Assessing α-Bisabolol as a Transmucosal Permeation Enhancer of Buccal Local Anesthetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Drugs
2.2. Manufacture and Quality Assessment of Buccal Anesthetic Films
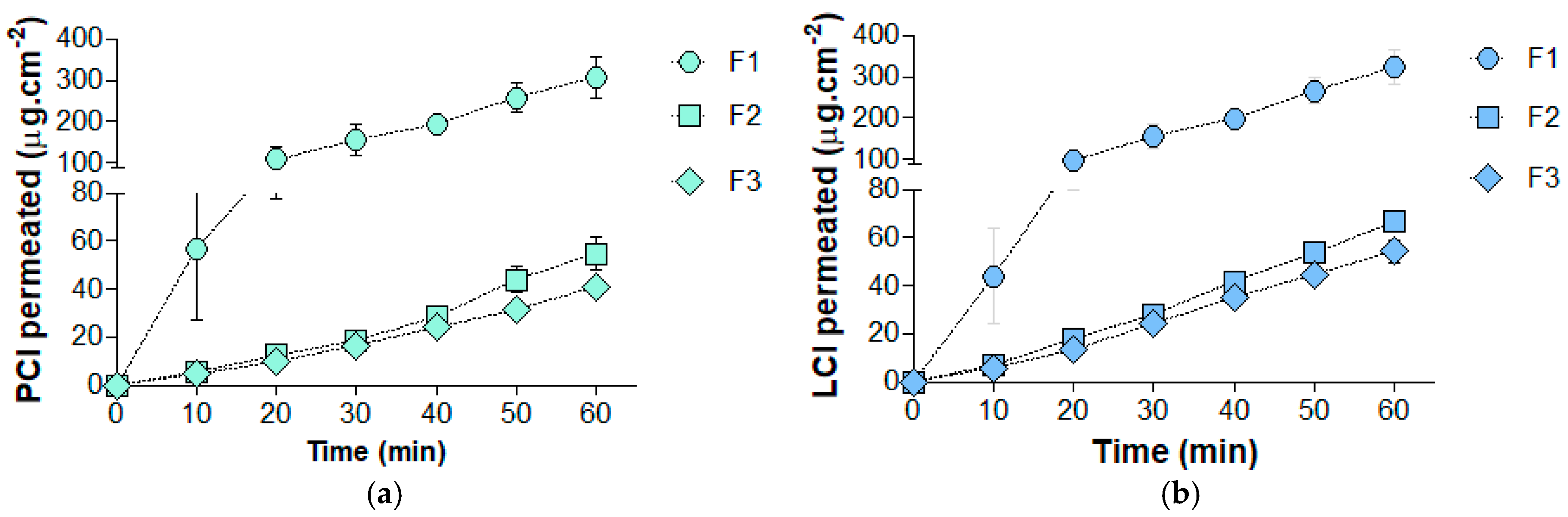
2.3. In Vitro Transmucosal Anesthetic Permeation
2.4. Assessing the Mechanical Properties
2.5. Data Analyses
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kudo, M. Initial Injection Pressure for Dental Local Anesthesia: Effects on Pain and Anxiety. Anesth. Prog. 2005, 52, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ogle, O.E.; Mahjoubi, G. Advances in Local Anesthesia in Dentistry. Dent. Clin. N. Am. 2011, 55, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.M.; Yagiela, J.A. Advanced Techniques and Armamentarium for Dental Local Anesthesia. Dent. Clin. N. Am. 2010, 54, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Gupta, S.K.; Newaskar, V.; Chandra, A. Advances in Dental Local Anesthesia Techniques and Devices: An Update. Natl. J. Maxillofac. Surg. 2013, 4, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Nuvvula, S.; Saikiran, K.V.; Elicherla, S.R.; Sahithi, V.; Nunna, M.; Challa, R.R. Local Anesthesia in Pediatric Dentistry: A Literature Review on Current Alternative Techniques and Approaches. J. South Asian Assoc. Pediatr. Dent. 2021, 4, 148–154. [Google Scholar] [CrossRef]
- Franz-Montan, M.; de Morais Ribeiro, L.N.; Volpato, M.C.; Cereda, C.M.S.; Groppo, F.C.; Tofoli, G.R.; de Araújo, D.R.; Santi, P.; Padula, C.; de Paula, E.; et al. Recent Advances and Perspectives in Topical Oral Anesthesia. Expert Opin. Drug Deliv. 2017, 14, 673–684. [Google Scholar] [CrossRef]
- McLenon, J.; Rogers, M.A.M.M. The Fear of Needles: A Systematic Review and Meta-Analysis. J. Adv. Nurs. 2019, 75, 30–42. [Google Scholar] [CrossRef]
- Adami, L.E.; de Freitas, O.; de Figueiredp, F.A.T.; Ferreira, M.P.; Macedo, A.P.; do Couto, R.O.; Pedrazzi, V. Needle-Free Anesthesia: Clinical Efficacy of a Mucoadhesive Patch for Atraumatic Anesthesia in Dental Procedures. Braz. Oral Res. 2021, 35, 1–14. [Google Scholar] [CrossRef]
- do Couto, R.O.; Cubayachi, C.; Calefi, P.L.; Lopez, R.F.V.; Pedrazzi, V.; De Gaitani, C.M.; de Freitas, O. Combining Amino Amide Salts in Mucoadhesive Films Enhances Needle-Free Buccal Anesthesia in Adults. J. Control. Release 2017, 266, 205–215. [Google Scholar] [CrossRef]
- Iwamatsu-Kobayashi, Y.; Watanabe, J.; Kusama, T.; Endo, H.; Ikeda, S.; Tokuda, K.; Igarashi, K.; Egusa, H. A 19-Year Study of Dental Needlestick and Sharps Injuries in Japan. Int. Dent. J. 2023, 73, 114–120. [Google Scholar] [CrossRef]
- Vaia, E.S.; Metz-Favre, C. Hypersensitivity to Excipients: Myth or Reality? Rev. Fr. Allergol. 2022, 62, 570–571. [Google Scholar] [CrossRef]
