Bovine Lactoferrin-Loaded Plasmonic Magnetoliposomes for Antifungal Therapeutic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Manganese Ferrite Nanoparticles
2.2. Preparation of Gold Nanoparticles
Functionalization with 11-Mercaptoundecanoic Acid and Octadecanethiol
2.3. Preparation of Plasmonic Magnetoliposomes (PMLs)
2.4. Bovine Lactoferrin Encapsulation
2.5. Preparation of Small Unilamellar Vesicles (SUVs) as Membrane Models
2.6. Characterization of Nanoparticles and Magnetoliposomes
2.6.1. UV–Vis–NIR (Ultraviolet–Visible–Near-Infrared) Absorption
2.6.2. X-ray Diffraction
2.6.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.6.4. Magnetic Properties and Hyperthermia
2.6.5. Photothermia Assays
2.6.6. Dinamic Light Scattering and Electrophoretic Light Scattering
2.6.7. Transmission Electron Microscopy
2.6.8. Fluorescence Spectroscopy Measurements
Fluorescence Emission Studies
Fusion Assays with Membrane Models
Anisotropy Measurements
2.6.9. Encapsulation Efficiency of Bovine Lactoferrin
2.6.10. Release Assays
2.7. Biological Assays
2.7.1. Growth Conditions
2.7.2. In Vitro Cytotoxicity Assays
2.7.3. Nanosystem Internalization Assays
2.7.4. Nanosystem Cellular Uptake under Inhibitory Conditions
3. Results and Discussion
3.1. Nanoparticles Characterization
3.1.1. UV–VIS–NIR Absorption Spectroscopy
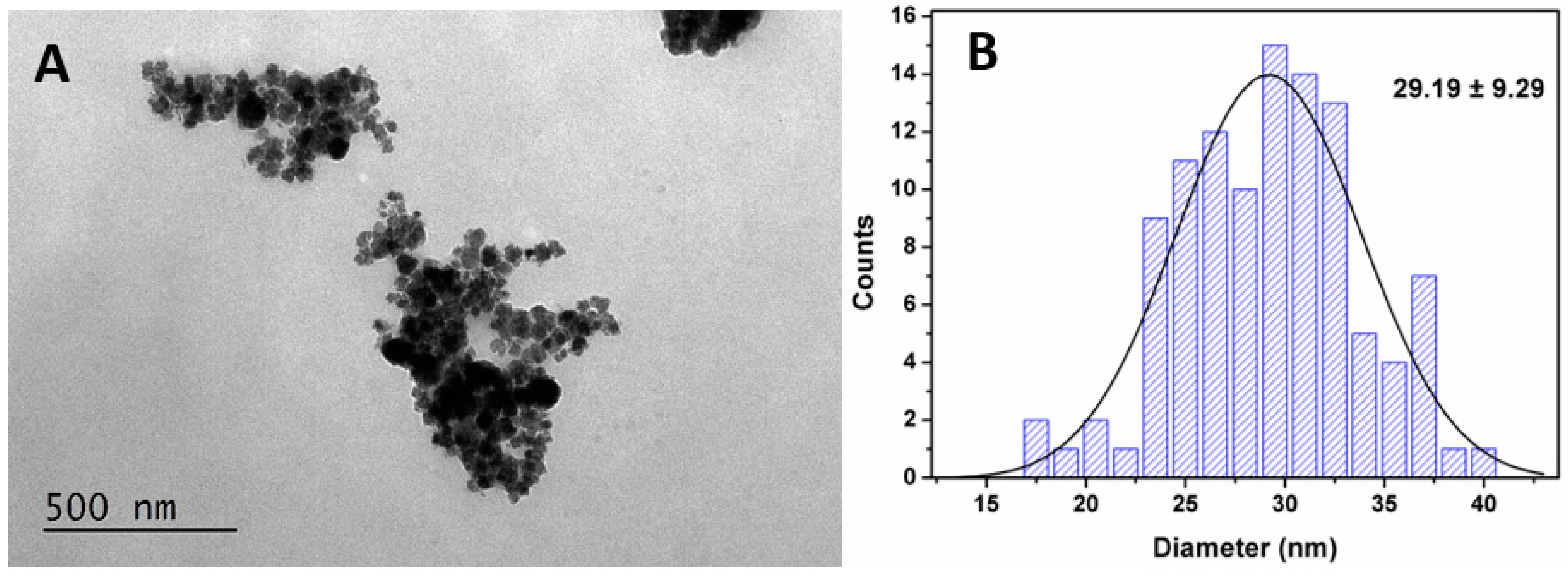
3.1.2. X-ray Diffraction Analysis of MnFe2O4 NPs
3.1.3. Magnetic and Structural Properties of MnFe2O4 Nanoparticles
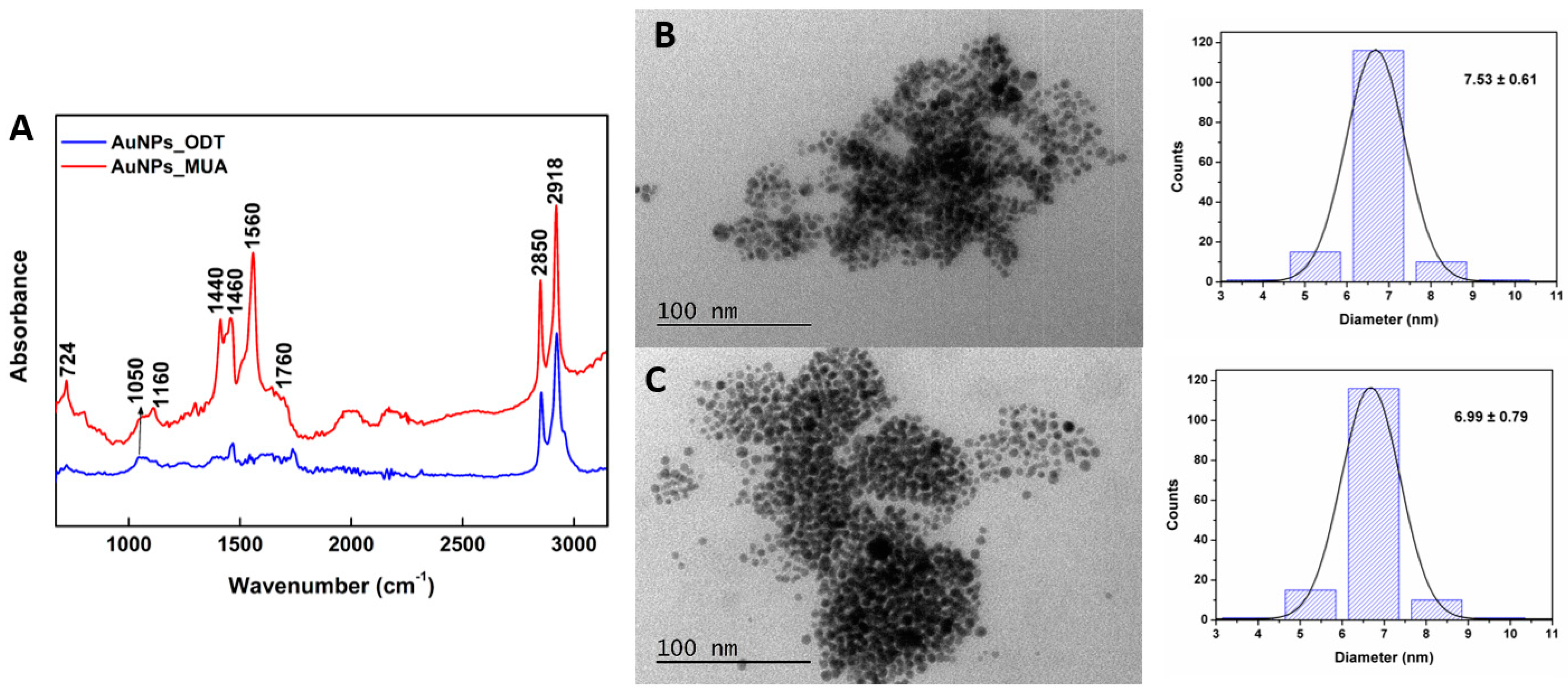
3.1.4. FTIR and Structural Characterization of Gold Nanoparticles
3.1.5. Specific Absorption Rate (SAR)
3.2. Characterization of Plasmonic Magnetoliposomes
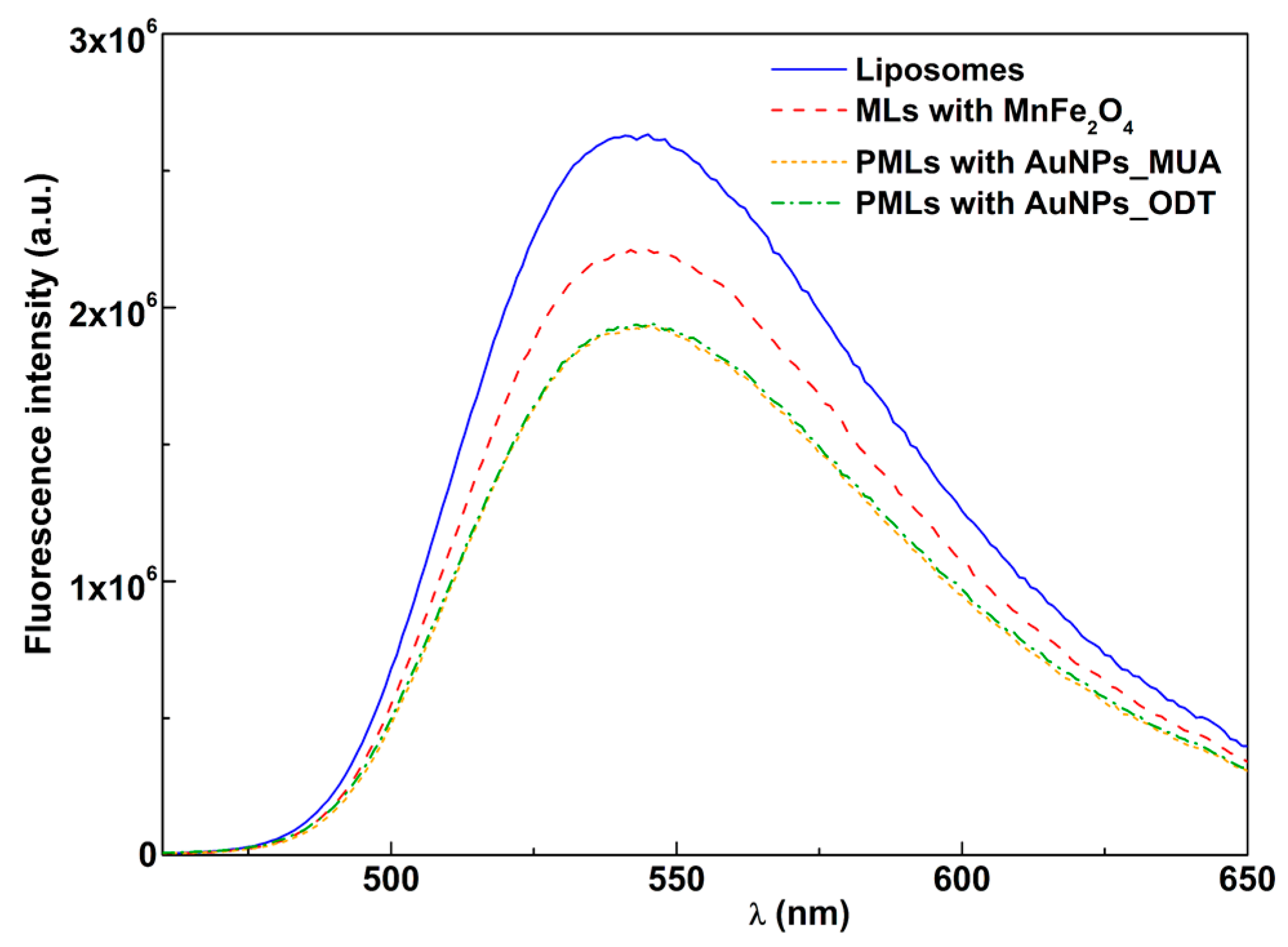
3.2.1. Fluorescence Quenching by Magnetic and Plasmonic NPs
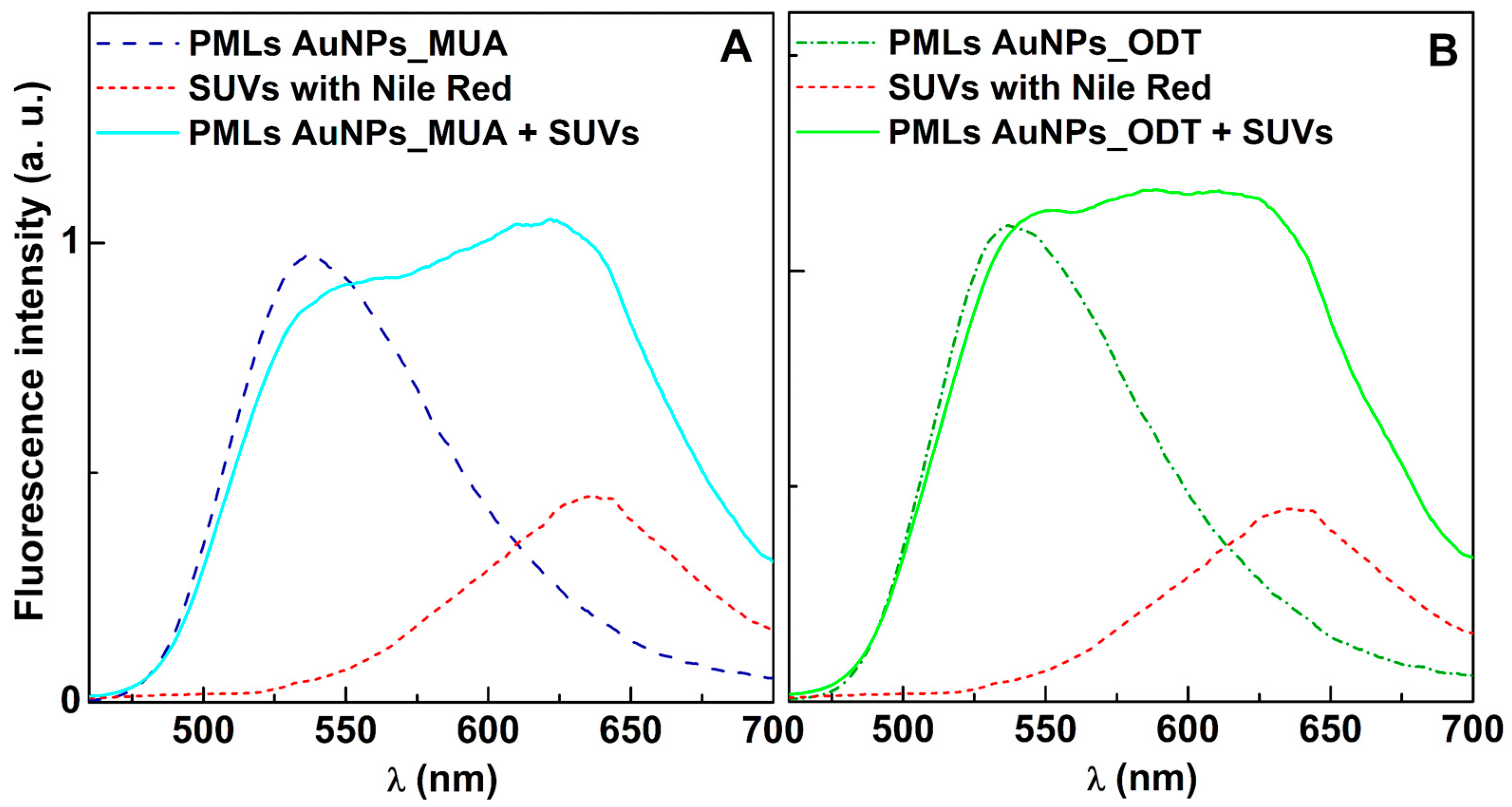
3.2.2. Fusion Assays with Membrane Models
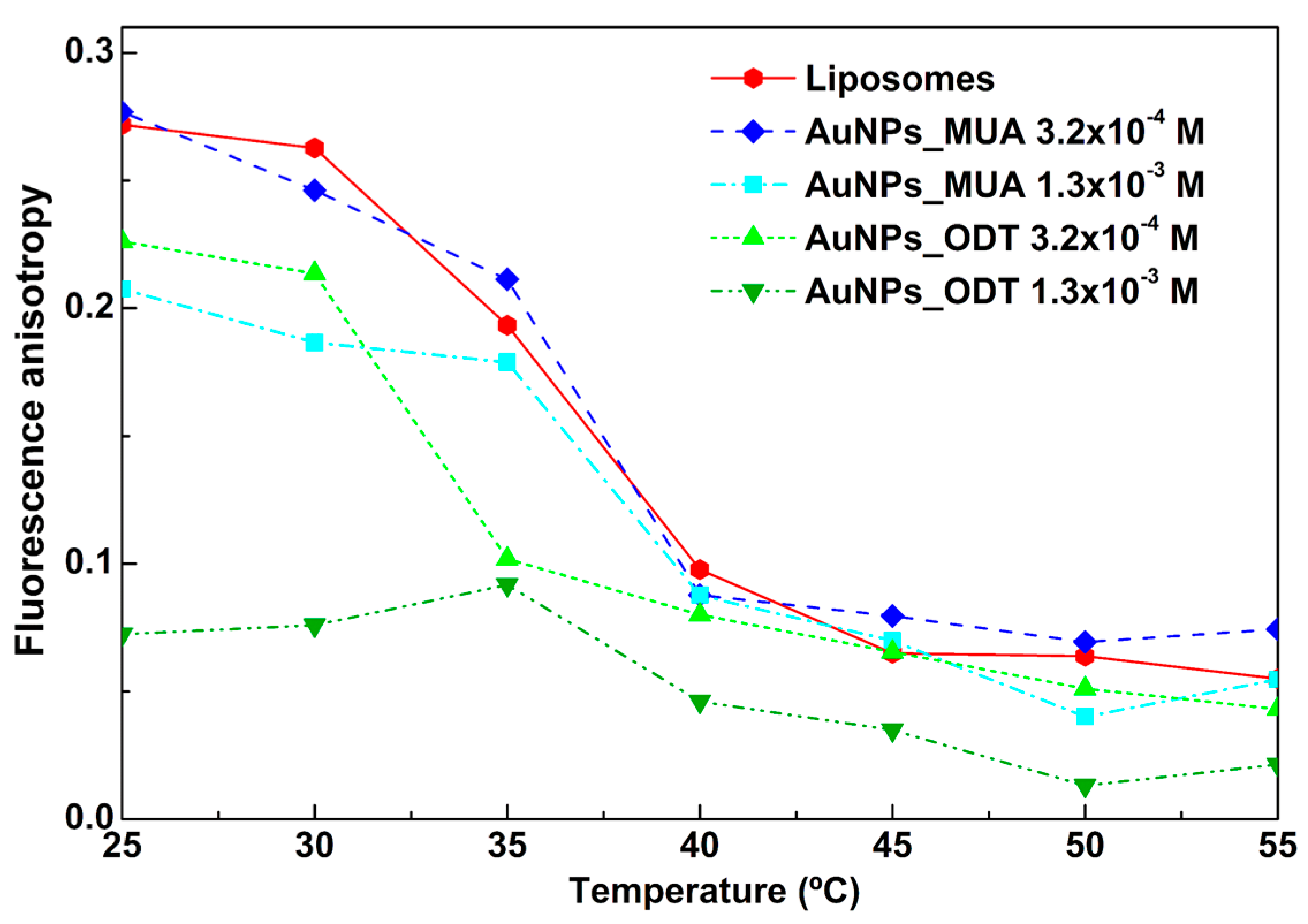
3.2.3. Influence of AuNPs in Phase Transition of DPPC
3.2.4. Structural and Surface Charge Characterization
3.3. bLf-Loaded Plasmonic Magnetoliposomes
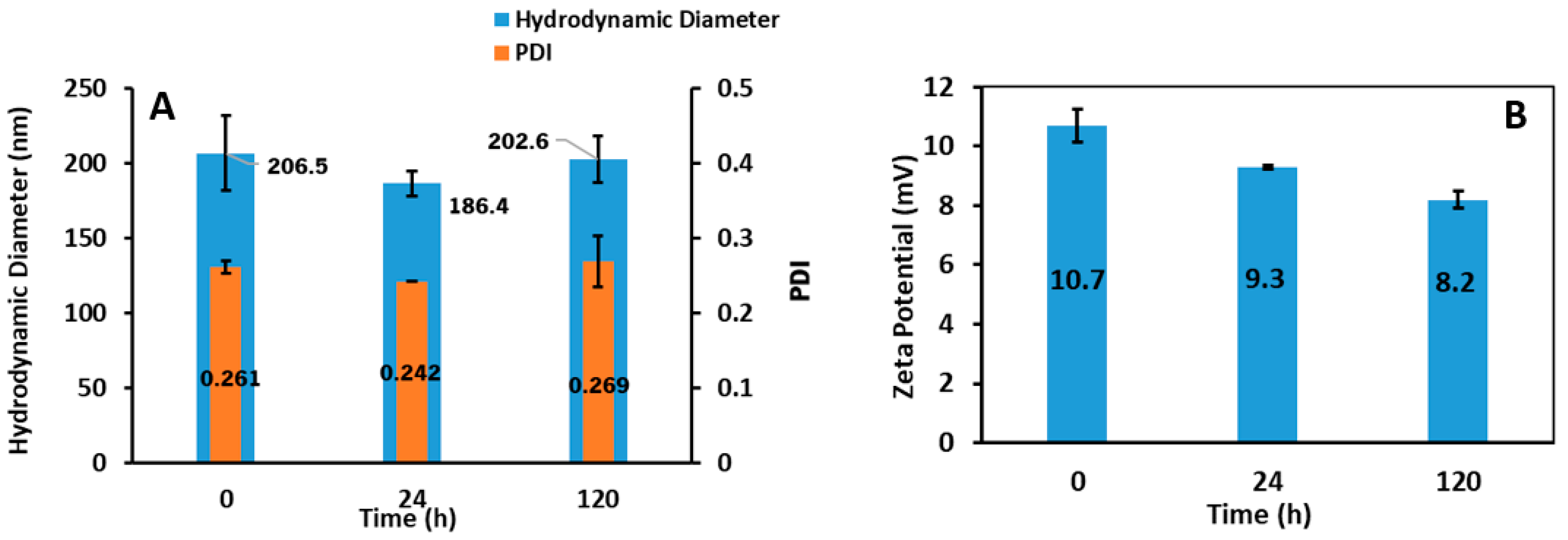
3.3.1. Hydrodynamic Diameter and Zeta Potential
3.3.2. bLf Encapsulation Efficiency and Release Profiles
3.4. Antifungal Activity of bLf-Loaded PMLs
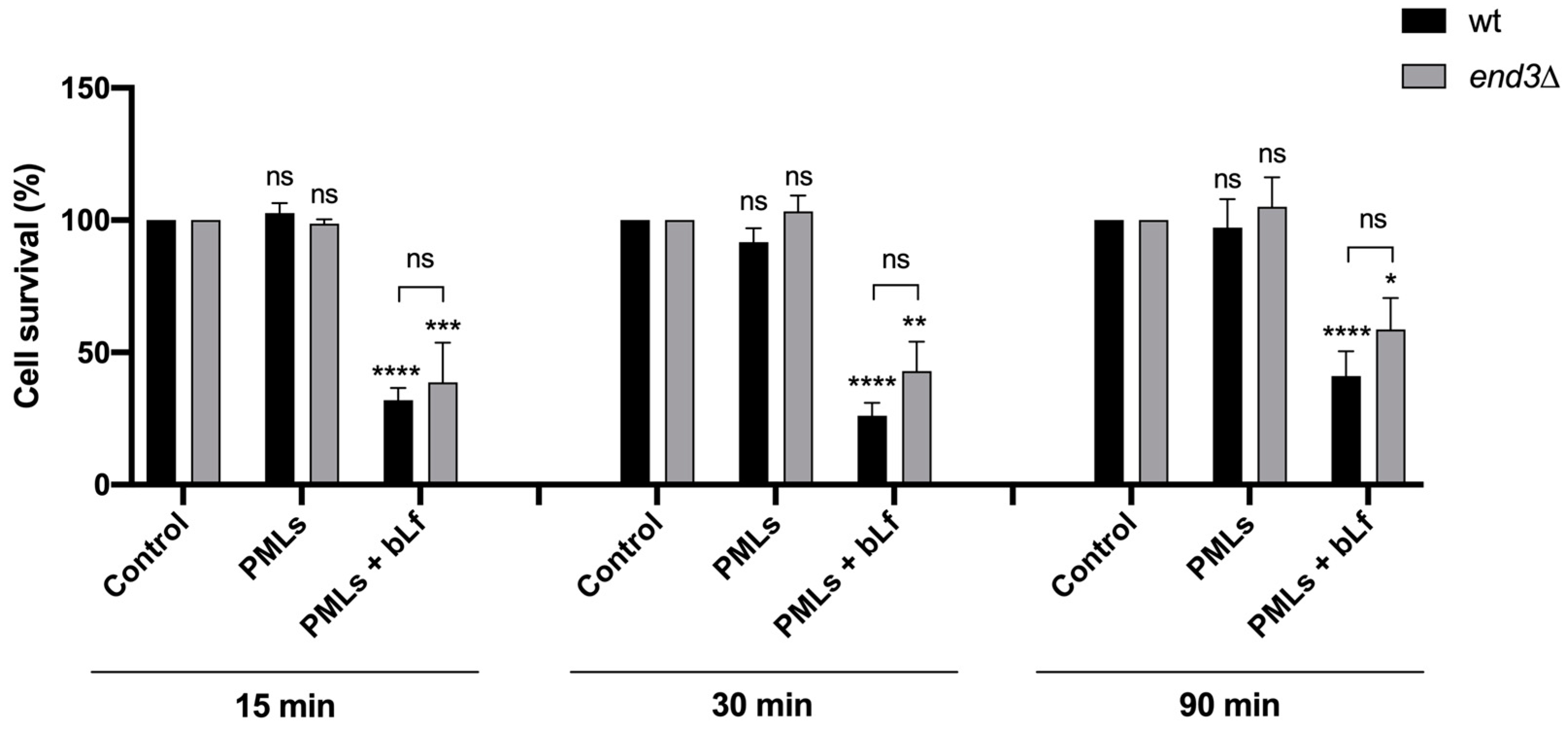
3.4.1. Evaluation of Cytotoxicity and Internalization of bLf-Loaded PMLs
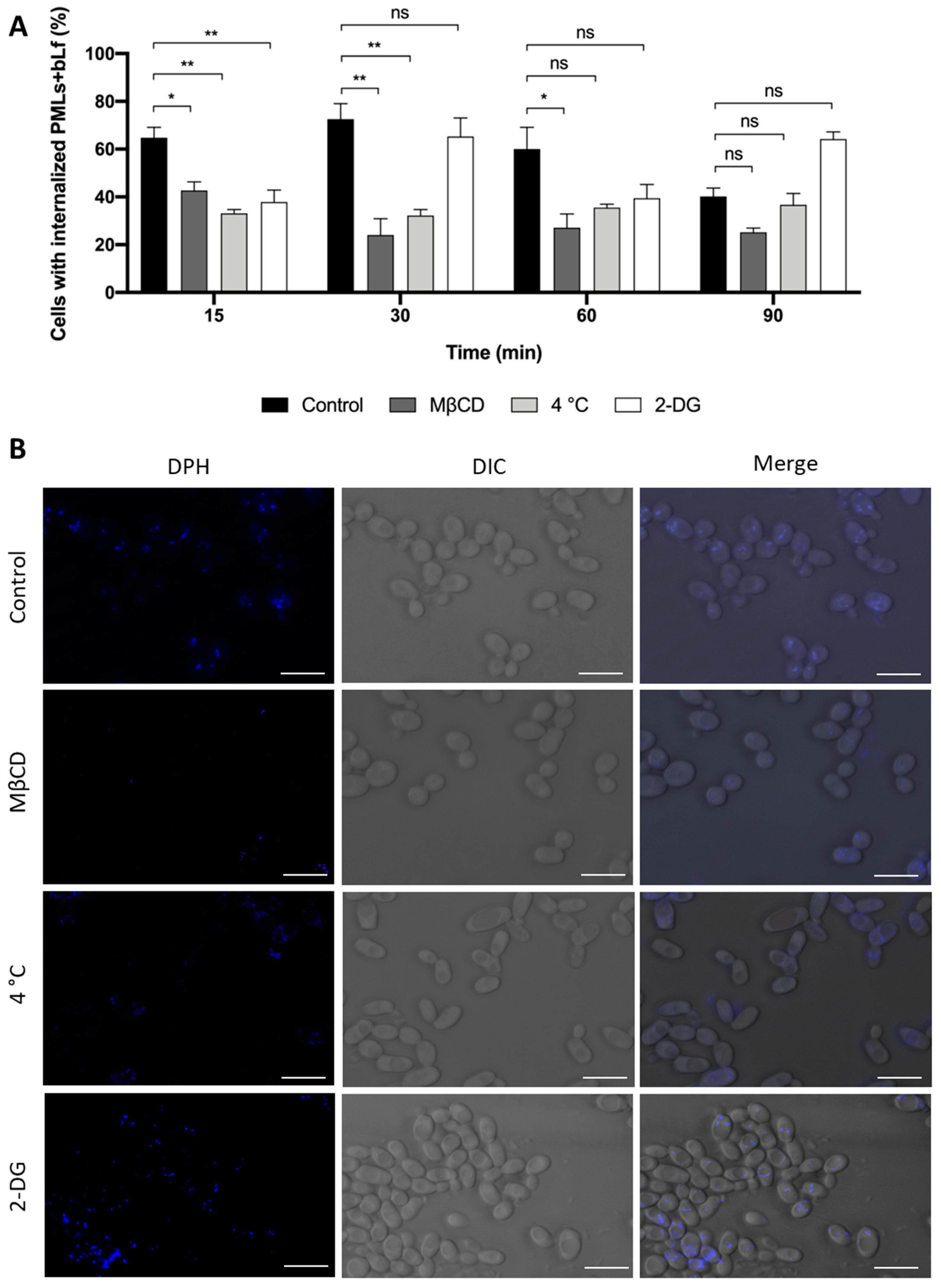
3.4.2. Study of the Internalization Mechanism of bLf-Loaded PMLs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niemirowicz, K.; Durnaś, B.; Piktel, E.; Bucki, R. Development of Antifungal Therapies Using Nanomaterials. Nanomedicine 2017, 12, 1891–1905. [Google Scholar] [CrossRef] [PubMed]
