Synthesis and Evaluation of 99mTc(CO)3 Complexes with Ciprofloxacin Dithiocarbamate for Infection Imaging
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis of Rhenium Complexes
2.2.1. Synthesis of Complex fac-[Re(CO)3(Cip-DTC)(H2O)] (1)
2.2.2. Synthesis of fac-[Re(CO)3(Cip-DTC)(PPh3)] (2)
2.2.3. One-Pot Synthesis of Rhenium Complexes 3–6 Using Re(CO)5Br
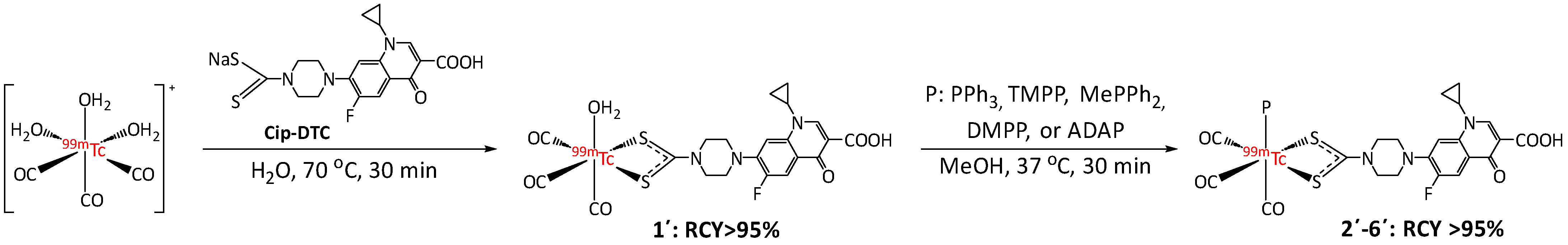
2.3. Preparation of Technetium-99m Complexes
2.3.1. Synthesis of Intermediate Complex fac-[99mTc(CO)3(Cip-DTC)(H2O)] (1′)
2.3.2. Synthesis of fac-[99mTc(CO)3(Cip-DTC)(P)] Complexes (2′–6′)
2.4. Stability Studies for 99mTc-Complexes
2.4.1. Stability in Reaction Mixture
2.4.2. Stability of Purified Complexes
2.4.3. Cysteine and Histidine Challenge Studies
2.5. Lipophilicity Studies for 99mTc-Complexes
2.6. Biodistribution Studies for 99mTc-Complexes
3. Results and Discussion
3.1. Preparation of Rhenium Complexes
3.2. Preparation of 99mTc-Complexes
3.3. Stability Studies
3.4. Lipophilicity Studies
3.5. Biodistribution Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahts, M.; Summers, B.; Gutta, A.; Pilloy, W.; Ebenhan, T. Recently developed radiopharmaceuticals for bacterial infection imaging. EJNMMI Radiopharm. Chem. 2024, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, A.A.; Sellmyer, M.A.; Gowrishankar, G.; Ruiz-Bedoya, C.A.; Tucker, E.W.; Palestro, C.J.; Hammoud, D.A.; Jain, S.K. Molecular imaging of bacterial infections: Overcoming the barriers to clinical translation. Sci. Transl. Med. 2019, 11, eaax8251. [Google Scholar] [CrossRef] [PubMed]
- Rathmann, S.M.; Ahmad, Z.; Slikboer, S.; Bilton, H.A.; Snider, D.P.; Valliant, J.F. The Radiopharmaceutical Chemistry of Technetium-99m. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 311–333. [Google Scholar]
- Kooshki, N.; Grambow-Velilla, J.; Mahida, B.; Benali, K.; Nguyen, C.; Cimadevilla, C.; Braham, W.; Pisani, A.; Iung, B.; Raffoul, R.; et al. Diagnostic performance of White Blood Cell SPECT imaging against intra-operative findings in patients with a suspicion of prosthetic valve endocarditis. J. Nucl. Cardiol. 2022, 29, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Blanc, P.; Bonnet, E.; Giordano, G.; Monteil, J.; Salabert, A.S.; Payoux, P. The use of labelled leucocyte scintigraphy to evaluate chronic periprosthetic joint infections: A retrospective multicentre study on 168 patients. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1625–1631. [Google Scholar] [CrossRef]
- Gotthardt, M.; Bleeker-Rovers, C.P.; Boerman, O.C.; Oyen, W.J.G. Imaging of Inflammation by PET, Conventional Scintigraphy, and Other Imaging Techniques. J. Nucl. Med. 2010, 51, 1937. [Google Scholar] [CrossRef]
