Short Communication: Rotavirus Group A Occurrence in Rural Water Source Samples in a Midwest Region State of Brazil, Comparing Wet and Dry Seasons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Sample Collection Sites during Dry and Rainy Season
2.2. RV Concentration
2.3. The Extraction of Viral Nucleic Acids of RV in the Samples
2.4. RV Positive Control
2.5. Molecular Analysis: The Detection and Quantification of RV in the Samples
2.6. RT-qPCR
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subekti, D.; Lesmana, M.; Tjaniadi, P.; Safari, N.; Frazier, E.; Simanjuntak, C.; Komalarini, S.; Taslim, J.; Campbell, J.R.; Oyofo, B.A. Incidence of Norwalk-like viruses, rotavirus and adenovirus infection in patients with acute gastroenteritis in Jakarta, Indonesia. FEMS Microbiol. Immunol. 2002, 33, 27–33. [Google Scholar] [CrossRef]
- Toze, S. PCR and the detection of microbial pathogens in water and wastewater. Wat. Res. 1999, 33, 3545–3556. [Google Scholar] [CrossRef]
- Bouseettine, R.; Hassou, N.; Bessi, H.; Ennaji, M.M. Waterborne Transmission of Enteric Viruses and Their Impact on Public Health. In Emerging and Reemerging Viral Pathogens—Volume 1: Fundamental and Basic Virology Aspects of Human, Animal and Plant Pathogens, 1st ed.; Ennaji, M.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 907–932. [Google Scholar]
- Ashbolt, N.J. Microbial Contamination of Drinking Water and Human Health from Community Water Systems. Curr. Environ. Health Rep. 2015, 2, 95–106. [Google Scholar] [CrossRef]
- Nóbrega, M.D.A.C.; Silva, N.Q.; Félix, T.S.; Silva, G.A.; Nóbrega, J.Y.L.; Soares, C.M.; Coelho, D.C. Análise físico-química e bacteriológica da água de abastecimento da cidade de São Domingos–PB. INTESA 2015, 9, 10. [Google Scholar]
- Health Canada. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—Enteric Viruses, 2nd ed.; Health Canada: Ottawa, ON, Canada, 2019; pp. 1–123. [Google Scholar]
- Upfold, N.S.; Luke, G.A.; Knox, C. Occurrence of Human Enteric Viruses in Water Sources and Shellfish: A Focus on Africa. Food Environ. Virol 2021, 13, 1–31. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Attoui, H.; Bányai, K.; Brussaard, C.P.D.; Danthi, P.; Del Vas, M.; Dermody, T.S.; Duncan, R.; Fang, Q.; Johne, R.; et al. ICTV Virus Taxonomy Profile: Sedoreoviridae 2022. J. Gen. Virol. 2022, 103, 001782. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Amimo, J.O.; Saif, L.J. Porcine Rotaviruses: Epidemiology, Immune Responses and Control Strategies. Viruses 2017, 9, 48. [Google Scholar] [CrossRef]
- Damasceno, J.L.; Andrade, G.; Santiago, M.B.; Martins, C.H.G.; Pires, R.H. Rotavirus and the emergence of new genotypes: A narrative review. ReBraM 2020, 23, 173–189. [Google Scholar]