- León-Buitimea, A.; Garza-Cervantes, J.A.; Gallegos-Alvarado, D.Y.; Osorio-Concepción, M.; Morones-Ramírez, J.R. Nanomaterial-Based Antifungal Therapies to Combat Fungal Diseases Aspergillosis, Coccidioidomycosis, Mucormycosis, and Candidiasis. Pathogens 2021, 10, 1303. [Google Scholar] [CrossRef]
- Moastafa, T.M.; El-Sissy, A.E.-D.E.; El-Saeed, G.K.; Koura, M.S.E.-D. Study on the Therapeutic Benefit on Lactoferrin in Patients with Colorectal Cancer Receiving Chemotherapy. Int. Sch. Res. Not. 2014, 2014, 184278. [Google Scholar] [CrossRef]
- El Amrousy, D.; El-Afify, D.; Elsawy, A.; Elsheikh, M.; Donia, A.; Nassar, M. Lactoferrin for Iron-Deficiency Anemia in Children with Inflammatory Bowel Disease: A Clinical Trial. Pediatr. Res. 2022, 92, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Superti, F.; Karadja, E.; De Seta, F. Randomised Clinical Trial in Women with Recurrent Vulvovaginal Candidiasis: Efficacy of Probiotics and Lactoferrin as Maintenance Treatment. Mycoses 2019, 62, 328–335. [Google Scholar] [CrossRef]
- Araujo, V.H.S.; Di Filippo, L.D.; Duarte, J.L.; Spósito, L.; de Camargo, B.A.F.; da Silva, P.B.; Chorilli, M. Exploiting Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery against Cutaneous Fungal Infections. Crit. Rev. Microbiol. 2021, 47, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Nami, S.; Aghebati-Maleki, A.; Aghebati-Maleki, L. Current Applications and Prospects of Nanoparticles for Antifungal Drug Delivery. EXCLI J. 2021, 20, 562–584. [Google Scholar] [CrossRef]
- Renzi, D.F.; de Almeida Campos, L.; Miranda, E.H.; Mainardes, R.M.; Abraham, W.-R.; Grigoletto, D.F.; Khalil, N.M. Nanoparticles as a Tool for Broadening Antifungal Activities. Curr. Med. Chem. 2021, 28, 1841–1873. [Google Scholar] [CrossRef]
- Soliman, G.M. Nanoparticles as Safe and Effective Delivery Systems of Antifungal Agents: Achievements and Challenges. Int. J. Pharm. 2017, 523, 15–32. [Google Scholar] [CrossRef]
- Hokken, M.W.J.; Zwaan, B.J.; Melchers, W.J.G.; Verweij, P.E. Facilitators of Adaptation and Antifungal Resistance Mechanisms in Clinically Relevant Fungi. Fungal Genet. Biol. 2019, 132, 103254. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Kaczyńska, K.; Kleczkowska, P.; Bukowska-ośko, I.; Kramkowski, K.; Sulejczak, D. The Lactoferrin Phenomenon—A Miracle Molecule. Molecules 2022, 27, 2941. [Google Scholar] [CrossRef]
- Andrés, M.T.; Fierro, J.F. Antimicrobial Mechanism of Action of Transferrins: Selective Inhibition of H+-ATPase. Antimicrob. Agents Chemother. 2010, 54, 4335–4342. [Google Scholar] [CrossRef] [PubMed]
- Santos-Pereira, C.; Andrés, M.T.; Chaves, S.R.; Fierro, J.F.; Gerós, H.; Manon, S.; Rodrigues, L.R.; Côrte-Real, M. Lactoferrin Perturbs Lipid Rafts and Requires Integrity of Pma1p-Lipid Rafts Association to Exert Its Antifungal Activity against Saccharomyces cerevisiae. Int. J. Biol. Macromol. 2021, 171, 343–357. [Google Scholar] [CrossRef]