- Caballero, M.L.; Quirce, S. Delayed Hypersensitivity Reactions Caused by Drug Excipients: A Literature Review. J. Investig. Allergol. Clin. Immunol. 2020, 30, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Caballero, M.L.; Quirce, S. Immediate Hypersensitivity Reactions Caused by Drug Excipients: A Literature Review. J. Investig. Allergol. Clin. Immunol. 2020, 30, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Shipp, L.; Liu, F.; Kerai-Varsani, L.; Okwuosa, T.C. Buccal Films: A Review of Therapeutic Opportunities, Formulations & Relevant Evaluation Approaches. J. Control. Release 2022, 352, 1071–1092. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, A.C.W.; Paiva, N.F.; Demonari, I.K.; Duarte, M.P.F.; do Couto, R.O.; de Freitas, O.; Vicentini, F.T.M.d.C. The Potential of Films as Transmucosal Drug Delivery Systems. Pharmaceutics 2023, 15, 2583. [Google Scholar] [CrossRef]
- Mantelle, J.A.; Lucking, D.; Kandos, D.P.; Fernandez, G. Oral Transmucosal Systems for Local Anesthetics: Dental and Oral Surgical Premedication. In Drug Delivery to the Oral Cavity: Molecules to Market; Taylor & Francis: London, UK, 2005; pp. 233–259. [Google Scholar]
- Bågesund, M.; Tabrizi, P. Lidocaine 20% Patch vs Lidocaine 5% Gel for Topical Anaesthesia of Oral Mucosa. Int. J. Paediatr. Dent. 2008, 18, 452–460. [Google Scholar] [CrossRef]
- Hersh, E.; Houpt, M.; Cooper, S.; Feldman, R.; Wolff, M.; Levin, L. Analgesic Efficacy and Safety of an Intraoral Lidocaine Patch. J. Am. Dent. Assoc. 1996, 127, 1626–1634. [Google Scholar] [CrossRef]
- Stecker, S.; Swift, J. Should a Mucoadhesive Patch (DentiPatch) Be Used for Gingival Anesthesia in Children? Anesth. Prog. 2002, 49, 3–8. [Google Scholar]
- Leopold, A.; Wilson, S.; Weaver, J.; Moursi, A. Pharmacokinetics of Lidocaine Delivered from a Transmucosal Patch in Children. Anesth. Prog. 2002, 49, 82–87. [Google Scholar]
- Kreider, K.; Stratmann, R.; Milano, M.; Agostini, F.; Munsell, M. Reducing Children’s Injection Pain: Lidocaine Patches versus Topical Benzocaine Gel. Pediatr. Dent. 2001, 23, 19–23. [Google Scholar]
- Carr, M.; Horton, J. Evaluation of a Transoral Delivery System for Topical Anesthesia. J. Am. Dent. Assoc. 2001, 132, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Vickers, E.R.; Marzbani, N.; Gerzina, T.M.; McLean, C.; Punnia-Moorthy, A.; Mather, L. Pharmacokinetics of EMLA Cream 5% Application to Oral Mucosa. Anesth. Prog. 1997, 44, 32–37. [Google Scholar] [PubMed]
- Al-Melh, M.; Andersson, L. Comparison of Topical Anesthetics (EMLA/Oraqix vs. Benzocaine) on Pain Experienced during Palatal Needle Injection. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Lagan, G.; McLure, H. Review of Local Anaesthetic Agents. Curr. Anaesth. Crit. Care 2005, 71, 59–74. [Google Scholar] [CrossRef]
- Malamed, S.F. Anesthetic Considerations in Dental Specialties. In Handbook of Local Anesthesia, 6th ed.; Elsevier: St. Louis, MO, USA, 2013; pp. 277–291. [Google Scholar]