- Calabria, F.F.; Guadagnino, G.; Cimini, A.; Leporace, M. PET/CT Imaging of Infectious Diseases: Overview of Novel Radiopharmaceuticals. Diagnostics 2024, 14, 1043. [Google Scholar] [CrossRef]
- Naqvi, S.A.R.; Drlica, K. Fluoroquinolones as imaging agents for bacterial infection. Dalton Trans. 2017, 46, 14452–14460. [Google Scholar] [CrossRef]
- Naqvi, S.A.R.; Roohi, S.; Iqbal, A.; Sherazi, T.A.; Zahoor, A.F.; Imran, M. Ciprofloxacin: From infection therapy to molecular imaging. Mol. Biol. Rep. 2018, 45, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Tzovas, G.; Papadimitriou, N.; Angelakarou, E.; Bompola, G.; Miliotou, A.N.; Kakafika, M.G.; Papadopoulou, L.C.; Iakovou, Ι.; Gabriel, C.; Sarigiannis, D.; et al. Synthesis and biological evaluation of 99mTc-tricarbonyl complexes of C-3 carboxy derivatives of fluoroquinolones in infection and tumor animal models. Inorg. Chim. Acta 2024, 562, 121894. [Google Scholar] [CrossRef]
- Kyprianidou, P.; Tsoukalas, C.; Chiotellis, A.; Papagiannopoulou, D.; Raptopoulou, C.P.; Terzis, A.; Pelecanou, M.; Papadopoulos, M.; Pirmettis, I. First example of well-characterized Re and 99mTc tricarbonyl complexes of ciprofloxacin and norfloxacin in the development of infection-specific imaging agents. Inorg. Chim. Acta 2011, 370, 236–242. [Google Scholar] [CrossRef]
- Shah, S.Q.; Khan, M.R.; Ali, S.M. Radiosynthesis of 99mTc(CO)3-Clinafloxacin Dithiocarbamate and Its Biological Evaluation as a Potential Staphylococcus aureus Infection Radiotracer. Nucl. Med. Mol. Imaging 2011, 45, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Welling, M.M.; Ferro-Flores, G.; Pirmettis, I.; Brouwer, P.J.M.C. Current Status of Imaging Infections with Radiolabeled Anti-Infective Agents. Anti-Infect. Agents Med. Chem. 2009, 8, 272–287. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb Perspect Med. 2016, 6, a025320. [Google Scholar] [CrossRef]
- Sonmezoglu, K.; Sonmezoglu, M.; Halac, M.; Akgün, I.; Türkmen, C.; Onsel, C.; Kanmaz, B.; Solanki, K.; Britton, K.E.; Uslu, I. Usefulness of 99mTc-ciprofloxacin (infecton) scan in diagnosis of chronic orthopedic infections: Comparative study with 99mTc-HMPAO leukocyte scintigraphy. J. Nucl. Med. 2001, 42, 567–574. [Google Scholar]
- Britton, K.E.; Vinjamuri, S.; Hall, A.V.; Solanki, K.; Siraj, Q.H.; Bomanji, J.; Das, S. Clinical evaluation of technetium-99m infecton for the localisation of bacterial infection. Eur. J. Nucl. Med. 1997, 24, 553–556. [Google Scholar] [CrossRef]
