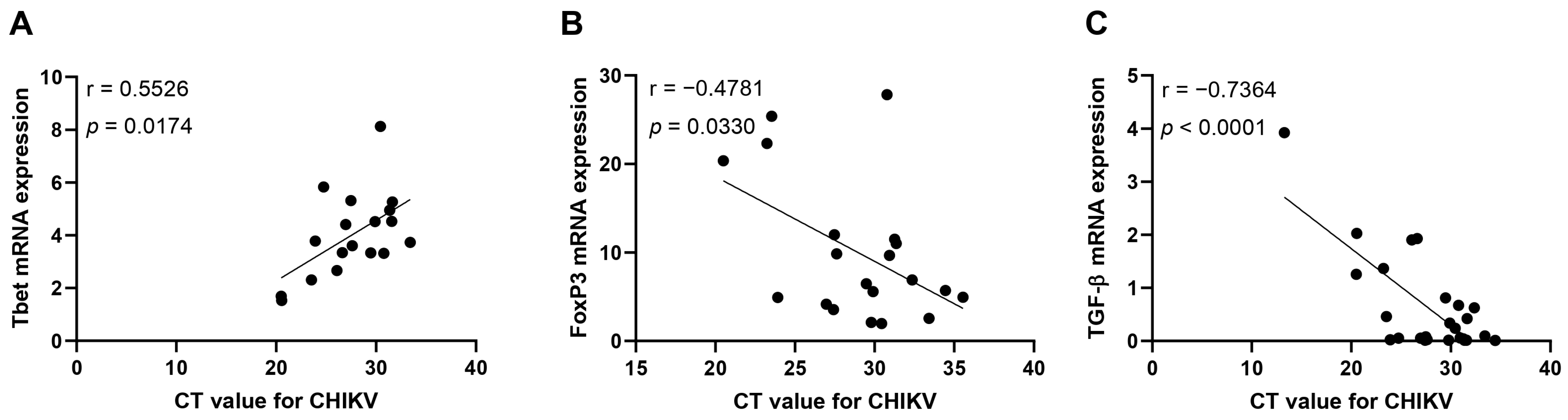
3.2. Immunological Association with Viral Load during Acute CHIKV Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Staples, J.E.; Breiman, R.F.; Powers, A.M. Chikungunya Fever: An Epidemiological Review of a Re-Emerging Infectious Disease. Clin. Infect. Dis. 2009, 49, 942–948. [Google Scholar] [CrossRef]
- Weaver, S.C.; Forrester, N.L. Chikungunya: Evolutionary History and Recent Epidemic Spread. Antivir. Res. 2015, 120, 32–39. [Google Scholar] [CrossRef]
- Coffey, L.L.; Failloux, A.B.; Weaver, S.C. Chikungunya virus-vector interactions. Viruses 2014, 6, 4628–4663. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Palomares, L.A.; Moreno-García, M.; Lanz-Mendoza, H.; Salazar, M.I. Molecular Basis for Arbovirus Transmission by Aedes aegypti Mosquitoes. Intervirology 2018, 61, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes aegypti vector competence studies: A review. Infect. Genet. Evol. 2019, 67, 191–209. [Google Scholar] [CrossRef]
- Zardini, A.; Menegale, F.; Gobbi, A.; Manica, M.; Guzzetta, G.; d’Andrea, V.; Marziano, V.; Trentini, F.; Montarsi, F.; Caputo, B.; et al. Estimating the potential risk of transmission of arboviruses in the Americas and Europe: A modelling study. Lancet Planet. Health 2024, 8, e30–e40. [Google Scholar] [CrossRef]
- Martina, B.E.; Koraka, P.; Osterhaus, A.D. Dengue virus pathogenesis: An integrated view. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Rothman, A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr. Opin. Infect. Dis. 2006, 19, 429–436. [Google Scholar] [CrossRef]
- Foo, S.S.; Chen, W.; Chan, Y.; Bowman, J.W.; Chang, L.C.; Choi, Y.; Yoo, J.S.; Ge, J.; Cheng, G.; Bonnin, A.; et al. The Systemic Inflammatory Landscape of Zika Virus Infection. PLoS Pathog. 2017, 13, e1006677. [Google Scholar] [CrossRef]
- Kam, Y.W.; Leite, J.A.; Lum, F.M.; Tan, J.J.; Lee, B.; Judice, C.C.; Teixeira, L.G.; Andreata-Santos, R.; Vinolo, M.A.R.; de Oliveira, C.; et al. Specific Biomarkers Associated with Neurological Complications and Congenital Central Nervous System Abnormalities from Zika Virus-Infected Patients in Brazil. J. Infect. Dis. 2017, 216, 1727–1736. [Google Scholar] [CrossRef]
- Bezerra, W.P.; Moizéis, R.N.C.; Salmeron, A.C.A.; Pereira, H.W.B.; de Araújo, J.M.G.; Guedes, P.M.M.; Fernandes, J.V.; Nascimento, M.S.L. Innate immune response in patients with acute Chikungunya disease. Med. Microbiol. Immunol. 2023, 212, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.S.; Kam, Y.W.; Lee, B.; Hapuarachchi, H.C.; Wimal, A.; Ng, L.C.; Ng, L.F. A Systematic Meta-analysis of Immune Signatures in Patients With Acute Chikungunya Virus Infection. J. Infect. Dis. 2015, 211, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.F.; Chow, A.; Sun, Y.J.; Kwek, D.J.; Lim, P.L.; Dimatatac, F.; Ng, L.C.; Ooi, E.E.; Choo, K.H.; Her, Z.; et al. IL-1beta, IL-6, and RANTES as biomarkers of Chikungunya severity. PLoS ONE 2009, 4, e4261. [Google Scholar] [CrossRef] [PubMed]