- Santos-Pereira, C.; Guedes, J.P.; Ferreira, D.; Rodrigues, L.R.; Côrte-Real, M. Lactoferrin Perturbs Intracellular Trafficking, Disrupts Cholesterol-Rich Lipid Rafts and Inhibits Glycolysis of Highly Metastatic Cancer Cells Harbouring Plasmalemmal V-ATPase. Int. J. Biol. Macromol. 2022, 220, 1589–1604. [Google Scholar] [CrossRef] [PubMed]
- Farid, A.; El Shemy, M.; Nafie, E.; Hegazy, A.; Abdelhiee, E. Anti-Inflammatory, Anti-Oxidant and Hepatoprotective Effects of Lactoferrin in Rats. Drug Chem. Toxicol. 2021, 44, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Einerhand, A.W.C.; van Loo-Bouwman, C.A.; Weiss, G.A.; Wang, C.; Ba, G.; Fan, Q.; He, B.; Smit, G. Can Lactoferrin, a Natural Mammalian Milk Protein, Assist in the Battle against COVID-19? Nutrients 2022, 14, 5274. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Samaranayake, Y.H.; Samaranayake, L.P.; Nikawa, H. In Vitro Susceptibility of Candida Species to Lactoferrin. Med. Mycol. 1999, 37, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, Y.H.; Samaranayake, L.P.; Wu, P.C.; So, M. The Antifungal Effect of Lactoferrin and Lysozyme on Candida krusei and Candida albicans. APMIS 1997, 105, 875–883. [Google Scholar] [CrossRef]
- Lahoz, E.; Pisacane, A.; Iannaccone, M.; Palumbo, D.; Capparelli, R. Fungistatic Activity of Iron-Free Bovin Lactoferrin against Several Fungal Plant Pathogens and Antagonists. Nat. Prod. Res. 2008, 22, 955–961. [Google Scholar] [CrossRef]
- Wang, J.; Xia, X.-M.; Wang, H.-Y.; Li, P.-P.; Wang, K.-Y. Inhibitory Effect of Lactoferrin against Gray Mould on Tomato Plants Caused by Botrytis Cinerea and Possible Mechanisms of Action. Int. J. Food Microbiol. 2013, 161, 151–157. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Weeks, K.; Carter, D.A. Lactoferrin Is Broadly Active against Yeasts and Highly Synergistic with Amphotericin B. Antimicrob. Agents Chemother. 2020, 64, e02284-19. [Google Scholar] [CrossRef] [PubMed]
- Velliyagounder, K.; Rozario, S.D.; Fine, D.H. The Effects of Human Lactoferrin in Experimentally Induced Systemic Candidiasis. J. Med. Microbiol. 2019, 68, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Liu, S.; Wang, H.; Su, H.; Liu, Z. Enhanced Antifungal Activity of Bovine Lactoferrin-Producing Probiotic Lactobacillus Casei in the Murine Model of Vulvovaginal Candidiasis. BMC Microbiol. 2019, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.E.; Carter, D.A. The Antifungal Activity of Lactoferrin and Its Derived Peptides: Mechanisms of Action and Synergy with Drugs against Fungal Pathogens. Front. Microbiol. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Troost, F.J.; Steijns, J.; Saris, W.H.M.; Brummer, R.J.M. Gastric Digestion of Bovine Lactoferrin In Vivo in Adults. J. Nutr. 2001, 131, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, M.; Jaśkiewicz, A.; Tarasiuk, A.; Fichna, J. Lactoferrin: An Overview of Its Main Functions, Immunomodulatory and Antimicrobial Role, and Clinical Significance. Crit. Rev. Food Sci. Nutr. 2022, 62, 6016–6033. [Google Scholar] [CrossRef]