- Britton, K.E.; Wareham, D.W.; Das, S.S.; Solanki, K.K.; Amaral, H.; Bhatnagar, A.; Katamihardja, A.H.; Malamitsi, J.; Moustafa, H.M.; Soroa, V.E.; et al. Imaging bacterial infection with 99mTc-ciprofloxacin (Infecton). J. Clin. Pathol. 2002, 55, 817–823. [Google Scholar] [CrossRef]
- Britton, K.E.; Wareham, D.W.; Das, S.S. Concerns about 99mTc-labelled ciprofloxacin for infection detection. Eur. J. Nucl. Med. 2001, 28, 779–781. [Google Scholar] [CrossRef]
- Kniess, T.; Laube, M.; Wüst, F.; Pietzsch, J. Technetium-99m based small molecule radiopharmaceuticals and radiotracers targeting inflammation and infection. Dalton Trans. 2017, 46, 14435–14451. [Google Scholar] [CrossRef]
- Oh, S.J.; Ryu, J.S.; Shin, J.W.; Yoon, E.J.; Ha, H.J.; Cheon, J.H.; Lee, H.K. Synthesis of 99mTc-ciprofloxacin by different methods and its biodistribution. Appl. Radiat. Isot. 2002, 57, 193–200. [Google Scholar] [CrossRef]
- Bai, J.-W.; Qiu, S.-Q.; Zhang, G.-J. Molecular and functional imaging in cancer-targeted therapy: Current applications and future directions. Signal Transduct. Ther. 2023, 8, 89. [Google Scholar] [CrossRef]
- Fang, S.; Jiang, Y.; Gan, Q.; Ruan, Q.; Xiao, D.; Zhang, J. Design, Preparation, and Evaluation of a Novel 99mTcN Complex of Ciprofloxacin Xanthate as a Potential Bacterial Infection Imaging Agent. Molecules 2020, 25, 5837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, S.; Guo, H.; Wang, X. Synthesis and biological evaluation of a novel 99mTc(CO)3 complex of ciprofloxacin dithiocarbamate as a potential agent to target infection. Bioorg. Med. Chem. Lett. 2010, 20, 3781–3784. [Google Scholar] [CrossRef] [PubMed]
- Roupa, I.; Flampouraris, C.; Shegani, A.; Ischyropoulou, M.; Makrypidi, K.; Raptopoulou, C.; Pirmettis, I.; Papadopoulos, M.S.; Psycharis, V.; Chiotellis, A. Synthesis and Characterization of Novel [2 + 1] Tricarbonyl Rhenium Complexes with the Hydrophilic Phosphine Ligands PTA and CAP. Bioinorg. Chem. Appl. 2022, 2022, 3117661. [Google Scholar] [CrossRef] [PubMed]
- Shegani, A.; Ischyropoulou, M.; Roupa, I.; Kiritsis, C.; Makrypidi, K.; Papasavva, A.; Raptopoulou, C.; Psycharis, V.; Hennkens, H.M.; Pelecanou, M.; et al. Synthesis and evaluation of new mixed “2 + 1” Re, 99mTc and 186Re tricarbonyl dithiocarbamate complexes with different monodentate ligands. Bioorg. Med. Chem. Lett. 2021, 47, 116373. [Google Scholar] [CrossRef]
- Shaikh, T.M.; Weng, C.-M.; Hong, F.-E. Secondary phosphine oxides: Versatile ligands in transition metal-catalyzed cross-coupling reactions. Coord. Chem. Rev. 2012, 256, 771–803. [Google Scholar] [CrossRef]