- Fiala, S.; Jones, S.A.; Brown, M.B. A Fundamental Investigation into the Effects of Eutectic Formation on Transmembrane Transport. Int. J. Pharm. 2010, 393, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J. Farmacologia Dos Anestésicos Locais. Rev. Bras. Anestesiol. 1994, 44, 75–82. [Google Scholar]
- Malamed, S.F. Handbook of Local Anesthesia, 5th ed.; Mosby: Maryland Heights, MO, USA, 2005; Volume 52, ISBN 978-0-323-02449-5. [Google Scholar]
- Wei, R.; Simon, L.; Hu, L.; Michniak-Kohn, B. Effects of Iontophoresis and Chemical Enhancers on the Transport of Lidocaine and Nicotine across the Oral Mucosa. Pharm. Res. 2012, 29, 961–971. [Google Scholar] [CrossRef]
- Hu, L.; Silva, S.M.C.; Damaj, B.B.; Martin, R.; Michniak-kohn, B.B. Transdermal and Transbuccal Drug Delivery Systems: Enhancement Using Iontophoretic and Chemical Approaches. Int. J. Pharm. 2011, 421, 53–62. [Google Scholar] [CrossRef]
- Lee, H.; Min, H.S.; Jang, M.; Kang, G.; Gong, S.; Lee, C.; Song, Y.W.; Jung, U.-W.; Lee, S.; Ryu, H.Y.; et al. Lidocaine-Loaded Dissolving Microneedle for Safe Local Anesthesia on Oral Mucosa for Dental Procedure. Expert Opin. Drug Deliv. 2023, 20, 851–861. [Google Scholar] [CrossRef]
- Zhu, T.; Yu, X.; Yi, X.; Guo, X.; Li, L.; Hao, Y.; Wang, W. Lidocaine-Loaded Hyaluronic Acid Adhesive Microneedle Patch for Oral Mucosal Topical Anesthesia. Pharmaceutics 2022, 14, 686. [Google Scholar] [CrossRef]
- Li, Q.; Yu, X.; Zheng, X.; Yang, J.; Hui, J.; Fan, D. Rapid Dissolution Microneedle Based on Polyvinyl Alcohol/Chitosan for Local Oral Anesthesia. Int. J. Biol. Macromol. 2024, 257, 128629. [Google Scholar] [CrossRef] [PubMed]
- Daly, S.; Claydon, N.C.A.; Newcombe, R.G.; Seong, J.; Addy, M.; West, N.X. Randomised Controlled Trial of a Microneedle Patch with a Topical Anaesthetic for Relieving the Pain of Dental Injections. J. Dent. 2021, 107, 103617. [Google Scholar] [CrossRef] [PubMed]
- Babakurd, F.M.; Azzawi, S.K.; Alkhouli, M.; Al-Nerabieah, Z. Evaluation of EMLA Cream with Microneedle Patches in Palatal Anesthesia in Children: A Randomized Controlled Clinical Trial. Sci. Rep. 2024, 14, 15295. [Google Scholar] [CrossRef]
- Cubayachi, C.; do Couto, R.O.; de Gaitani, C.M.; Pedrazzi, V.; de Freitas, O.; Lopez, R.F.V. Needle-Free Buccal Anesthesia Using Iontophoresis and Amino Amide Salts Combined in a Mucoadhesive Formulation. Colloids Surf. B Biointerfaces 2015, 136, 1193–1201. [Google Scholar] [CrossRef]
- Couto, R.O.; Cubayachi, C.; Lopez, R.F.V.V.; de Gaitani, C.M.; Pedrazzi, V.; de Freitas, O.; De Gaitani, C.M.; Pedrazzi, V.; De Freitas, O. A Simple and High-Resolution HPLC-PDA Method for Simultaneous Quantification of Local Anesthetics in in Vitro Buccal Permeation Enhancement Studies. Biomed. Chromatogr. 2016, 30, 857–866. [Google Scholar] [CrossRef]
- Seeni, R.Z.; Zheng, M.; Lio, D.C.S.; Wiraja, C.; Mohd Yusoff, M.F.B.; Koh, W.T.Y.; Liu, Y.; Goh, B.T.; Xu, C. Targeted Delivery of Anesthetic Agents to Bone Tissues Using Conductive Microneedles Enhanced Iontophoresis for Painless Dental Anesthesia. Adv. Funct. Mater. 2021, 31, 2105686. [Google Scholar] [CrossRef]