- Abad, I.; Conesa, C.; Sánchez, L. Development of Encapsulation Strategies and Composite Edible Films to Maintain Lactoferrin Bioactivity: A Review. Materials 2021, 14, 7358. [Google Scholar] [CrossRef]
- Yao, X.; Bunt, C.; Cornish, J.; Quek, S.Y.; Wen, J. Preparation, Optimization and Characterization of Bovine Lactoferrin-Loaded Liposomes and Solid Lipid Particles Modified by Hydrophilic Polymers Using Factorial Design. Chem. Biol. Drug Des. 2014, 83, 560–575. [Google Scholar] [CrossRef]
- Emerich, D.F.; Thanos, C.G. Nanotechnology and Medicine. Expert. Opin. Biol. Ther. 2003, 3, 655–663. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of Magnetic Nanoparticles in Biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, M.S.; Aleya, L.; Kim, H.; Mathew, B. Magnetic Nanoparticles for Hyperthermia in Cancer Treatment: An Emerging Tool. Environ. Sci. Pollut. Res. 2020, 27, 19214–19225. [Google Scholar] [CrossRef]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold Nanoparticle Based Photothermal Therapy: Development and Application for Effective Cancer Treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Khafaji, M.; Zamani, M.; Golizadeh, M.; Bavi, O. Inorganic Nanomaterials for Chemo/Photothermal Therapy: A Promising Horizon on Effective Cancer Treatment. Biophys. Rev. 2019, 11, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Nejadnik, H.; Daldrup-Link, H.E. Next-Generation Superparamagnetic Iron Oxide Nanoparticles for Cancer Theranostics. Drug Discov. Today 2017, 22, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Abdelmoneem, M.A.; Hassanin, I.A.; Elwakil, M.M.A.; Elnaggar, M.A.; Mokhtar, S.; Fang, J.-Y.; Elkhodairy, K.A. Lactoferrin, a Multi-Functional Glycoprotein: Active Therapeutic, Drug Nanocarrier & Targeting Ligand. Biomaterials 2020, 263, 120355. [Google Scholar] [CrossRef]
- El-Fakharany, E.M. Nanoformulation of Lactoferrin Potentiates Its Activity and Enhances Novel Biotechnological Applications. Int. J. Biol. Macromol. 2020, 165, 970–984. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Silva, J.F.G.; Hilliou, L.; Moura, C.; Coutinho, P.J.G.; Martins, J.A.; Testa-Anta, M.; Salgueiriño, V.; Correa-Duarte, M.A.; Ferreira, P.M.T.; et al. Impact of Citrate and Lipid-Functionalized Magnetic Nanoparticles in Dehydropeptide Supramolecular Magnetogels: Properties, Design and Drug Release. Nanomaterials 2020, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Ansar, S.; Chakraborty, S.; Kitchens, C. PH-Responsive Mercaptoundecanoic Acid Functionalized Gold Nanoparticles and Applications in Catalysis. Nanomaterials 2018, 8, 339. [Google Scholar] [CrossRef]