- Burt, F.J.; Chen, W.; Miner, J.J.; Lenschow, D.J.; Merits, A.; Schnettler, E.; Kohl, A.; Rudd, P.A.; Taylor, A.; Herrero, L.J.; et al. Chikungunya virus: An update on the biology and pathogenesis of this emerging pathogen. Lancet Infect. Dis. 2017, 17, e107–e117. [Google Scholar] [CrossRef]
- Teo, T.H.; Lum, F.M.; Claser, C.; Lulla, V.; Lulla, A.; Merits, A.; Rénia, L.; Ng, L.F. A pathogenic role for CD4+ T cells during Chikungunya virus infection in mice. J. Immunol. 2013, 190, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.S.; Baldoni, N.R.; Cardoso, C.S.; Oliveira, C.D.L. Biomarkers of severity and chronification in chikungunya fever: A systematic review and meta-analysis. Rev. Inst. Med. Trop. 2021, 63, e16. [Google Scholar] [CrossRef]
- Liu, X.; Poo, Y.S.; Alves, J.C.; Almeida, R.P.; Mostafavi, H.; Tang, P.C.H.; Bucala, R.; Teixeira, M.M.; Taylor, A.; Zaid, A.; et al. Interleukin-17 Contributes to Chikungunya Virus-Induced Disease. mBio 2022, 13, e0028922. [Google Scholar] [CrossRef]
- Fox, J.M.; Diamond, M.S. Immune-Mediated Protection and Pathogenesis of Chikungunya Virus. J. Immunol. 2016, 197, 4210–4218. [Google Scholar] [CrossRef]
- Kulkarni, S.P.; Ganu, M.; Jayawant, P.; Thanapati, S.; Ganu, A.; Tripathy, A.S. Regulatory T cells and IL-10 as modulators of chikungunya disease outcome: A preliminary study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2475–2481. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Panella, A.J.; Velez, J.O.; Lambert, A.J.; Campbell, G.L. Chikungunya virus in US travelers returning from India, 2006. Emerg. Infect. Dis. 2007, 13, 764–767. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, I.S.B.; Tanabe, E.L.L.; Santos, E.C.; Martins, W.V.; Araújo, I.M.T.C.; Cavalcante, M.C.A.; Lima, A.R.V.; Câmara, N.O.S.; Anderson, L.; Yunusov, D.; et al. Cellular and Molecular Immune Response to Chikungunya Virus Infection. Front. Cell. Infect. Microbiol. 2018, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Long, K.M.; Heise, M.T. Protective and Pathogenic Responses to Chikungunya Virus Infection. Curr. Trop. Med. Rep. 2015, 2, 13–21. [Google Scholar] [CrossRef]
- Traverse, E.M.; Millsapps, E.M.; Underwood, E.C.; Hopkins, H.K.; Young, M.; Barr, K.L. Chikungunya Immunopathology as It Presents in Different Organ Systems. Viruses 2022, 14, 1786. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Kumar, A.; Hasan, A.; Mehta, D.; Kumar, R.; Sharma, C.; Sunil, S. Disease Resolution in Chikungunya-What Decides the Outcome? Front. Immunol. 2020, 11, 695. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Mikkelsen, H.; Jungersen, G. Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies. J. Immunol. Res. 2019, 2019, 1356540. [Google Scholar] [CrossRef]