- Desselberger, U. Viral gastroenteritis. Medicine 2017, 45, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Barardi, C.R.M.; Viancelli, A.; Rigotto, C.; Corrêa, A.A.; Moresco, V.; Souza, D.S.M.; ElMahdy, M.E.I.; Fongaro, G.; Pilotto, M.R.; Nascimento, M.A. Monitoring viruses in environmental samples. Int. J. Environ. Sci. Eng. Res. 2012, 3, 62–79. [Google Scholar]
- Masukawa, M.L.T.; Moriwaki, A.M.; Santana, R.G.; Uchimura, N.S.; Uchimura, T.T. Impacto da vacinação oral de Rotavírus Humano nas taxas de hospitalizações em crianças. Acta Paul. Enferm. 2015, 28, 243–249. [Google Scholar] [CrossRef]
- Stuempfig, N.D.; Seroy, J. Viral Gastroenteritis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Katayama, H.; Shimasaki, A.; Ohgaki, S. Development of a virus concentration method and its application to detection of enterovirus and Norwalk virus from coastal seawater. Appl. Environ. Microbiol. 2002, 68, 1033–1039. [Google Scholar] [CrossRef]
- Vecchia, A.D.; Fleck, J.D.; Comerlato, J.; Kluge, M.; Bergamaschi, B.; da Silva, J.V.S.; da Luz, R.B.; Teixeira, T.F.; Garbinatto, G.N.; Oliveira, D.V.; et al. First description of Adenovirus, Enterovirus, Rotavirus and Torqueteno virus in water samples collected from the ArroioDilúvio, Porto Alegre, Brazil. Braz. J. Biol. 2012, 72, 323–329. [Google Scholar] [CrossRef]
- Steyer, A.; Torkar, K.G.; Gutiérrez-Aguirre, I.; Poljšak-Prijatelj, M. High prevalence of enteric viruses in untreated individual drinking water sources and surface water in Slovenia. Int. J. Hyg. Environ. Health. 2011, 214, 392–398. [Google Scholar] [CrossRef]
- Castells, M.; Schild, C.; Caffarena, D.; Bok, M.; Giannitti, F.; Armendano, J.; Riet-Correa, F.; Victoria, M.; Parreño, V.; Colina, R. Prevalence and viability of group A rotavirus in dairy farm water sources. J. Appl. Microbiol. 2018, 124, 922–929. [Google Scholar] [CrossRef]
- Faccin-Galhardi, L.C.; Lopes, N.; Espada, S.F.; Linhares, R.E.C.; Pelayo, J.S.; Nozawa, C. Waterborne Viral Pathogens: Detection, Control And Monitoring Of Water Quality For Human Consumption. Soc. Bras. Virol. 2013, 18, 3. [Google Scholar] [CrossRef]
- Shaheen, M.N.F. Prevalence, Genotyping, and Seasonality of Rotavirus in Environmental Water Samples and Stool Samples of Gastroenteritis Children in Egypt (2010–2016). J. Inf. Dis. Trav. Med. 2019, 3, 1–8. [Google Scholar] [CrossRef]
- SESGO. Gerência de Vigilância Epidemiológica das Doenças Transmissíveis. Coordenação de Controle das Doenças Hídricas e Alimentares; Assunto: Sazonalidade do rotavírus; Informe Técnico N.º 04/2014; Secretaria de Estado de Saúde de Goiás. Superintendência de Vigilância em Saúde: Goiânia, Brazil, 2014. [Google Scholar]