- Rodrigues, A.R.O.; Ramos, J.M.F.; Gomes, I.T.; Almeida, B.G.; Araújo, J.P.; Queiroz, M.J.R.P.; Coutinho, P.J.G.; Castanheira, E.M.S. Magnetoliposomes Based on Manganese Ferrite Nanoparticles as Nanocarriers for Antitumor Drugs. RSC Adv. 2016, 6, 17302–17313. [Google Scholar] [CrossRef]
- Loura, L.M.S.; Fernandes, F.; Fernandes, A.C.; Ramalho, J.P.P. Effects of Fluorescent Probe NBD-PC on the Structure, Dynamics and Phase Transition of DPPC. A Molecular Dynamics and Differential Scanning Calorimetry Study. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.; Gensch, T.; Hviid, L.; van der Horst, M.A.; Hellingwerf, K.J.; van Thor, J.J. Transient Exposure of Hydrophobic Surface in the Photoactive Yellow Protein Monitored with Nile Red. Biophys. J. 2002, 82, 1632–1643. [Google Scholar] [CrossRef]
- Gooneh-Farahani, S.; Naghib, S.M.; Naimi-Jamal, M.R. A Novel and Inexpensive Method Based on Modified Ionic Gelation for PH-Responsive Controlled Drug Release of Homogeneously Distributed Chitosan Nanoparticles with a High Encapsulation Efficiency. Fibers Polym. 2020, 21, 1917–1926. [Google Scholar] [CrossRef]
- Acosta-Zaldívar, M.; Andrés, M.T.; Rego, A.; Pereira, C.S.; Fierro, J.F.; Côrte-Real, M. Human Lactoferrin Triggers a Mitochondrial- and Caspase-Dependent Regulated Cell Death in Saccharomyces cerevisiae. Apoptosis 2016, 21, 163–173. [Google Scholar] [CrossRef]
- Raths, S.; Rohrer, J.; Crausaz, F.; Riezman, H. End3 and End4: Two Mutants Defective in Receptor-Mediated and Fluid-Phase Endocytosis in Saccharomyces cerevisiae. J. Cell Biol. 1993, 120, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.; Santos-Pereira, C.; Soares, I.; Martins, V.; Terra-Matos, J.; Côrte-Real, M.; Lúcio, M.; Oliveira, M.E.C.D.R.; Gerós, H. Resveratrol-Loaded Lipid Nanocarriers Are Internalized by Endocytosis in Yeast. J. Nat. Prod. 2019, 82, 1240–1249. [Google Scholar] [CrossRef]
- Cabrera, L.I.; Somoza, Á.; Marco, J.F.; Serna, C.J.; Morales, M.P. Synthesis and Surface Modification of Uniform MFe2O4 (M = Fe, Mn, and Co) Nanoparticles with Tunable Sizes and Functionalities. J. Nanoparticle Res. 2012, 14, 873. [Google Scholar] [CrossRef]
- Rafique, M.Y.; Pan, L.Q.; Javed, Q.U.A.; Iqbal, M.Z.; Qiu, H.M.; Farooq, M.H.; Guo, Z.G.; Tanveer, M. Growth of Monodisperse Nanospheres of MnFe2O4 with Enhanced Magnetic and Optical Properties. Chin. Phys. B 2013, 22, 107101. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Ishida, T.; Yanaga, Y.; Yamada, S.; Takahashi, Y. A Versatile Method for Surface Functionalization and Hydrophobization of Gold Nanoparticles. Appl. Surf. Sci. 2021, 546, 148932. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.; Friedel, P.; Kleeberg, R. IUCr Commission on Powder Diffraction. Newsletter 1998, 20, 5–8. [Google Scholar]
