Titanium Dioxide Nanomaterials: Progress in Synthesis and Application in Drug Delivery
Abstract
:1. Introduction
2. Synthesis of TiO2 Nanomaterials
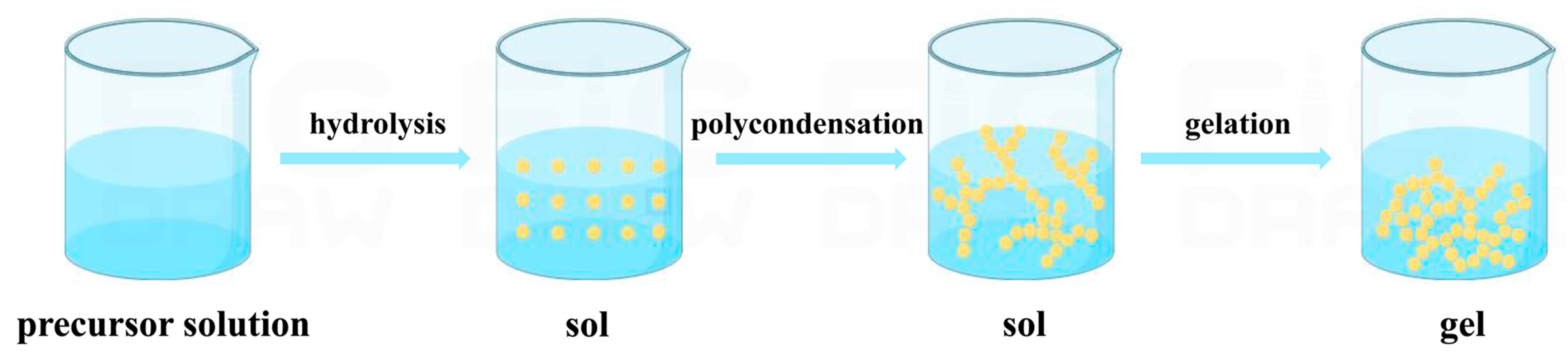
2.1. Sol-Gel Method
2.2. Hydrothermal Method
2.3. Template Method
2.3.1. Hard Template Method
2.3.2. Soft Template Method
2.4. Gas-State Method
2.5. Solid-State Method
2.6. Green Synthesis Method
2.7. Other Methods
3. Functionalization of TiO2
3.1. Physical Surface Modification
3.2. Chemical Surface Modification
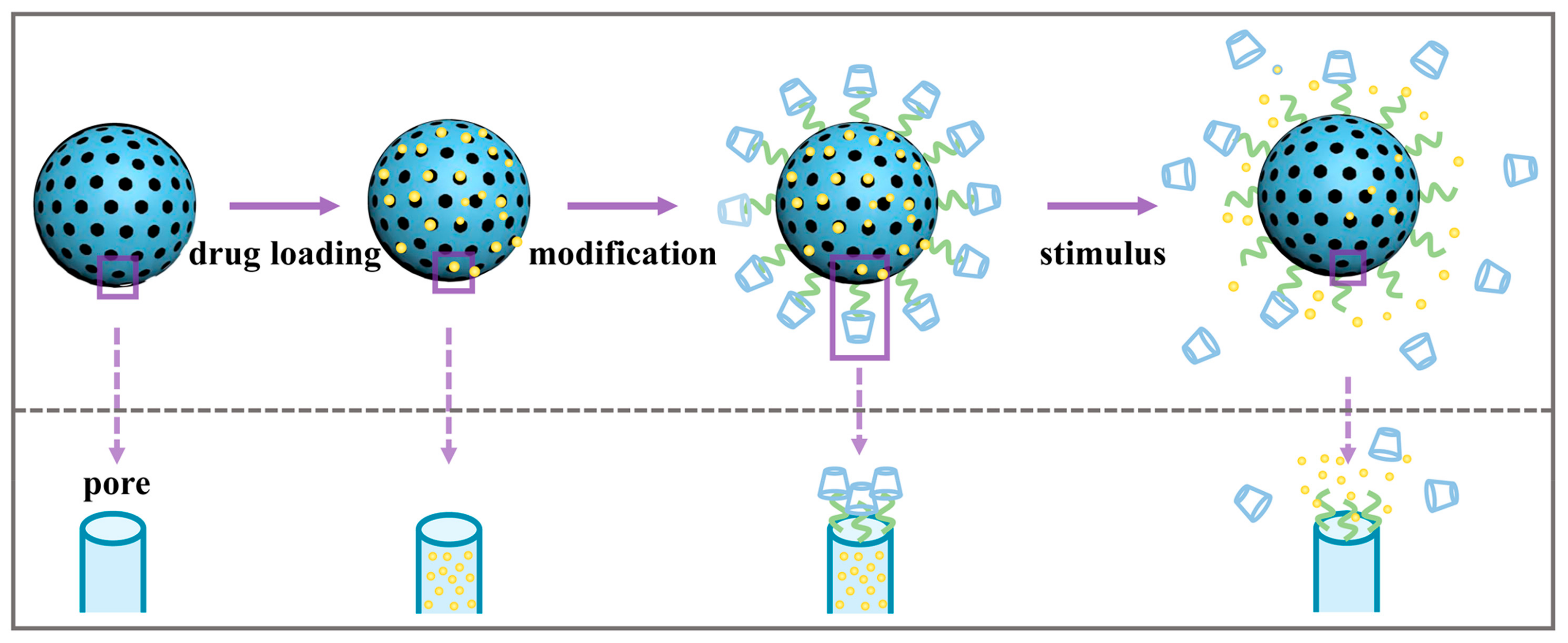
4. Sustained Drug Delivery
4.1. Pore Structure and Morphology
4.2. Interaction Force
4.3. Diffusion Inhibition Effect
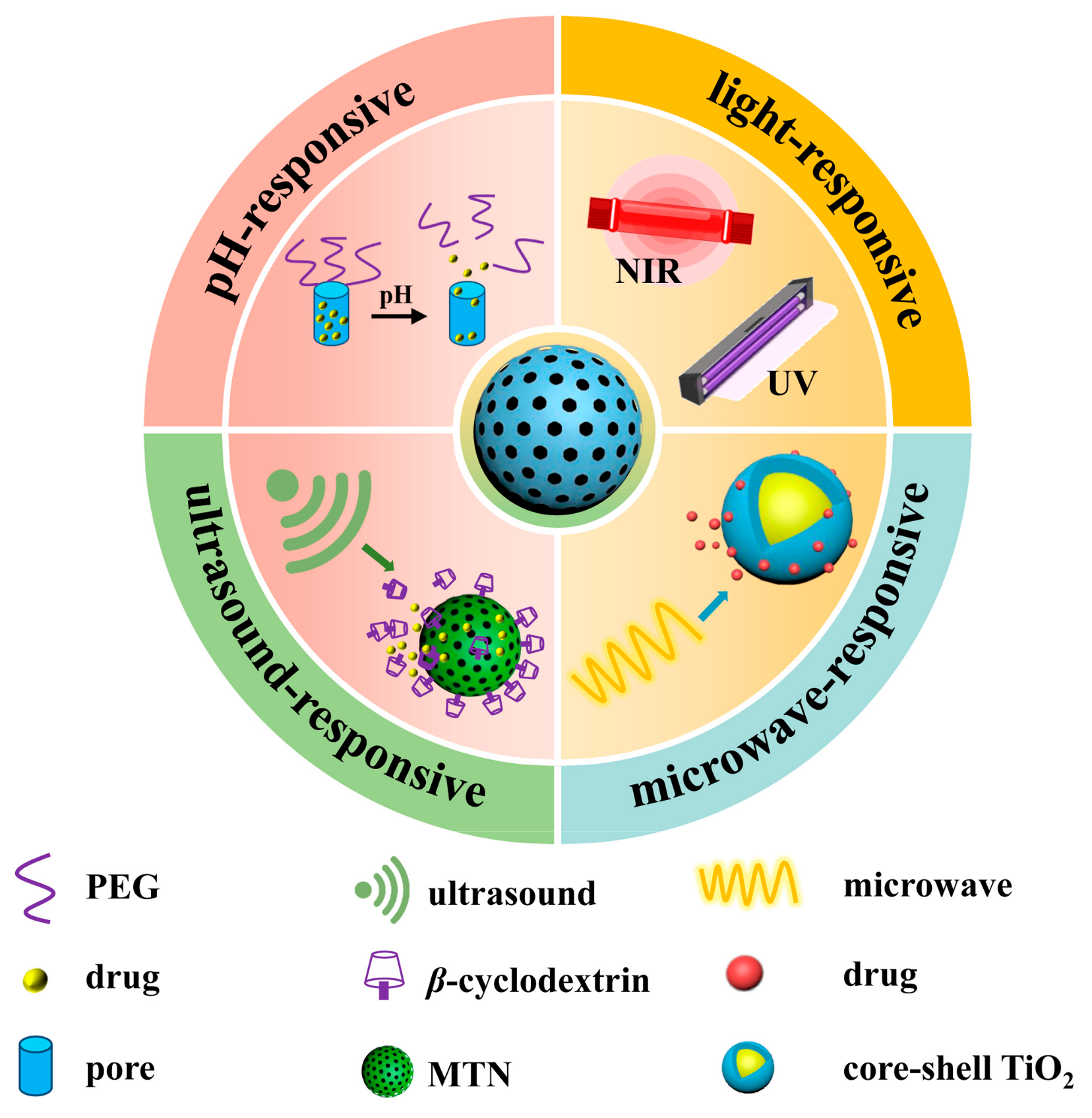
5. Controlled Drug Delivery
5.1. pH-Responsive
5.2. Light-Responsive
5.3. Microwave-Responsive
5.4. Ultrasound-Responsive
5.5. Dual- or Multiple-Stimuli-Responsive
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Bonifácio, B.V.; da Silva, P.B.; Ramos, M.A.D.; Negri, K.M.S.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Al Ragib, A.; Chakma, R.; Dewan, K.; Islam, T.; Kormoker, T.; Idris, A.M. Current advanced drug delivery systems: Challenges and potentialities. J. Drug Deliv. Sci. Technol. 2022, 76, 103727. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, K.J.; Yu, C.H.; Huang, Q.L.; Du, Y.Z. Nano-drug delivery systems in wound treatment and skin regeneration. J. Nanobiotechnol. 2019, 17, 82. [Google Scholar] [CrossRef]
- Liu, J.Y.; Li, S.Q.; Wang, J.; Li, N.N.; Zhou, J.N.; Chen, H.X. Application of Nano Drug Delivery System (NDDS) in Cancer Therapy: A Perspective. Recent Pat. Anti-Cancer Drug Discov. 2023, 18, 125–132. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.R.; Kumar, A.; Tan, A.; Jin, S.B.; Mozhi, A.; Liang, X.J. pH-Sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef]
- Parra-Nieto, J.; del Cid, M.A.G.; de Cárcer, I.A.; Baeza, A. Inorganic Porous Nanoparticles for Drug Delivery in Antitumoral Therapy. Biotechnol. J. 2021, 16, 2000150. [Google Scholar] [CrossRef]
- Luther, D.C.; Huang, R.; Jeon, T.; Zhang, X.Z.; Lee, Y.W.; Nagaraj, H.; Rotello, V.M. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv. Drug Deliv. Rev. 2020, 156, 188–213. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Hemasa, A.L.; Freag, M.S. Hybrid protein-inorganic nanoparticles: From tumor-targeted drug delivery to cancer imaging. J. Control Release 2016, 243, 303–322. [Google Scholar] [CrossRef]