- Hoarau, J.J.; Jaffar Bandjee, M.C.; Krejbich Trotot, P.; Das, T.; Li-Pat-Yuen, G.; Dassa, B.; Denizot, M.; Guichard, E.; Ribera, A.; Henni, T.; et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol. 2010, 184, 5914–5927. [Google Scholar] [CrossRef]
- Nutman, T.B. Looking beyond the induction of Th2 responses to explain immunomodulation by helminths. Parasite Immunol. 2015, 37, 304–313. [Google Scholar] [CrossRef]
- Henry, E.K.; Inclan-Rico, J.M.; Siracusa, M.C. Type 2 cytokine responses: Regulating immunity to helminth parasites and allergic inflammation. Curr. Pharmacol. Rep. 2017, 3, 346–359. [Google Scholar] [CrossRef]
- Paiva, I.A.; Badolato-Corrêa, J.; Familiar-Macedo, D.; de-Oliveira-Pinto, L.M. Th17 Cells in Viral Infections-Friend or Foe? Cells 2021, 10, 1159. [Google Scholar] [CrossRef]
- Sánchez-Vargas, L.A.; Hernández-Flores, K.G.; Thomas-Dupont, P.; Izaguirre-Hernández, I.Y.; Sánchez-Marce, E.E.; Remes-Ruiz, R.; Fonseca-Coronado, S.; Hernández-Romano, P.A.; Flores-Collins, M.E.; Vivanco-Cid, H. Characterization of the IL-17 and CD4+ Th17 Cells in the Clinical Course of Dengue Virus Infections. Viruses 2020, 12, 1435. [Google Scholar] [CrossRef]
- Almeida, R.S.; Ferreira, M.L.B.; Sonon, P.; Cordeiro, M.T.; Sadissou, I.; Diniz, G.T.N.; Militão-Albuquerque, M.F.P.; Franca, R.F.O.; Donadi, E.A.; Lucena-Silva, N. Cytokines and Soluble HLA-G Levels in the Acute and Recovery Phases of Arbovirus-Infected Brazilian Patients Exhibiting Neurological Complications. Front. Immunol. 2021, 12, 582935. [Google Scholar] [CrossRef]
- Beringer, A.; Miossec, P. Systemic effects of IL-17 in inflammatory arthritis. Nat. Rev. Rheumatol. 2019, 15, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Zhou, C.; Nandakumar, K.S. Molecular and Cellular Pathways Contributing to Joint Damage in Rheumatoid Arthritis. Mediat. Inflamm. 2020, 2020, 3830212. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, N.G.; MeloVilar, K.; Duarte, A.L.B.P.; Rêgo, M.J.B.M.; Pereira, M.C.; da Rocha Pitta, I.; Marques, C.D.L.; Pitta, M.G.R. IL-27 in patients with Chikungunya fever: A possible chronicity biomarker? Acta Trop. 2019, 196, 48–51. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Miyara, M.; Costantino, C.; Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef]
- Rojas, J.M.; Avia, M.; Martín, V.; Sevilla, N. IL-10: A Multifunctional Cytokine in Viral Infections. J. Immunol. Res. 2017, 2017, 6104054. [Google Scholar] [CrossRef]