- Stobnicka-Kupiec, A.; Górny, R.L. Seasonal prevalence of potentially infectious enteric viruses in surface waters below treated wastewater discharge. Ann. Agric. Environ. Med. 2022, 29, 523–528. [Google Scholar] [CrossRef]
- Fong, T.T.; Lipp, E.K. Enteric Viruses of Humans and Animals in Aquatic Environments: Health Risks, Detection, and Potential Water Quality Assessment Tools. Microbiol. Mol. Biol. 2005, 69, 357–371. [Google Scholar] [CrossRef]
- Cioffi, B.; Monini, M.; Salamone, M.; Pellicanò, R.; Di Bartolo, I.; Guida, M.; La Rosa, G.; Fusco, G. Environmental surveillance of human enteric viruses in wastewaters, groundwater, surface water and sediments of Campania Region. Reg. Stud. Mar. Sci. 2022, 38, 101368. [Google Scholar] [CrossRef]
- Meng, Z.; Birch, C.; Heath, R.; Gust, I. Physicochemical Stability and Inactivation of Human and Simian Rotaviruses. Appl. Environ. Microbiol. 1987, 53, 727–730. [Google Scholar] [CrossRef]
- Espinosa, A.C.; Mazari-Hiriart, M.; Espinosa, R.; Maruri-Avidal, L.; Méndez, E.; Arias, C.F. Infectivity and genome persistence of rotavirus and astrovirus in groundwater and surface water. Water Res. 2008, 42, 2618–2628. [Google Scholar] [CrossRef]
- Fongaro, G.; Nascimento, M.A.; Viancelli, A.; Tonetta, D.; Petrucio, M.M.; Barardi, C.R.M. Surveillance of human viral contamination and physicochemical profiles in a surface water lagoon. Water Sci. Technol. 2012, 66, 2682–2689. [Google Scholar] [CrossRef]
- Bortagaray, V.; Girardi, V.; Pou, S.; Lizasoain, A.; Tort, L.F.L.; Spilki, F.R.; Colina, R.; Victoria, M. Detection, Quantifcation, and Microbial Risk Assessment of Group A Rotavirus in Rivers from Uruguay. Food Environ. Virol. 2020, 12, 89–98. [Google Scholar] [CrossRef]
- Fisman, D. Seasonality of viral infections: Mechanisms and unknowns. Clin. Microbiol. Infect. 2012, 18, 946–954. [Google Scholar] [CrossRef]
- Borchardt, M.A.; Bradbury, K.R.; Gotkowitz, M.B.; Cherry, J.A.; Parker, B.L. Human enteric viruses in groundwater from a confined bedrock aquifer. Environ. Sci. Technol. 2007, 41, 6606–6612. [Google Scholar] [CrossRef]
- Staggemeier, R.; Bortoluzzi, M.; Heck, T.M.S.; da Luz, R.B.; Fabres, R.B.; Soliman, M.C.; Rigotto, C.; Baldasso, N.A.; Spilki, F.R.; Almeida, S.E.M. Animal and human enteric viruses in water and sediment samples from dairy farms. Agric. Water Manag. 2015, 152, 135–141. [Google Scholar] [CrossRef]
- Sorensen, J.P.R.; Aldous, P.; Bunting, S.Y.; McNally, S.; Townsend, B.R.; Barnett, M.J.; Harding, T.; Ragione, R.M.L.; Stuart, M.E.; Tipper, H.J.; et al. Seasonality of enteric viruses in groundwater-derived public water sources. Water Res. 2021, 207, 117813. [Google Scholar] [CrossRef]
- Gotkowitz, M.B.; Bradbury, K.R.; Borchardt, M.A.; Zhu, J.; Spencer, S.K. Effects of Climate and Sewer Condition on Virus Transport to Groundwater. Environ. Sci. Technol. 2016, 50, 8497–8504. [Google Scholar] [CrossRef]