- Papagiannopoulou, D.; Triantis, C.; Vassileiadis, V.; Raptopoulou, C.P.; Psycharis, V.; Terzis, A.; Pirmettis, I.; Papadopoulos, M.S. Synthesis, structural characterization and radiochemistry of di- and tricarbonyl Re(I) and 99mTc(I) complexes with 8-hydroxyquinoline or 8-mercaptoquinoline and triphenylphosphine. Polyhedron 2014, 68, 46–52. [Google Scholar] [CrossRef]
- Riondato, M.; Camporese, D.; Martín, D.; Suades, J.; Alvarez-Larena, A.; Mazzi, U. Synthesis and Characterisation of [Re(CO)3(SS)(P)] Complexes: A [2+1] Concept for 99mTc- and 188Re-Radiopharmaceutical Applications. Eur. J. Inorg. Chem. 2005, 2005, 4048–4055. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, H.; Zhang, S.; Lin, Y.; Wang, X. Synthesis and biodistribution of a novel 99mTcN complex of ciprofloxacin dithiocarbamate as a potential agent for infection imaging. Bioorg. Med. Chem. Lett. 2008, 18, 5168–5170. [Google Scholar] [CrossRef]
- Schmidt, S.P.; Trogler, W.C.; Basolo, F.; Urbancic, M.A.; Shapley, J.R. Pentacarbonylrhenium Halides. In Inorganic Syntheses; Angelici, R.J., Ed.; Wiley & Sons: Hoboken, NJ, USA, 1990; pp. 160–165. [Google Scholar]
- Alberto, R.; Egli, A.; Abram, U.; Hegetschweiler, K.; Gramlich, V.; Schubiger, P.A. Synthesis and reactivity of [NEt4]2[ReBr3(CO)3]. Formation and structural characterization of the clusters [NEt4][Re3(µ3-OH)(µ-OH)3(CO)9] and [NEt4][Re2(µ-OH)3(CO)6] by alkaline titration. J. Chem. Soc. Dalton Trans. 1994, 19, 2815–2820. [Google Scholar] [CrossRef]
- Alberto, R.; Schibli, R.; Schubiger, A.P.; Abram, U.; Pietzsch, H.J.; Johannsen, B. First Application of fac-[99mTc(OH2)3(CO)3]+ in Bioorganometallic Chemistry: Design, Structure, and In Vitro Affinity of a 5-HT1A Receptor Ligand Labeled with 99mTc. J. Am. Chem. Soc. 1999, 121, 6076–6077. [Google Scholar] [CrossRef]
- Makrypidi, K.; Kiritsis, C.; Roupa, I.; Triantopoulou, S.; Shegani, A.; Paravatou-Petsotas, M.; Chiotellis, A.; Pelecanou, M.; Papadopoulos, M.; Pirmettis, I. Evaluation of Rhenium and Technetium-99m Complexes Bearing Quinazoline Derivatives as Potential EGFR Agents. Molecules 2023, 28, 1786. [Google Scholar] [CrossRef] [PubMed]
- Kyprianidou, P.; Tsoukalas, C.; Patsis, G.; Papagiannopoulou, D.; Nikolić, N.; Janković, D.; Djokić, D.; Raptopoulou, C.P.; Pelecanou, M.; Papadopoulos, M.; et al. Rhenium(I) and technetium-99m(I) fac-tricarbonyl complexes with 4-(imidazolin-2-yl)-3-thiabutanoic acid derivatives as tridentate ligands: Synthesis and structural characterization. Polyhedron 2009, 28, 3171–3176. [Google Scholar] [CrossRef]
- Shegani, A.; Triantis, C.; Nock, B.A.; Maina, T.; Kiritsis, C.; Psycharis, V.; Raptopoulou, C.; Pirmettis, I.; Tisato, F.; Papadopoulos, M.S. Rhenium(I) Tricarbonyl Complexes with (2-Hydroxyphenyl)diphenylphosphine as PO Bidentate Ligand. Inorg. Chem. 2017, 56, 8175–8186. [Google Scholar] [CrossRef] [PubMed]