- Gupta, J.; Quadros, M.; Momin, M. Mesoporous silica nanoparticles: Synthesis and multifaceted functionalization for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2023, 81, 104305. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Saha, S.; Ali, M.R.; Khaleque, M.A.; Bacchu, M.S.; Aly, M.A.S.; Khan, M.Z.H. Metal oxide nanocarrier for targeted drug delivery towards the treatment of global infectious diseases: A review. J. Drug Deliv. Sci. Technol. 2023, 86, 104728. [Google Scholar] [CrossRef]
- Haghighi, F.H.; Mercurio, M.; Cerra, S.; Salamone, T.A.; Bianymotlagh, R.; Palocci, C.; Spica, V.R.; Fratoddi, I. Surface modification of TiO2 nanoparticles with organic molecules and their biological applications. J. Mater. Chem. B 2023, 11, 2334–2366. [Google Scholar] [CrossRef]
- Malehmir, S.; Abedini, A.; Sobhani-Nasab, A.; Eshraghi, R.; Akbari, M.; Atapour, A.; Hasan-Abad, A.M. A review of biogenic routes for the manufacture of manganese oxide nanostructures and its anti-cancer, drug delivery, anti-bacterial, and bioimaging potentials. Inorg. Chem. Commun. 2023, 156, 111306. [Google Scholar] [CrossRef]
- Sattari, S.; Adeli, M.; Beyranvand, S.; Nemati, M. Functionalized Graphene Platforms for Anticancer Drug Delivery. Int. J. Nanomed. 2021, 16, 5955–5980. [Google Scholar] [CrossRef]
- An, N.; Qin, C.Y.; Li, Y.W.; Tan, T.; Yuan, Z.Y.; Zhang, H.; Wu, Y.; Yao, B.C.; Rao, Y.J. Graphene-Fiber Biochemical Sensors: Principles, Implementations, and Advances. Photonic Sens. 2021, 11, 123–139. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rajabzadeh-Khosroshahi, M.; Eshaghi, M.M.; Rahmani, E.; Motasadizadeh, H.; Arshad, R.; Rahdar, A.; Pandey, S. TiO2-based nanocomposites for cancer diagnosis and therapy: A comprehensive review. J. Drug Deliv. Sci. Technol. 2023, 82, 104370. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, X.B.; Zhang, G. TiO2-based catalysts for photothermal catalysis: Mechanisms, materials and applications. J. Clean. Prod. 2022, 381, 135156. [Google Scholar] [CrossRef]