- Cordeiro Lima Fernandes, P.; David de Moura, L.; Freitas de Lima, F.; Henrique Rodrigues da Silva, G.; Isaias Carvalho Souza, R.; de Paula, E. Lipid Nanocapsules Loaded with Prilocaine and Lidocaine and Incorporated in Gel for Topical Application. Int. J. Pharm. 2021, 602, 120675. [Google Scholar] [CrossRef]
- da Silva, C.B.; dos Santos, C.P.; Serpe, L.; Sanchez, J.B.; Ferreira, L.E.N.; de Melo, N.F.S.; Groppo, F.C.; Fraceto, L.F.; Volpato, M.C.; Franz-Montan, M. Polymeric Nanocapsules Loaded with Lidocaine: A Promising Formulation for Topical Dental Anesthesia. Pharmaceuticals 2024, 17, 485. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Baroni, D.; Brunetto, G.; Sobral, V.R.V.; Da Silva, C.M.G.; Venâncio, P.; Zago, P.W.; Cereda, C.M.S.; Volpato, M.C.; De Araújo, D.R.; et al. Liposomal Lidocaine Gel for Topical Use at the Oral Mucosa: Characterization, in Vitro Assays and in Vivo Anesthetic Efficacy in Humans. J. Liposome Res. 2015, 25, 11–19. [Google Scholar] [CrossRef]
- Muniz, B.V.; Baratelli, D.; Di Carla, S.; Serpe, L.; da Silva, C.B.; Guilherme, V.A.; Ribeiro, L.N.d.M.; Cereda, C.M.S.; de Paula, E.; Volpato, M.C.; et al. Hybrid Hydrogel Composed of Polymeric Nanocapsules Co-Loading Lidocaine and Prilocaine for Topical Intraoral Anesthesia. Sci. Rep. 2018, 8, 17972. [Google Scholar] [CrossRef]
- Ribeiro, L.N.M.; Franz-Montan, M.; Breitkreitz, M.C.; Alcântara, A.C.S.; Castro, S.R.; Guilherme, V.A.; Barbosa, R.M.; de Paula, E. Nanostructured Lipid Carriers as Robust Systems for Topical Lidocaine-Prilocaine Release in Dentistry. Eur. J. Pharm. Sci. 2016, 93, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.N.M.; Franz-Montan, M.; Alcântara, A.C.S.; Breitkreitz, M.C.; Castro, S.R.; Guilherme, V.A.; Muniz, B.V.; Rodrigues da Silva, G.H.; de Paula, E. Hybrid Nanofilms as Topical Anesthetics for Pain-Free Procedures in Dentistry. Sci. Rep. 2020, 10, 11341. [Google Scholar] [CrossRef] [PubMed]
- Khongkhunthian, S.; Sastraruji, T.; Klayraung, S.; Okonogi, S. Efficacy of Anesthetic Rice Nanogel on Pain Reduction in Human Oral Cavity. Drug Discov. Ther. 2018, 12, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Abu-Huwaij, R.; Assaf, S.; Salem, M.; Sallam, A. Potential Mucoadhesive Dosage Form of Lidocaine Hydrochloride: II. In Vitro and in Vivo Evaluation. Drug Dev. 2007, 33, 437–448. [Google Scholar] [CrossRef]
- Cavallari, C.; Fini, A.; Ospitali, F. Mucoadhesive Multiparticulate Patch for the Intrabuccal Controlled Delivery of Lidocaine. Eur. J. Pharm. Biopharm. 2013, 83, 405–414. [Google Scholar] [CrossRef]
- Kottke, D.; Majid, H.; Breitkreutz, J.; Burckhardt, B.B. Development and Evaluation of Mucoadhesive Buccal Dosage Forms of Lidocaine Hydrochloride by Ex-Vivo Permeation Studies. Int. J. Pharm. 2020, 581, 119293. [Google Scholar] [CrossRef]