- Konkel, J.E.; Zhang, D.; Zanvit, P.; Chia, C.; Zangarle-Murray, T.; Jin, W.; Wang, S.; Chen, W. Transforming Growth Factor-β Signaling in Regulatory T Cells Controls T Helper-17 Cells and Tissue-Specific Immune Responses. Immunity 2017, 46, 660–674. [Google Scholar] [CrossRef]
- Gois, B.M.; Peixoto, R.F.; Guerra-Gomes, I.C.; Palmeira, P.H.S.; Dias, C.N.S.; Araújo, J.M.G.; Veras, R.C.; Medeiros, I.A.; Azevedo, F.L.A.A.; Boyton, R.J.; et al. Regulatory T cells in acute and chronic human Chikungunya infection. Microbes Infect. 2022, 24, 104927. [Google Scholar] [CrossRef] [PubMed]
| Probe or Primer | Sequence (5′-3′) | Position in the Genome |
|---|---|---|
| Prob VCHIK 6919P | AGGTACGCGCTTCAAGTTCGGCG | 6919–6941 |
| VCHIK 6856F (+) | TCACTCCCTGTTGGACTTGATAGA | 6856–6879 |
| VCHIK 6981R (−) | TTGACGAACAGAGTTAGGAACATACC | 6981–6956 |
| Primer | Forward | Reverse | MT 1 |
|---|---|---|---|
| β-actin | TGACTCAGGATTTAAAAACTGGAA | GCCACATTGTGAACTTTGGG | 56.5 |
| GATA-3 | GTCCCTTTCGACTTGCATTT | TATCCATCGCGTTTAGGCTTC | 56.9 |
| T-bet | AATGCCGAGATTACTCAGCTG | AAAGTTCTCCCGGAATCCTT | 56.9 |
| RORγT | TGACCAGATTGTGCTTCTCAAA | TCCTAACCAGCACCACTTCCAT | 58.2 |
| PU.1 | AGAAGAAGATCCGCCTGTACCA | GTGCTTGGACGAGAACTGGAA | 60.0 |
| AHR | CAGCGTCAGTTACCTGAGAGCCAAG | CGCAAACAAAGCCAACTGAGGTGGAAG | 65.1 |
| FoxP3 | AGGAAAGGAGGATGGACGAA | AGGCAAGACAGTGGAAACCT | 57.8 |
| STAT3 | CTGTTTCAGAGGCTAGGTTGTT | AGGTGAGGGACCTTTAGACAC | 59.1 |
| IL-4 | AACAGCCTCACAGAGCAGAAGAC | GTGTTCTTGGAGGCAGCAAAG | 61.0 |
| IL-9 | CTTCTGGCCATGGTCCTTAC | CATGGTCTGGTGCAGTTGTC | 59.8 |
| IL-10 | AGATCTCCGAGATGCCTTCA | ATTCTTCACCTGCTCCACGG | 58.8 |
| IL-17 | CAATGACCTGGAAATACCAA | TGAAGGCATGTGAAATCGAGA | 54.9 |
| IL-22 | TTCCAGCAGCCCTATATCACC | GCTCACTCATACTGACTCCGTG | 60.9 |
| IFN-γ | ATGCAGAGCCAAATTGTCTCC | AGGCAGGACAACCATTACTGG | 59.0 |
| TGF-β | ATTGAGGGCTTTCGCCTTAG | TGTGTTATCCCTGCTGTCACAG | 58.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, R.M.d.M.; de Melo, M.F.; Fernandes, J.V.; Valverde, J.G.; Matta Guedes, P.M.; de Araújo, J.M.G.; Nascimento, M.S.L. Acute Chikungunya Virus Infection Triggers a Diverse Range of T Helper Lymphocyte Profiles. Viruses 2024, 16, 1387. https://doi.org/10.3390/v16091387
Brito RMdM, de Melo MF, Fernandes JV, Valverde JG, Matta Guedes PM, de Araújo JMG, Nascimento MSL. Acute Chikungunya Virus Infection Triggers a Diverse Range of T Helper Lymphocyte Profiles. Viruses. 2024; 16(9):1387. https://doi.org/10.3390/v16091387
Chicago/Turabian StyleBrito, Ramayana Morais de Medeiros, Marília Farias de Melo, José Veríssimo Fernandes, Joanna Gardel Valverde, Paulo Marcos Matta Guedes, Josélio Maria Galvão de Araújo, and Manuela Sales Lima Nascimento. 2024. "Acute Chikungunya Virus Infection Triggers a Diverse Range of T Helper Lymphocyte Profiles" Viruses 16, no. 9: 1387. https://doi.org/10.3390/v16091387