- Wang, T.Y.; Jiang, H.T.; Wan, L.; Zhao, Q.F.; Jiang, T.Y.; Wang, B.; Wang, S.L. Potential application of functional porous TiO2 nanoparticles in light-controlled drug release and targeted drug delivery. Acta Biomater. 2015, 13, 354–363. [Google Scholar] [CrossRef]
- Torres, C.C.; Campos, C.H.; Diáz, C.; Jiménez, V.A.; Vidal, F.; Guzmán, L.; Alderete, J.B. PAMAM-grafted TiO2 nanotubes as novel versatile materials for drug delivery applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 65, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Li, L.F.; Xie, C.L.; Xiao, X.F. Polydopamine modified TiO2 nanotube arrays as a local drug delivery system for ibuprofen. J. Drug Deliv. Sci. Technol. 2020, 56, 135156. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Joo, J.B.; Lu, Z.; Dahl, M.; Gan, Y.; Yin, Y. Water-assisted crystallization of mesoporous anatase TiO2 nanospheres. Nanoscale 2016, 8, 9113–9117. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.B.; Zhang, Q.; Dahl, M.; Lee, I.; Goebl, J.; Zaera, F.; Yin, Y. Control of the nanoscale crystallinity in mesoporous TiO2 shells for enhanced photocatalytic activity. Energy Environ. Sci. 2012, 5, 6321–6327. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, T.; Wang, L.; Sun, C.; Jiang, T.; Cheng, G.; Wang, S. Development of an amorphous mesoporous TiO2 nanosphere as a novel carrier for poorly water-soluble drugs: Effect of different crystal forms of TiO2 carriers on drug loading and release behaviors. Microporous Mesoporous Mater. 2012, 153, 124–130. [Google Scholar] [CrossRef]
- Chen, S.; Shen, L.L.; Huang, D.; Du, J.; Fan, X.X.; Wei, A.L.; Jia, L.; Chen, W.Y. Facile synthesis, microstructure, formation mechanism, in vitro biocompatibility, and drug delivery property of novel dendritic TiO2 nanofibers with ultrahigh surface area. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111100. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A review on the synthesis of TiO2 nanoparticles by solution route. Cent. Eur. J. Chem. 2012, 10, 279–294. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.P.; Wu, Z.X.; Wang, J.X.; Li, B.; Feng, S.S.; Deng, Y.H.; Zhang, F.; Zhao, D.Y. A Versatile Kinetics-Controlled Coating Method To Construct Uniform Porous TiO2 Shells for Multifunctional Core-Shell Structures. J. Am. Chem. Soc. 2012, 134, 11864–11867. [Google Scholar] [CrossRef]