- Clitherow, K.H.; Murdoch, C.; Spain, S.G.; Handler, A.M.; Colley, H.E.; Stie, M.B.; Mørck Nielsen, H.; Janfelt, C.; Hatton, P.V.; Jacobsen, J. Mucoadhesive Electrospun Patch Delivery of Lidocaine to the Oral Mucosa and Investigation of Spatial Distribution in a Tissue Using MALDI-Mass Spectrometry Imaging. Mol. Pharm. 2019, 16, 3948–3956. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Monou, P.K.; Bouropoulos, N.; Boetker, J.; Rantanen, J.; Jacobsen, J.; Vizirianakis, I.S.; Fatouros, D.G. Fabrication of Mucoadhesive Buccal Films for Local Administration of Ketoprofen and Lidocaine Hydrochloride by Combining Fused Deposition Modeling and Inkjet Printing. J. Pharm. Sci. 2020, 109, 2757–2766. [Google Scholar] [CrossRef]
- Padula, C.; Pozzetti, L.; Traversone, V.; Nicoli, S.; Santi, P. In Vitro Evaluation of Mucoadhesive Films for Gingival Administration of Lidocaine. AAPS PharmSciTech 2013, 14, 1279–1283. [Google Scholar] [CrossRef]
- Bebawy, G.; Sokar, M.S.; Abdallah, O.Y. Buccal Lidocaine Mucoadhesive Patches for Pediatrics’ Teething Pain: Overcoming Possible Hazards of Oral Gels. Pharm. Dev. Technol. 2024, 1–9. [Google Scholar] [CrossRef]
- Favacho, H.A.S.; do Couto, R.O.; Duarte, M.P.F.; Peixoto, M.P.G.; Lopez, R.F.V.; Pedrazzi, V.; de Gaitani, C.M.; de Freitas, O. Synergy between Surfactants and Mucoadhesive Polymers Enhances the Transbuccal Permeation of Local Anesthetics from Freeze-Dried Tablets. Mater. Sci. Eng. C 2020, 108, 110373. [Google Scholar] [CrossRef] [PubMed]
- do Couto, R.O.; Cubayachi, C.; Duarte, M.P.F.; Lopez, R.F.V.; Pedrazzi, V.; De Gaitani, C.M.; de Freitas, O. Towards the Advance of a Novel Iontophoretic Patch for Needle-Free Buccal Anesthesia. Mater. Sci. Eng. C 2021, 122, 111778. [Google Scholar] [CrossRef] [PubMed]
- Amra, K.; Momin, M.; Desai, N.; Khan, F. Therapeutic Benefits of Natural Oils along with Permeation Enhancing Activity. Int. J. Dermatol. 2022, 61, 484–507. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, L.; Zhang, L.; Li, J.; Gu, J.; Gong, H.; Guo, P.; Tong, W. Enhancement and Mechanism of Transdermal Absorption of Terpene-Induced Propranolol Hydrochloride. Arch. Pharm. Res. 2011, 34, 1477–1485. [Google Scholar] [CrossRef]
- Quintanilha, N.P.; Costa, I.D.S.M.; de Souza Ramos, M.F.; de Oliveira Miguel, N.C.; Pierre, M.B.R. α-Bisabolol Improves 5-Aminolevulinic Acid Retention in Buccal Tissues: Potential Application in the Photodynamic Therapy of Oral Cancer. J. Photochem. Photobiol. B Biol. 2017, 174, 298–305. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Chemical Permeation Enhancement. In Enhancement in Drug Delivery; Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 233–254. ISBN 9781420004816. [Google Scholar]
- Ramazani, E.; Akaberi, M.; Emami, S.A.; Tayarani-Najaran, Z. Pharmacological and Biological Effects of Alpha-Bisabolol: An Updated Review of the Molecular Mechanisms. Life Sci. 2022, 304, 120728. [Google Scholar] [CrossRef]