- Murphy, H.M.; McGinnis, S.; Blunt, R.; Stokdyk, J.; Wu, J.W.; Cagle, A.; Denno, D.M.; Spencer, S.; Firnstahl, A.; Borchardt, M.A. Septic Systems and Rainfall Influence Human Fecal Marker and Indicator Organism Occurrence in Private Wells in Southeastern Pennsylvania. Environ. Sci. Technol. 2020, 54, 3159–3168. [Google Scholar] [CrossRef]
- Gonella, J.M.; Tonani, K.A.A.; Fregonesi, B.M.; Machado, C.S.; Trevilato, R.B.; Zagui, G.S.; Alves, R.I.S.; Munõz, S.I.S. Adenovírus e rotavírusemáguassuperficiais do córrego Ribeiro Preto, São Paulo, Brasil. O Mundo Saúde 2016, 40, 474–480. [Google Scholar] [CrossRef]
- Lima, F.S.; Scalize, O.S.; Gabriel, E.F.M.; Gomes, R.P.; Gama, A.R.; Demoliner, M.; Spilki, F.R.; Vieira, J.D.G.; Carneiro, L.C. Escherichia coli, Species C Human Adenovirus, and Enterovirus in Water Samples Consumed in Rural Areas of Goiás, Brazil. Food Environ. Virol. 2021, 14, 77–88. [Google Scholar] [CrossRef]
- Spilki, F.R. Crise hídrica, saúde e parâmetros de qualidademicrobiológica da água no Brasil. Rev. USP 2015, 106, 71–78. [Google Scholar] [CrossRef]
- Mackowiak, M.; Leifels, M.; Hamza, I.A.; Jurzik, L.; Wingender, J. Distribution of Escherichia coli, coliphages and enteric viruses in water, epilithic biofilms and sediments of an urban river in Germany. Sci. Total Environ. 2018, 626, 650–659. [Google Scholar] [CrossRef]
- Amaral, L.A.; Filho, A.N.; Junior, O.D.R.; Ferreira, F.L.A.; Barros, L.S.S. Água de consumohumanocomofator de risco à saúdeempropriedadesrurais. Rev. Saude Publica 2003, 37, 510–514. [Google Scholar] [CrossRef]
- da Luz, R.B.; Staggemeier, R.; Fratta, L.X.S.; Longo, L.; Schutz, R.; Soliman, M.C.; Kluge, M.; Fabres, R.B.; Schenkel, G.C.; Bruni, F.P.; et al. Contaminação viral e bacterianaemáguassubterrâneasnaporçãoaflorante do Aquífero Guaraní, município de Ivoti, RS. Rev. Ambient. Agua 2017, 12, 871–880. [Google Scholar] [CrossRef]
- John, D.E.; Rose, J.B. Review of Factors Affecting Microbial Survival in Groundwater. Environ. Sci. Technol. 2005, 39, 7345–7356. [Google Scholar] [CrossRef]
- Krauss, S.; Griebler, C. Pathogenic Microrganims and Viruses in Groundwater; Acatech: Berlin, Germany, 2011. [Google Scholar]
- Bosch, A. Human Viruses in Water; Elsevier Science: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Gibson, K.E.; Opryszko, M.C.; Schissler, J.T.; Guo, Y.; Schwab, K.J. Evaluation of Human Enteric Viruses in Surface Water and Drinking Water Resources in Southern Ghana. Am. J. Trop. Med. Hyg. 2011, 84, 20–29. [Google Scholar] [CrossRef]
- Pinon, A.; Vialette, M. Survival of Viruses in Water. Intervirology 2018, 61, 214–222. [Google Scholar] [CrossRef]
| Municipalities (Community) | Latitude | Longitude | Water Samples/Site | Season |