- Celzard, A.; Mareche, J.F. Applications of the Sol-gel Process Using Well-Tested Recipes. Am. Chem. Soc. 2002, 79, 854–859. [Google Scholar] [CrossRef]
- Li, Y.; White, T.; Lim, S.H. Structure control and its influence on photoactivity and phase transformation of TiO2 nano-particles. Rev. Adv. Mater. Sci. 2003, 5, 211–215. [Google Scholar]
- Li, B.R.; Wang, X.H.; Yan, M.Y.; Li, L.T. Preparation and characterization of nano-TiO2 powder. Mater. Chem. Phys. 2003, 78, 184–188. [Google Scholar] [CrossRef]
- Park, G.; Kim, M.; Lee, H. Steam-modified sol-gel method for TiO2-based nanosized particles. Ceram. Int. 2022, 48, 25656–25660. [Google Scholar] [CrossRef]
- Jaroenworaluck, A.; Sunsaneeyametha, W.; Kosachan, N.; Stevens, R. Characteristics of silica-coated TiO2 and its UV absorption for sunscreen cosmetic applications. Surf. Interface Anal. 2006, 38, 473–477. [Google Scholar] [CrossRef]
- Réti, B.; Major, Z.; Szarka, D.; Boldizsár, T.; Horváth, E.; Magrez, A.; Forró, L.; Dombi, A.; Hernádi, K. Influence of TiO2 phase composition on the photocatalytic activity of TiO2/MWCNT composites prepared by combined sol-gel/hydrothermal method. J. Mol. Catal. A Chem. 2016, 414, 140–147. [Google Scholar] [CrossRef]
- Calabrese, C.; Maertens, A.; Piras, A.; Aprile, C.; Liotta, L.F. Novel Sol-Gel Synthesis of TiO2 Spherical Porous Nanoparticles Assemblies with Photocatalytic Activity. Nanomaterials 2023, 13, 1928. [Google Scholar] [CrossRef]
- Wu, J.H.; Tu, J.L.; Hu, K.; Xiao, X.J.; Li, L.; Yu, S.Z.; Xie, Y.C.; Wu, H.; Yang, Y.Y. Sol-gel-derived bayberry-like SiO2@TiO2 multifunctional antireflective coatings for enhancing photovoltaic power generation. Colloid. Surf. A 2022, 654, 130173. [Google Scholar] [CrossRef]
- Usman, A.K.; Cursaru, D.L.; Branoiu, G.; Somoghi, R.; Manta, A.M.; Matei, D.; Mihai, S. A Modified Sol-Gel Synthesis of Anatase {001}-TiO2/Au Hybrid Nanocomposites for Enhanced Photodegradation of Organic Contaminants. Gels 2022, 8, 728. [Google Scholar] [CrossRef]
- Sondezi, N.; Njengele-Tetyana, Z.; Matabola, K.P.; Makhetha, T.A. Sol-Gel-Derived TiO2 and TiO2/Cu Nanoparticles: Synthesis, Characterization, and Antibacterial Efficacy. ACS Omega 2024, 9, 15959–15970. [Google Scholar] [CrossRef]