- Ruan, J.; Liu, C.; Wang, J.; Zhong, T.; Quan, P.; Fang, L. Efficacy and Safety of Permeation Enhancers: A Kinetic Evaluation Approach and Molecular Mechanism Study in the Skin. Int. J. Pharm. 2022, 626, 122155. [Google Scholar] [CrossRef]
- Ganem-quintanar, A.; Kalia, Y.N.; Falson-rieg, F.; Buri, P.; Bernard, C.; Lyon, I.; Rockefeller, A. Mechanisms of Oral Permeation Enhancement. Int. J. Pharm. 1997, 156, 127–142. [Google Scholar] [CrossRef]
- Naveen, C.; Kiran, Y.; Rao, P.V.; Rao, T.R. Chemical Enhancers in Buccal and Sublingual Delivery. Int. J. Pharm. Sci. Nanotechnol. 2011, 4, 1307–1319. [Google Scholar]
- Del Consuelo, I.D.; Jacques, Y.; Pizzolato, G.-P.; Guy, R.H.; Falson, F.; Diaz-Del Consuelo, I.; Jacques, Y.; Pizzolato, G.-P.; Guy, R.H.; Falson, F. Comparison of the Lipid Composition of Porcine Buccal and Esophageal Permeability Barriers. Arch. Oral Biol. 2005, 50, 981–987. [Google Scholar] [CrossRef]
- Del Consuelo, I.D.; Pizzolato, G.-P.; Falson, F.; Guy, R.H.; Jacques, Y. Evaluation of Pig Esophageal Mucosa as a Permeability Barrier Model for Buccal Tissue. J. Pharm. Sci. 2005, 94, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Mashru, R.C.; Sutariya, V.B.; Sankalia, M.G.; Parikh, P.P. Development and Evaluation of Fast-Dissolving Film of Salbutamol Sulphate. Drug Dev. Ind. Pharm. 2005, 31, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing Margareth. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary PH: A Diagnostic Biomarker. J. Indian Soc. Periodontol. 2013, 17, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Franz-Montan, M.; Serpe, L.; Martinelli, C.C.M.; da Silva, C.B.; dos Santos, C.P.; Novaes, P.D.; Volpato, M.C.; de Paula, E.; Lopez, R.F.V.; Groppo, F.C. Evaluation of Different Pig Oral Mucosa Sites as Permeability Barrier Models for Drug Permeation Studies. Eur. J. Pharm. Sci. 2016, 81, 52–59. [Google Scholar] [CrossRef]
- De Araújo, J.S.M.; Volpato, M.C.; Muniz, B.V.; Xavier, G.G.A.; Martinelli, C.C.M.; Lopez, R.F.V.; Groppo, F.C.; Franz-Montan, M. Resistivity Technique for the Evaluation of the Integrity of Buccal and Esophageal Epithelium Mucosa for in Vitro Permeation Studies: Swine Buccal and Esophageal Mucosa Barrier Models. Pharmaceutics 2021, 13, 643. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2002; Volume 14. [Google Scholar]
- Ferreira, L.F.M.; Thomaz, D.V.; Duarte, M.P.F.; Lopez, R.F.V.; Pedrazzi, V.; de Freitas, O.; Do Couto, R.O. Quality by Design-Driven Investigation of the Mechanical Properties of Mucoadhesive Films for Needleless Anesthetics Administration. Revista de Ciências Farmacêutica Básica e Aplicadas 2021, 42, 1–12. [Google Scholar] [CrossRef]
- Padula, C.; Nicoli, S.; Colombo, P.; Santi, P. Single-Layer Transdermal Film Containing Lidocaine: Modulation of Drug Release. Eur. J. Pharm. Biopharm. 2007, 66, 422–428. [Google Scholar] [CrossRef]