|---|---|---|---|---|
| Simolândia (Castelo, Retiro e Três rios) | 14°28′18″ S | 46°29′11″ W | 5 | Dry and Rainy season |
| Nova Roma (Magalhães) | 13°44′25″ S | 46°52′52″ W | 1 | Dry season |
| Mineiros (Cedro) | 17°34′43″ S | 52°32′33′′ W | 7 | Dry season |
| Mimoso de Goiás (Queixo Dantas) | 15°3′29″ S | 48°9′33″ W | 2 | Dry season |
| Cavalcante (São Domingos) | 13°47′51″ S | 47°27′20″ W | 3 | Dry and Rainy season |
| Flores de Goiás (Canabrava) | 14°26′18″ S | 47°2′55″ W | 14 | Dry and Rainy season |
| Monte Alegre de Goiás (Pelotas) | 13°25′14.16″ S | 47°9′46.08″ W | 14 | Dry and Rainy season |
| Cidade Ocidental (Mesquita) | 16°6′19″ S | 47°57′0″ W | 4 | Dry season |
| São João d’Aliança (Forte) | 14°42′31″ S | 47°31′17″ W | 2 | Dry season |
| Niquelândia (Rafael Machado) | 14°27′28″ S | 48°27′59″ W | 2 | Dry season |
| Colinas do sul (José de Coleto) | 14°8′47″ S | 48°4′19″ W | 1 | Dry season |
| Campos Belos (Taquarussu) | 13°1′31″ S | 46°45′54″ W | 13 | Dry and Rainy season |
| Iaciara (Extrema) | 14°5′45″ S | 46°37′55″ W | 19 | Dry and Rainy season |
| Padre Bernardo (Sumidouro) | 15°9′36″ S | 48°17′2″ W | 25 | Dry and Rainy season |
| São Miguel do Araguaia (Lageado settlements) | 13°16′30″ S | 50°9′46″ W | 27 | Dry and Rainy season |
| Silvânia (São Sebastião da Garganta settlements) | 16°39′32″ S | 48°36′28″ W | 27 | Dry and Rainy season |
| Mineiros (Pouso Alegre) | 17°34′08″ S | 52°33′03″ W | 1 | Rainy season |
| Uruaçu (São Lourenço) | 14°31′30″ S | 49°08′27″ W | 11 | Rainy season |
| Goianésia (Itajá ii) | 15°19′1″ S | 49°7′1″ W | 10 | Rainy season |
| Professor Jamil (Rochedo) | 17°15′03″ S | 49°14′38″ W | 20 | Rainy season |
| Silvânia (João de Deus) | 16°39′32″ S | 48°36′28″ W | 5 | Rainy season |
| Nova Crixás (Landi) | 14°05′52″ S | 50°20′17″ W | 8 | Rainy season |
| Água limpa (Arraial da ponte) | 18°04′26″ S | 48°45′43″ W | 1 | Rainy season |
| Goiandira (Povoado Veríssimo) | 18°07′58″ S | 48°05′06″ W | 1 | Rainy season |
| Gameleira de Goiás (Olhos d’Agua) | 16°27′50″ S | 48°40′12″ W | 4 | Rainy season |
| Barro alto (Santo Antônio da Laguna) | 14°58′15″ S | 48°54′57″ W | 9 | Rainy season |
| Niquelândia (Povoado Vermelho) | 14°28′26″ S | 48°27′36″ W | 2 | Rainy season |
| Divinópolis de Goiás (Vazante) | 13°17′42″ S | 46°23′34″ W | 2 | Rainy season |
| Alto paraíso de Goiás (Povoado Moinho) | 14°07′58″ S | 47°30′36″ W | 4 | Rainy season |
| Posse (Baco Pari) | 14°05′34″ S | 46°22′08″ W | 2 | Rainy season |
| Total | 246 |
| Virus | Target Gene | Name | Sequence | Polarity | Position | Ta * (°C) | Amplicon (bp) |
|---|---|---|---|---|---|---|---|
| GARV | VP6 | ROTAFEEVALE-FW | 5′-GATGTCCTGTACTCCTTGT-3′ | Sense | 7–25 a | 60° | 160 |
| ROTAFEEVALE-REV | 5′-GGTAGATTACCAATTCCTCC-3′ | Reverse | 148–167 a |