- Kader, S.; Al-Mamun, M.R.; Suhan, M.B.K.; Shuchi, S.B.; Islam, M.S. Enhanced photodegradation of methyl orange dye under UV irradiation using MoO3 and Ag doped TiO2 photocatalysts. Environ. Technol. Innov. 2022, 27, 102476. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of titanium oxide nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Thapa, R.; Maiti, S.; Rana, T.H.; Maiti, U.N.; Chattopadhyay, K.K. Anatase TiO2 nanoparticles synthesis via simple hydrothermal route: Degradation of Orange II, Methyl Orange and Rhodamine B. J. Mol. Catal. A Chem. 2012, 363, 223–229. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Sun, D.D.; Guo, P.; Leckie, J.O. One-step fabrication and high photocatalytic activity of porous TiO2 hollow aggregates by using a low-temperature hydrothermal method without templates. Chem. A Eur. J. 2007, 13, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Bao, Y.; Kang, Q.; Liu, C.; Zhang, W.; Zhu, Q. Solvent-controlled synthesis and photocatalytic activity of hollow TiO2 microspheres prepared by the solvothermal method. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127931. [Google Scholar] [CrossRef]
- Cui, X.-G.; Chen, H.; Ye, Q.-B.; Cui, X.-Y.; Cui, X.-J.; Cui, H.-J.; Shen, G.-Z.; Li, M.-J.; Lin, J.-T.; Sun, Y.-X. Porous Titanium Dioxide Spheres for Drug Delivery and Sustained Release. Front. Mater. 2021, 8, 649237. [Google Scholar] [CrossRef]
- Sun, W.J.; Zheng, H.N.; Ma, J.B.; Xi, Z.Y.; Wang, B.X.; Hao, C.C. Preparation and electrorheological properties of eggshell-like TiO2 hollow spheres via one step template-free solvothermal method. Colloid. Surf. A 2020, 601, 125055. [Google Scholar] [CrossRef]
- Song, Y.D.; Li, B.; Shi, Y.Y.; Wang, Q.Y.; Jin, R.C.; Huang, B.B.; Dai, Y.; Gao, S.M. Preparation of fusiform Ti3+ self-doped TiO2 nanoparticles by mixed solvothermal method and its photoelectrochemical properties. Mater. Lett. 2019, 252, 134–137. [Google Scholar] [CrossRef]
- Rani, N.; Dehiya, B.S. Influence of anionic and non-ionic surfactants on the synthesis of core-shell Fe3O4@TiO2 nanocomposite synthesized by hydrothermal method. Ceram. Int. 2020, 46, 23516–23525. [Google Scholar] [CrossRef]