- Mahrous, G.M.; Shazly, G.; Zidan, D.E.; Zaher, A.A.A.; El-Mahdy, M. Formulation and Evaluation of Buccoadhesive Films of Lidocaine Hydrochloride. J. Adv. Biomed. Pharm. Sci. 2020, 3, 53–59. [Google Scholar] [CrossRef]
- Kohda, Y.; Kobayashi, H.; Baba, Y.; Yuasa, H.; Ozeki, T.; Kanaya, Y.; Sagara, E. Controlled Release of Lidocaine Hydrochloride from Buccal Mucosa-Adhesive Films with Solid Dispersion. Int. J. Pharm. 1997, 158, 147–155. [Google Scholar] [CrossRef]
- Abu-Huwaij, R.; Assaf, S.; Salem, M.; Sallam, A. Mucoadhesive Dosage Form of Lidocaine Hydrochloride: I. Mucoadhesive and Physicochemical Characterization. Drug Dev. Ind. Pharm. 2007, 33, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.; Gutta, K.; Prodduturi, S.; Munjal, M.; Stodghill, S. Characterization of Cellulosic Hot-Melt Extruded Films Containing Lidocaine. Eur. J. Pharm. Biopharm. 2005, 59, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Nakamori, T.; Arakawa, Y.; Iida, K.; Danjo, K. Development of Polymer Film Dosage Forms of Lidocaine for Buccal Administration: II. Comparison of Preparation Methods. J. Pharm. Sci. 2002, 91, 2424–2432. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Taguchi, H.; Iida, K.; Danjo, K. Development of Polymer Film Dosage Forms of Lidocaine for Buccal Administration I. Penetration Rate and Release Rate. J. Control. Release 2001, 77, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al-Dhubiab, B.E.; Alhaider, I.A. In Vitro Techniques to Evaluate Buccal Films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef]
- Dixit, R.P.; Puthli, S.P. Oral Strip Technology: Overview and Future Potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef]
- Preis, M.; Woertz, C.; Schneider, K.; Kukawka, J.; Broscheit, J.; Roewer, N.; Breitkreutz, J. Design and Evaluation of Bilayered Buccal Film Preparations for Local Administration of Lidocaine Hydrochloride. Eur. J. Pharm. Biopharm. 2014, 86, 552–561. [Google Scholar] [CrossRef]
- Sohi, H.; Ahuja, A.; Ahmad, F.J.; Khar, R.K. Critical Evaluation of Permeation Enhancers for Oral Mucosal Drug Delivery. Drug Dev. Ind. Pharm. 2010, 36, 254–282. [Google Scholar] [CrossRef]
- Kokate, A.; Li, X.; Williams, P.J.; Singh, P.; Jasti, B.R. In Silico Prediction of Drug Permeability across Buccal Mucosa. Pharm. Res. 2009, 26, 1130–1139. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.A.; Ojha, S. Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol. Nutrients 2022, 14, 1370. [Google Scholar] [CrossRef]
- Morales, J.O.; Mcconville, J.T. Manufacture and Characterization of Mucoadhesive Buccal Films. Eur. J. Pharm. Biopharm. 2011, 77, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Alaei, S.; Omidian, H. Mucoadhesion and Mechanical Assessment of Oral Films. Eur. J. Pharm. Sci. 2021, 159, 105727. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A.; O’Donnell, P.B.; McGinity, J.W. Mechanical Properties of Polymeric Films Prepared from Aqueous Dispersions. In Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms; McGinity, J.W., Felton, L.A., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2008; pp. 105–128. ISBN 9780849387883. [Google Scholar]