| Position | Cip | Cip-DTC | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|
| H-2/H-5 | 2.90 | 4.57 | 4.11 | 3.60 | 3.67 3.58 | 3.76 3.47 | 3.85 | 4.15 3.99 |
| H-3/H4 | 3.24 | 3.17 | 3.44 | 3.27 2.89 | 3.34 2.80 | 3.39 3.09 | 3.43 3.19 | 3.45 3.32 |
| H-7 | 7.54 | 7.42 | 7.58 | 7.39 | 7.37 | 7.48 | 7.48 | 7.44 |
| H-10 | 7.90 | 7.80 | 7.92 | 7.81 | 7.81 | 7.88 | 7.87 | 7.82 |
| H-12 | 8.66 | 8.54 | 8.63 | 8.54 | 8.53 | 8.60 | 8.59 | 8.55 |
| H-16 | 3.83 | 3.61 | 3.75 | 3.65 | 3.57 | 3.72 | 3.70 | 3.57 |
| H-17/H-18 | 1.31 1.18 | 1.28 1.00 | 1.33 1.15 | 1.30 1.04 | 1.30 1.02 | 1.32 1.11 | 1.32 1.08 | 1.27 1.00 |
| P–Ph3 | 7.51 7.44 | 7.32 7.28 7.05 6.84 | 7.56 7.48 | 7.56 7.47 7.40 | ||||
| P–CH3 | 2.28 | 1.93 | ||||||
| O–CH3 | 3.71 | |||||||
| NCH2N | 4.47 | |||||||
| PCH2N | 4.20 |
| Compound | M: Re | M: 99mTc | |
|---|---|---|---|
| (tR min) | (tR min) | LogD7.4 | |
| fac-[M(CO)3(Cip-DTC)(H2O)] (1, 1′) | 16.8 | 17.1 | 1.2 |
| fac-[M(CO)3(Cip-DTC)(PPh3)] (2, 2′) | 26.1 | 26.4 | 2.3 |
| fac-[M(CO)3(Cip-DTC)(TMPP)] (3, 3′) | 27.1 | 27.5 | 2.7 |
| fac-[M(CO)3(Cip-DTC)(MePPh2)] (4, 4′) | 23.2 | 23.6 | 1.8 |
| fac-[M(CO)3(Cip-DTC)(DMPP)] (5, 5′) | 22.3 | 22.7 | 2.0 |
| fac-[M(CO)3(Cip-DTC)(ADAP)] (6, 6′) | 17.1 | 17.3 | 1.6 |
| Tissue | 1′ | 2′ | 3′ | |||
|---|---|---|---|---|---|---|
| 15 min | 120 min | 15 min | 120 min | 15 min | 120 min | |
| Blood | 14.07 ± 0.83 | 7.76 ± 0.29 | 4.51 ± 1.32 | 5.19 ± 0.81 | 2.54 ± 0.25 | 6.71 ± 0.50 |
| Liver | 22.05 ± 1.40 | 25.96 ± 2.23 | 32.36 ± 3.58 | 27.16 ± 4.02 | 47.48 ± 1.30 | 27.58 ± 0.66 |
| Heart | 1.93 ± 0.23 | 1.58 ± 0.21 | 1.23 ± 0.36 | 3.97 ± 0.25 | 0.74 ± 0.32 | 3.48 ± 0.23 |
| Kidneys | 5.16 ± 0.32 | 5.76 ± 0.65 | 1.30 ± 0.13 | 6.50 ± 0.36 | 1.40 ± 0.57 | 5.65 ± 0.78 |
| Stomach | 0.91 ± 0.20 | 1.42 ± 0.30 | 0.55 ± 0.23 | 1.23 ± 0.14 | 0.64 ± 0.61 | 1.21 ± 0.32 |
| Intestines | 0.71 ± 0.11 | 1.81 ± 0.15 | 0.14 ± 0.03 | 1.08 ± 0.07 | 0.36 ± 0.25 | 3.52 ± 0.19 |
| Spleen | 9.79 ± 1.69 | 5.24 ± 0.45 | 38.44 ± 3.92 | 37.02 ± 4.43 | 33.88 ± 2.81 | 23.10 ± 1.74 |
| Muscle | 0.18 ± 0.02 | 0.18 ± 0.01 | 0.90 ± 0.09 | 0.68 ± 0.05 | 0.13 ± 0.05 | 0.34 ± 0.02 |
| Lungs | 6.41 ± 1.14 | 4.33 ± 0.41 | 6.66 ± 1.45 | 10.20 ± 0.12 | 9.58 ± 0.86 | 8.67 ± 0.72 |
| Pancreas | 0.91 ± 0.04 | 0.82 ± 0.06 | 0.47 ± 0.20 | 1.04 ± 0.04 | 0.29 ± 0.13 | 0.93 ± 0.20 |
| Infection | 1.06 ± 0.20 | 1.80 ± 0.39 | 2.93 ± 0.09 | 3.12 ± 0.14 | 0.27 ± 0.11 | 1.14 ± 0.05 |
| Aseptic Infl. | 0.78 ± 0.51 | 1.33 ± 0.09 | 1.21 ± 0.07 | 0.86 ± 0.10 | 0.15 ± 0.07 | 0.52 ± 0.23 |