- Jahdi, M.; Mishra, S.B.; Nxumalo, E.N.; Mhlanga, S.D.; Mishra, A.K. Synergistic effects of sodium fluoride (NaF) on the crystallinity and band gap of Fe-doped TiO2 developed via microwave-assisted hydrothermal treatment. Opt. Mater. 2020, 104, 109844. [Google Scholar] [CrossRef]
- Cao, Z.Z.; Yin, Q.; Zhang, Y.X.; Li, Y.; Yu, C.J.; Zhang, M.K.; Fan, B.B.; Shao, G.; Wang, H.L.; Xu, H.L.; et al. Heterostructure composites of TiO2 and CdZnS nanoparticles decorated on Ti3C2TX nanosheets and their enhanced photocatalytic performance by microwave hydrothermal method. J. Alloys Compd. 2022, 918, 165681. [Google Scholar] [CrossRef]
- Sahu, K.; Dhonde, M.; Murty, V.V.S. Microwave-assisted hydrothermal synthesis of Cu-doped TiO2 nanoparticles for efficient dye-sensitized solar cell with improved open-circuit voltage. Int. J. Energy Res. 2020, 45, 5423–5432. [Google Scholar] [CrossRef]
- Shi, Y.F.; Wan, Y.; Zhao, D.Y. Ordered mesoporous non-oxide materials. Chem. Soc. Rev. 2011, 40, 3854–3878. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.H.; Chen, K.; Tüysüz, H. Protocol for the Nanocasting Method: Preparation of Ordered Mesoporous Metal Oxides. Chem. Mater. 2017, 29, 40–52. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Ye, K.; Long, H.B.; Wang, X.; Ru, H.Q. Preparation of hierarchically mesoporous silica microspheres with ordered mesochannels and ultra-large intra-particulate pores. Microporous Mesoporous Mater. 2016, 234, 267–276. [Google Scholar] [CrossRef]
- Wang, W.; Sun, R.; Zhu, L.; Wu, C.; Ru, H. Hierarchical mesoporous silica microspheres as unique hard-template for preparation of hierarchical mesoporous TiO2 microspheres with trimodal mesoporosities via nanocasting. Ceram. Int. 2019, 45, 16521–16529. [Google Scholar] [CrossRef]
- Li, Z.; He, J.; Ma, H.; Zang, L.; Li, D.; Guo, S.; Ci, Y. Preparation of heterogeneous TiO2/g-C3N4 with a layered mosaic stack structure by use of montmorillonite as a hard template approach: TC degradation, kinetic, mechanism, pathway and DFT investigation. Appl. Clay Sci. 2021, 207, 106107. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, X.Q.; Chen, Q.H.; Xu, H.L.; Dai, M.; Zhang, M.; Wang, W.Y.; Song, H. Microstructural modification of hollow TiO2 nanospheres and their photocatalytic performance. Appl. Surf. Sci. 2021, 535, 147641. [Google Scholar] [CrossRef]