| Component (%, m·m−1) | Films | ||
|---|---|---|---|
| F1 | F2 | F3 | |
| PCl | 3.0 | 3.0 | 3.0 |
| LCl | 3.0 | 3.0 | 3.0 |
| HPMC K100 LV | 3.0 | 3.0 | 3.0 |
| PEG 400 * | 0.9 | 0.9 | 0.9 |
| α-Bisabolol ** | - | 0.9 | 1.8 |
| PBS pH 7.0 | qsp 100 | qsp 100 | qsp 100 |
| Films | |||||
|---|---|---|---|---|---|
| F1 | F2 | F3 | Literature Specification | ||
| Quality attributes | Mass (mg) | 18.9 ± 2.6 | 17.7 ± 0.4 | 18.9 ± 0.5 | Changeable [9,45,48,55,73,74,75,76,77,78,79] |
| Thickness (µm) | 292.5 ± 61.0 | 344.8 ± 34.0 | 397.6 ± 49.0 | 50 to 1000 µm $ [80] | |
| PCl content (mg·g−1) | 291.7 ± 21.0 | 344.6 ± 22.0 | 381.6 ± 6.3 | 85–115% $ [81] | |
| LCl content (mg·g−1) | 291.2 ± 15.1 | 347.4 ± 24.0 | 379.5 ± 5.0 | 85–115% $ [81] | |
| Mechanical properties | EB (%) | 123.0 ± 4.0 | 31.6 ± 3.6 C | 33.5 ± 5.2 C | 0.9–137.1 [72,82] |
| TS (MPa) | 10.0 ± 1.0 | 3.5 ± 0.3 C | 3.5 ± 0.8 C | 0.6–70.4 [72,82] | |
| FB (N) | 18.0 ± 4.0 | 7.6 ± 0.7 C | 8.2 ± 2.3 C | 2.3–34.8 [72,82] | |
| YM (MPa) | 0.08 ± 0.005 | 0.11 ± 0.004 A | 0.11 ± 0.02 A | −14.4–9.7 [72] | |
| Films | |||
|---|---|---|---|
| Parameter | F1 | F2 | F3 |
| JSS PCL (µg·cm−2·min−1) | 5.0 ± 0.3 | 1.0 ± 0.2 C | 0.7 ± 0.1 C |
| JSS LCL (µg·cm−2·min−1) | 5.6 ± 0.4 | 1.2 ± 0.1 C | 1.0 ± 0.1 C |
| Lt PCl (min) | 1.7 ± 0.3 | 7.2 ± 3.4 A | 5.8 ± 0.2 A |
| Lt LCl (min) | 4.0 ± 1.0 | 4.5 ± 1.1 | 5.1 ± 0.1 |
| Kp × 10−4 PCL (cm·min−1) | 8.9 ± 0.8 | 1.4 ± 0.3 C | 0.9 ± 0.1 C |
| Kp × 10−4 LCL (cm·min−1) | 9.9 ± 0.7 | 1.7 ± 0.3 C | 1.2 ± 0.2 C |
| Qepit PCL (µg·cm−2) | 1277.8 ± 53.4 | 567.9 ± 55.9 C | 522.8 ± 69.4 C |
| Qepit LCL (µg·cm−2) | 1254.4 ± 34.1 | 696.8 ± 83.3 C | 625.1 ± 97.9 C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Couto, R.O.; Thomaz, D.V.; Duarte, M.P.F.; Lopez, R.F.V.; Pedrazzi, V.; de Freitas, O.; Tartaglia, G.M. Assessing α-Bisabolol as a Transmucosal Permeation Enhancer of Buccal Local Anesthetics. Pharmaceutics 2024, 16, 1198. https://doi.org/10.3390/pharmaceutics16091198
do Couto RO, Thomaz DV, Duarte MPF, Lopez RFV, Pedrazzi V, de Freitas O, Tartaglia GM. Assessing α-Bisabolol as a Transmucosal Permeation Enhancer of Buccal Local Anesthetics. Pharmaceutics. 2024; 16(9):1198. https://doi.org/10.3390/pharmaceutics16091198
Chicago/Turabian Styledo Couto, Renê Oliveira, Douglas Vieira Thomaz, Maira Perez Ferreira Duarte, Renata Fonseca Vianna Lopez, Vinícius Pedrazzi, Osvaldo de Freitas, and Gianluca Martino Tartaglia. 2024. "Assessing α-Bisabolol as a Transmucosal Permeation Enhancer of Buccal Local Anesthetics" Pharmaceutics 16, no. 9: 1198. https://doi.org/10.3390/pharmaceutics16091198