| Urine * | 0.15 ± 0.08 | 1.57 ± 0.98 | 3.95 ± 1.30 | 4.04 ± 1.23 | 0.00 ± 0.00 | 0.01 ± 0.02 |
| Tissue | 4′ | 5′ | 6′ | |||
|---|---|---|---|---|---|---|
| 15 min | 120 min | 15 min | 120 min | 15 min | 120 min | |
| Blood | 3.20 ± 0.47 | 1.48 ± 0.11 | 0.91 ± 0.03 | 0.66 ± 0.05 | 2.18 ± 0.06 | 1.33 ± 0.10 |
| Liver | 42.11 ± 1.70 | 31.09 ± 0.72 | 35.51 ± 0.97 | 31.27 ± 5.71 | 20.95 ± 1.56 | 22.98 ± 2.70 |
| Heart | 4.36 ± 0.46 | 8.85 ± 0.63 | 2.98 ± 0.46 | 4.30 ± 1.47 | 4.27 ± 0.42 | 1.94 ± 0.31 |
| Kidneys | 5.92 ± 0.60 | 11.10 ± 0.70 | 3.20 ± 0.35 | 5.69 ± 1.44 | 8.05 ± 0.22 | 4.47 ± 0.52 |
| Stomach | 1.11 ± 0.50 | 2.23 ± 0.37 | 0.53 ± 0.22 | 2.33 ± 0.44 | 0.98 ± 0.29 | 3.14 ± 0.69 |
| Intestines | 1.27 ± 0.16 | 7.97 ± 0.12 | 0.61 ± 0.17 | 9.98 ± 1.22 | 3.22 ± 0.32 | 10.24 ± 1.11 |
| Spleen | 21.59 ± 0.98 | 8.14 ± 0.93 | 18.04 ± 0.73 | 7.64 ± 1.22 | 6.40 ± 0.66 | 2.55 ± 0.27 |
| Muscle | 0.56 ± 0.07 | 1.48 ± 0.05 | 0.40 ± 0.06 | 1.34 ± 0.09 | 1.01 ± 0.12 | 0.69 ± 0.05 |
| Lungs | 11.15 ± 0.19 | 9.41 ± 1.15 | 9.87 ± 0.89 | 5.37 ± 0.47 | 7.13 ± 0.35 | 3.06 ± 0.10 |
| Pancreas | 1.38 ± 0.21 | 3.21 ± 0.28 | 0.83 ± 0.18 | 3.68 ± 0.48 | 3.70 ± 0.26 | 2.27 ± 0.37 |
| Infection | 0.88 ± 0.15 | 2.83 ± 0.30 | 0.94 ± 0.31 | 3.10 ± 0.46 | 0.94 ± 0.09 | 0.69 ± 0.09 |
| Aseptic Infl. | 0.77 ± 0.12 | 1.88 ± 0.26 | 0.51 ± 0.14 | 2.37 ± 0.34 | 1.10 ± 0.06 | 0.66 ± 0.10 |
| Urine * | 0.01 ± 0.00 | 0.04 ± 0.03 | 0.03 ± 0.01 | 0.32 ± 0.12 | 0.37 ± 0.08 | 1.49 ± 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papasavva, A.; Pirmettis, N.N.; Shegani, A.; Papadopoulou, E.; Kiritsis, C.; Georgoutsou-Spyridonos, M.; Mastellos, D.C.; Chiotellis, A.; Kyprianidou, P.; Pelecanou, M.; et al. Synthesis and Evaluation of 99mTc(CO)3 Complexes with Ciprofloxacin Dithiocarbamate for Infection Imaging. Pharmaceutics 2024, 16, 1210. https://doi.org/10.3390/pharmaceutics16091210
Papasavva A, Pirmettis NN, Shegani A, Papadopoulou E, Kiritsis C, Georgoutsou-Spyridonos M, Mastellos DC, Chiotellis A, Kyprianidou P, Pelecanou M, et al. Synthesis and Evaluation of 99mTc(CO)3 Complexes with Ciprofloxacin Dithiocarbamate for Infection Imaging. Pharmaceutics. 2024; 16(9):1210. https://doi.org/10.3390/pharmaceutics16091210
Chicago/Turabian StylePapasavva, Afroditi, Nektarios N. Pirmettis, Antonio Shegani, Eleni Papadopoulou, Christos Kiritsis, Maria Georgoutsou-Spyridonos, Dimitrios C. Mastellos, Aristeidis Chiotellis, Patricia Kyprianidou, Maria Pelecanou, and et al. 2024. "Synthesis and Evaluation of 99mTc(CO)3 Complexes with Ciprofloxacin Dithiocarbamate for Infection Imaging" Pharmaceutics 16, no. 9: 1210. https://doi.org/10.3390/pharmaceutics16091210