- Castilho, M.L.; Vieira, L.S.; Campos, A.P.C.; Achete, C.A.; Cardoso, M.A.G.; Raniero, L. The Efficiency Analysis of Gold Nanoprobes by FT-IR Spectroscopy Applied to the Non-Cross-Linking Colorimetric Detection of Paracoccidioides Brasiliensis. Sens. Actuators B Chem. 2015, 215, 258–265. [Google Scholar] [CrossRef]
- Nguyen, K.C. Quantitative Analysis of COOH-Terminated Alkanethiol SAMs on Gold Nanoparticle Surfaces. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 045008. [Google Scholar] [CrossRef]
- Iacovita, C.; Florea, A.; Dudric, R.; Pall, E.; Moldovan, A.; Tetean, R.; Stiufiuc, R.; Lucaciu, C. Small versus Large Iron Oxide Magnetic Nanoparticles: Hyperthermia and Cell Uptake Properties. Molecules 2016, 21, 1357. [Google Scholar] [CrossRef]
- Pradhan, P.; Giri, J.; Samanta, G.; Sarma, H.D.; Mishra, K.P.; Bellare, J.; Banerjee, R.; Bahadur, D. Comparative Evaluation of Heating Ability and Biocompatibility of Different Ferrite-Based Magnetic Fluids for Hyperthermia Application. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81B, 12–22. [Google Scholar] [CrossRef]
- Stettner, J.; Frank, P.; Griesser, T.; Trimmel, G.; Schennach, R.; Gilli, E.; Winkler, A. A Study on the Formation and Thermal Stability of 11-MUA SAMs on Au(111)/Mica and on Polycrystalline Gold Foils. Langmuir 2009, 25, 1427–1433. [Google Scholar] [CrossRef]
- Fernandes, N.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Overview of the Application of Inorganic Nanomaterials in Cancer Photothermal Therapy. Biomater. Sci. 2020, 8, 2990–3020. [Google Scholar] [CrossRef]
- Dembskey, N.; Abrahamse, H. The Efficacy of Phototherapy for the Treatment of Onychomycosis: An Observational Study. Photonics 2021, 8, 350. [Google Scholar] [CrossRef]
- Pacheco, A.R.F.; Cardoso, B.D.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Carvalho, V.M.; Rodrigues, R.O.; Coutinho, P.J.G.; Castelo-Grande, T.; Augusto, P.A.; et al. Development of PH-Sensitive Magnetoliposomes Containing Shape Anisotropic Nanoparticles for Potential Application in Combined Cancer Therapy. Nanomaterials 2023, 13, 1051. [Google Scholar] [CrossRef]
- Lentz, B.R. Membrane “Fluidity” as Detected by Diphenylhexatriene Probes. Chem. Phys. Lipids 1989, 50, 171–190. [Google Scholar] [CrossRef]
- Lentz, B.R. Use of Fluorescent Probes to Monitor Molecular Order and Motions within Liposome Bilayers. Chem. Phys. Lipids 1993, 64, 99–116. [Google Scholar] [CrossRef]