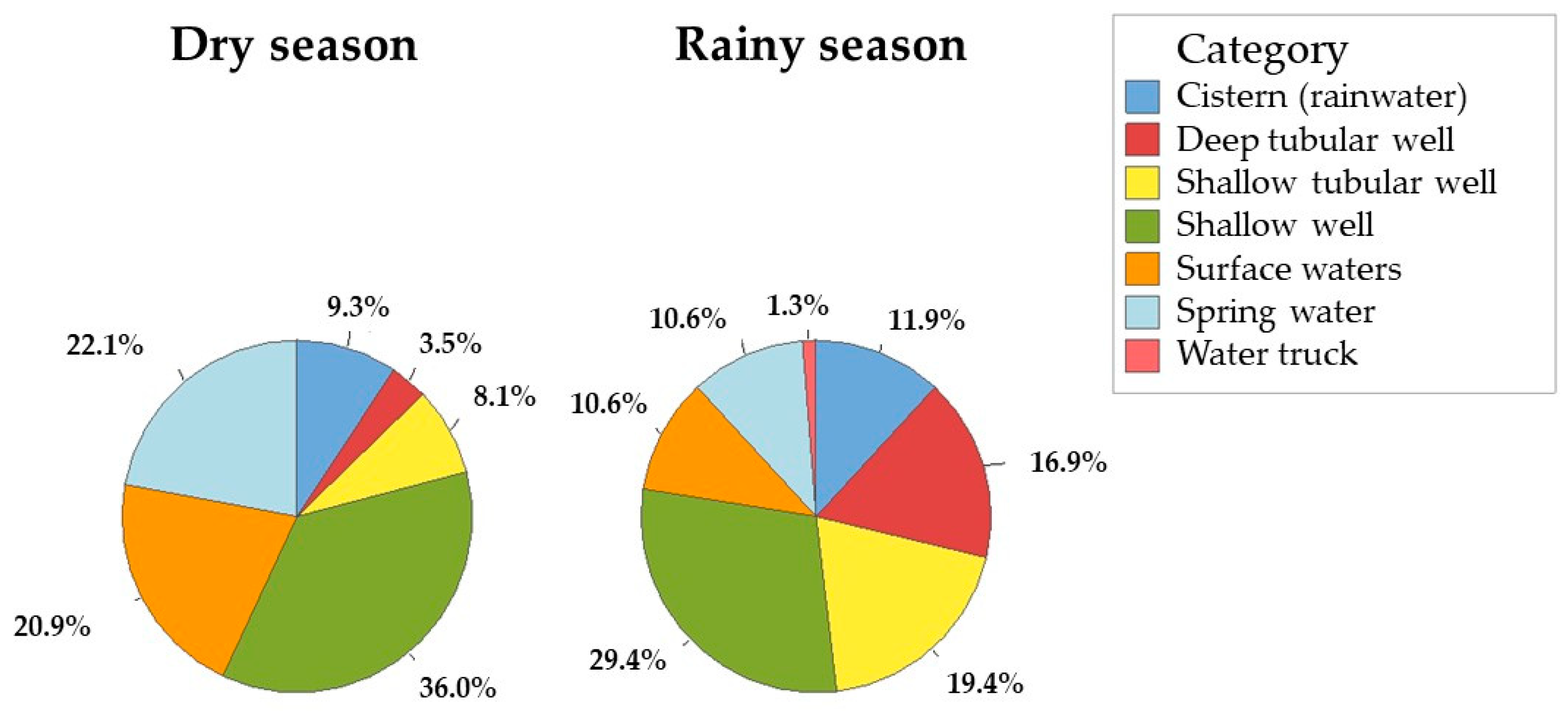
| Season | Font Type | Count | Positive | χ2 (p-Value) | Fisher’s p-Value |
|---|---|---|---|---|---|
| Dry season | Cistern (rainwater) | 8 (9.3%) | 4 | 3.43 (0.064) | 0.145 |
| Rainy season | Cistern (rainwater) | 19 (11.9%) | 3 | ||
| Dry season | Deep tubular well | 3 (3.5%) | 1 | 0.017 (0.894) | 1 |
| Rainy season | Deep tubular well | 27 (16.9%) | 3 | ||
| Dry season | Shallow tubular well | 7 (8.1%) | 4 | (1.52) 0.218 | 0.387 |
| Rainy season | Shallow tubular well | 31 (19.4%) | 10 | ||
| Dry season | Shallow well | 31 (36%) | 23 | (21.43) < 0.001 | <0.001 |
| Rainy season | Shallow well | 47 (29.4%) | 10 | ||
| Dry season | Surface waters | 18 (20.9%) | 8 | (10.28) 0.002 | 0.003 |
| Rainy season | Surface waters | 17 (10.6%) | 0 | ||
| Dry season | Spring water | 19 (22.1%) | 7 | (4.97) 0.026 | 0.044 |
| Rainy season | Spring water | 17 (10.6%) | 1 | ||
| Rainy season | Water truck | 2 (1.3%) | 0 | No estimate | 1 |
| Dry season | Total | 86 (100%) | 47 | (30.81) < 0.001 | <0.001 |
| Rainy season | Total | 160 (100%) | 32 |
| Font Type | OR | Lower | Upper | p-Value |
|---|---|---|---|---|
| Cistern (rainwater) | 5.33 | 0.83 | 34.09 | 0.17 |
| Deep tubular well | 1.18 | 0.09 | 15.03 | 0.59 |
| Shallow tubular well | 2.80 | 0.52 | 14.95 | 0.42 |
| Shallow well | 10.63 | 3.63 | 30.87 | <0.0001 |
| Surface waters | - | - | - | - |
| Water truck | - | - | - | - |
| Spring | 9.33 | 1.01 | 86.36 | 0.05 |
| Total | 4.82 | 2.71 | 8.56 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picciola Bordoni, G.; Candido Gonçalves Barbosa, L.; Santos Lima, F.; de Oliveira Santos, M.; Gonçalves Vieira, J.D.; Reis Oliveira, T.; Scalize, P.S.; Carneiro, L.C. Short Communication: Rotavirus Group A Occurrence in Rural Water Source Samples in a Midwest Region State of Brazil, Comparing Wet and Dry Seasons. Viruses 2024, 16, 1452. https://doi.org/10.3390/v16091452
Picciola Bordoni G, Candido Gonçalves Barbosa L, Santos Lima F, de Oliveira Santos M, Gonçalves Vieira JD, Reis Oliveira T, Scalize PS, Carneiro LC. Short Communication: Rotavirus Group A Occurrence in Rural Water Source Samples in a Midwest Region State of Brazil, Comparing Wet and Dry Seasons. Viruses. 2024; 16(9):1452. https://doi.org/10.3390/v16091452
Chicago/Turabian StylePicciola Bordoni, Graziela, Lucas Candido Gonçalves Barbosa, Fernando Santos Lima, Mônica de Oliveira Santos, José Daniel Gonçalves Vieira, Thais Reis Oliveira, Paulo Sérgio Scalize, and Lilian Carla Carneiro. 2024. "Short Communication: Rotavirus Group A Occurrence in Rural Water Source Samples in a Midwest Region State of Brazil, Comparing Wet and Dry Seasons" Viruses 16, no. 9: 1452. https://doi.org/10.3390/v16091452