- Kanjana, N.; Maiaugree, W.; Poolcharuansin, P.; Laokul, P. Size controllable synthesis and photocatalytic performance of mesoporous TiO2 hollow spheres. J. Mater. Sci. Technol. 2020, 48, 105–113. [Google Scholar] [CrossRef]
- Monnier, A.; Schüth, F.; Huo, Q.; Kumar, D.; Margolese, D.; Maxwell, R.S.; Stucky, G.D.; Krishnamurty, M.; Petroff, P.; Firouzi, A.; et al. Cooperative Formation of Inorganic-Organic Interfaces in the Synthesis of Silicate Mesostructures. Science 1993, 261, 1299–1303. [Google Scholar] [CrossRef]
- Lan, K.; Liu, Y.; Zhang, W.; Liu, Y.; Elzatahry, A.; Wang, R.C.; Xia, Y.Y.; Al-Dhayan, D.; Zheng, N.F.; Zhao, D.Y. Uniform Ordered Two-Dimensional Mesoporous TiO2 Nanosheets from Hydrothermal-Induced Solvent-Confined Monomicelle Assembly. J. Am. Chem. Soc. 2018, 140, 4135–4143. [Google Scholar] [CrossRef]
- Hung, I.M.; Wang, Y.; Huang, C.-F.; Fan, Y.-S.; Han, Y.-J.; Peng, H.-W. Effects of templating surfactant concentrations on the mesostructure of ordered mesoporous anatase TiO2 by an evaporation-induced self-assembly method. J. Eur. Ceram. Soc. 2010, 30, 2065–2072. [Google Scholar] [CrossRef]
- Lee, J.-H.; Chakraborty, D.; Chatterjee, S.; Cho, E.-B. Role of polymer template in crystal structure and photoactivity of Cu-TiO2 heterojunction nanostructures towards environmental remediation. Environ. Res. 2023, 232, 116352. [Google Scholar] [CrossRef]
- Fan, J.; Boettcher, S.W.; Stucky, G.D. Nanoparticle assembly of ordered multicomponent mesostructured metal oxides via a versatile sol-gel process. Chem. Mater. 2006, 18, 6391–6396. [Google Scholar] [CrossRef]
- Xiong, H.; Zhou, H.; Qi, C.; Liu, Z.; Zhang, L.; Zhang, L.; Qiao, Z.-A. Polymer-oriented evaporation induced self-assembly strategy to synthesize highly crystalline mesoporous metal oxides. Chem. Eng. J. 2020, 398, 125527. [Google Scholar] [CrossRef]
- Wisutiratanamanee, A.; Poochinda, K.; Poompradub, S. Low-temperature particle synthesis of titania/silica/natural rubber composites for antibacterial properties. Adv. Powder Technol. 2017, 28, 1263–1269. [Google Scholar] [CrossRef]
- Yang, K.; Zhong, S.; Zhou, X.M.; Tang, S.Y.; Qiao, K.; Ma, K.; Song, L.; Yue, H.R.; Liang, B. Controllable Al2O3 coating makes TiO2 photocatalysts active under visible light by pulsed chemical vapor deposition. Chem. Eng. Sci. 2023, 277, 118792. [Google Scholar] [CrossRef]