- Biltonen, R.L.; Lichtenberg, D. The Use of Differential Scanning Calorimetry as a Tool to Characterize Liposome Preparations. Chem. Phys. Lipids 1993, 64, 129–142. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.R.O.; Gomes, I.T.; Almeida, B.G.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Magnetic Liposomes Based on Nickel Ferrite Nanoparticles for Biomedical Applications. Phys. Chem. Chem. Phys. 2015, 17, 18011–18021. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Shin, S.H.R.; Abezgauz, L.L.; Lewis, S.A.; Chirsan, A.M.; Danino, D.D.; Bishop, K.J.M. Integration of Gold Nanoparticles into Bilayer Structures via Adaptive Surface Chemistry. J. Am. Chem. Soc. 2013, 135, 5950–5953. [Google Scholar] [CrossRef]
- Chen, H.; Tang, L.; Qin, Y.; Yin, Y.; Tang, J.; Tang, W.; Sun, X.; Zhang, Z.; Liu, J.; He, Q. Lactoferrin-Modified Procationic Liposomes as a Novel Drug Carrier for Brain Delivery. Eur. J. Pharm. Sci. 2010, 40, 94–102. [Google Scholar] [CrossRef]
- Colletier, J.P.; Chaize, B.; Winterhalter, M.; Fournier, D. Protein Encapsulation in Liposomes: Efficiency Depends on Interactions between Protein and Phospholipid Bilayer. BMC Biotechnol. 2002, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the Use of the Weibull Function for the Discernment of Drug Release Mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Noyes, A.A.; Whitney, W.R. The Rate of Solution of Solid Substances in Their Own Solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key Principles and Methods for Studying the Endocytosis of Biological and Nanoparticle Therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, B.; Verma, N.; Prina-Mello, A.; Williams, Y.; Davies, A.M.; Bakos, G.; Tormey, L.; Edwards, C.; Hanrahan, J.; Salvati, A.; et al. Activation of Stress-Related Signalling Pathway in Human Cells upon SiO2 Nanoparticles Exposure as an Early Indicator of Cytotoxicity. J. Nanobiotechnol. 2011, 9, 29. [Google Scholar] [CrossRef]
- Fernando, L.P.; Kandel, P.K.; Yu, J.; McNeill, J.; Ackroyd, P.C.; Christensen, K.A. Mechanism of Cellular Uptake of Highly Fluorescent Conjugated Polymer Nanoparticles. Biomacromolecules 2010, 11, 2675–2682. [Google Scholar] [CrossRef]
- Habib, F.S.; Fouad, E.A.; Abdel-Rhaman, M.S.; Fathalla, D. Liposomes as an Ocular Delivery System of Fluconazole: In-Vitro Studies. Acta Ophthalmol. 2010, 88, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, O.; Ulgen, K.O.; Ercan, N.I. The Toxicity of Polystyrene-Based Nanoparticles in Saccharomyces cerevisiae Is Associated with Nanoparticle Charge and Uptake Mechanism. Chem. Res. Toxicol. 2021, 34, 1055–1068. [Google Scholar] [CrossRef]
- Harisa, G.I.; Badran, M.M.; Alanazi, F.K.; Attia, S.M. An Overview of Nanosomes Delivery Mechanisms: Trafficking, Orders, Barriers and Cellular Effects. Artif. Cells Nanomed. Biotechnol. 2018, 46, 669–679. [Google Scholar] [CrossRef]







| Hc (Oe) | Ms (emu/g) | Mr (emu/g) | Mr/Ms | |
|---|---|---|---|---|
| MnFe2O4 NPs | 5.45 | 65.32 | 0.52 | 0.008 |
| NPs | Hydrodynamic Diameter ± SD (nm) | PDI ± SD | Zeta Potential ± SD (mV) |
|---|---|---|---|
| AuNPs | 84 ± 4 | 0.27 ± 0.01 | −39.9 ± 1.9 |
| AuNPs_MUA | 101 ± 39 | 0.27 ± 0.06 | −35.0 ± 1.3 |
| AuNPs_ODT | 450 ± 36 | 0.02 ± 0.01 | −6.6 ± 5.7 |
| Nanoparticles | Mechanism | ΔT (°C) | ΔT/Δt (°C/min) | SAR (W/g) |
|---|---|---|---|---|
| MnFe2O4 | Magnetic hyperthermia | 17.5 | 0.05 | 4.2 |
| MnFe2O4 | Photothermia | 3.99 | 0.52 | 2177 |
| AuNPs_MUA | Photothermia | 4.58 | 0.80 | 3349 |
| PMLs | Photothermia | 5.52 | 0.52 | 1088 |
| Lipid | System | Hydrodynamic Diameter ± SD (nm) | PDI ± SD | Zeta Potential ± SD (mV) | |||
|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 0 h | 24 h | 0 h | 24 h | ||
| DPPC | Liposomes | 186 ± 14 | 165 ± 25 | 0.28 ± 0.01 | 0.30 ± 0.05 | −13.2 ± 0.2 | −13.6 ± 0.8 |
| PMLs with AuNPs_MUA | 195 ± 7 | 203 ± 10 | 0.27 ± 0.04 | 0.29 ± 0.08 | 31.1 ± 0.8 | 30.8 ± 0.4 | |
| PMLs with AuNPs_ODT | 261 ± 10 | 302 ± 55 | 0.279 ± 0.007 | 0.248 ± 0.03 | 28.1 ± 0.8 | 31.2 ± 0.3 | |
| Egg-PC | Liposomes | 132 ± 11 | 140 ± 21 | 0.24 ± 0.03 | 0.27 ± 0.01 | −27 ± 2 | −28 ± 1 |
| PMLs with AuNPs_MUA | 207 ± 18 | 215 ± 10 | 0.27 ± 0.04 | 0.26 ± 0.02 | −12.6 ± 0.5 | −8.7 ± 0.6 | |
| PMLs with AuNPs_ODT | 337 ± 32 | 334 ± 7 | 0.28 ± 0.04 | 0.26 ± 0.02 | −16.5 ± 0.4 | −14.9 ± 0.2 | |
| Assay | EE (%) | Mean ± SD (%) |
|---|---|---|
| I | 94.7 | |
| II | 95.4 | 95.6 ± 1.0 |
| III | 96.6 |
| Weibull | First-Order | ||||
|---|---|---|---|---|---|
| a | b | R2 | k (min−1) | R2 | |
| Tris-HCl buffer | 0.396 | 0.695 | 0.999 | 0.505 | 0.987 |
| YEPD medium | 0.511 | 0.757 | 0.999 | 0.417 | 0.984 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, M.; Rodrigues, A.R.O.; Amaral, L.; Côrte-Real, M.; Santos-Pereira, C.; Castanheira, E.M.S. Bovine Lactoferrin-Loaded Plasmonic Magnetoliposomes for Antifungal Therapeutic Applications. Pharmaceutics 2023, 15, 2162. https://doi.org/10.3390/pharmaceutics15082162
Pereira M, Rodrigues ARO, Amaral L, Côrte-Real M, Santos-Pereira C, Castanheira EMS. Bovine Lactoferrin-Loaded Plasmonic Magnetoliposomes for Antifungal Therapeutic Applications. Pharmaceutics. 2023; 15(8):2162. https://doi.org/10.3390/pharmaceutics15082162
Chicago/Turabian StylePereira, Mélanie, Ana Rita O. Rodrigues, Leslie Amaral, Manuela Côrte-Real, Cátia Santos-Pereira, and Elisabete M. S. Castanheira. 2023. "Bovine Lactoferrin-Loaded Plasmonic Magnetoliposomes for Antifungal Therapeutic Applications" Pharmaceutics 15, no. 8: 2162. https://doi.org/10.3390/pharmaceutics15082162
APA StylePereira, M., Rodrigues, A. R. O., Amaral, L., Côrte-Real, M., Santos-Pereira, C., & Castanheira, E. M. S. (2023). Bovine Lactoferrin-Loaded Plasmonic Magnetoliposomes for Antifungal Therapeutic Applications. Pharmaceutics, 15(8), 2162. https://doi.org/10.3390/pharmaceutics15082162