- Lang, J.H.; Takahashi, K.; Kubo, M.; Shimada, M. Ag-Doped TiO2 Composite Films Prepared Using Aerosol-Assisted, Plasma-Enhanced Chemical Vapor Deposition. Catalysts 2022, 12, 365. [Google Scholar] [CrossRef]
- Hudandini, M.; Kusdianto, K.; Kubo, M.; Shimada, M. Gas-Phase Fabrication and Photocatalytic Activity of TiO2 and TiO2-CuO Nanoparticulate Thin Films. Materials 2024, 17, 1149. [Google Scholar] [CrossRef]
- Hasan, M.M.; Haseeb, A.; Saidur, R.; Masjuki, H.H.; Hamdi, M. Influence of substrate and annealing temperatures on optical properties of RF-sputtered TiO2 thin films. Opt. Mater. 2010, 32, 690–695. [Google Scholar] [CrossRef]
- González-García, L.; González-Valls, I.; Lira-Cantu, M.; Barranco, A.; González-Elipe, A.R. Aligned TiO2 nanocolumnar layers prepared by PVD-GLAD for transparent dye sensitized solar cells. Energy Environ. Sci. 2011, 4, 3426–3435. [Google Scholar] [CrossRef]
- Othman, S.H.; Rashid, S.A.; Ghazi, T.I.M.; Abdullah, N. Effect of Postdeposition Heat Treatment on the Crystallinity, Size, and Photocatalytic Activity of TiO2 Nanoparticles Produced via Chemical Vapour Deposition. J. Nanomater. 2010, 2010, 512785. [Google Scholar] [CrossRef]
- Li, G.; Bai, R.B.; Zhao, X.S. Coating of TiO2 Thin Films on the Surface of SiO2 Microspheres: Toward Industrial Photocatalysis. Ind. Eng. Chem. Res. 2008, 47, 8228–8232. [Google Scholar] [CrossRef]
- Evans, P.; Sheel, D.W. Photoactive and antibacterial TiO2 thin films on stainless steel. Surf. Coat. Technol. 2007, 201, 9319–9324. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C. High Temperature Stable Anatase Phase Titanium Dioxide Films Synthesized by Mist Chemical Vapor Deposition. Nanomaterials 2020, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tadé, M.O.; Shao, Z.P. Nitrogen-doped simple and complex oxides for photocatalysis: A review. Prog. Mater. Sci. 2018, 92, 33–63. [Google Scholar] [CrossRef]
- Alotaibi, A.M.; Williamson, B.A.D.; Sathasivam, S.; Kafizas, A.; Alqahtani, M.; Sotelo-Vazquez, C.; Buckeridge, J.; Wu, J.; Nair, S.P.; Scanlon, D.O.; et al. Enhanced Photocatalytic and Antibacterial Ability of Cu-Doped Anatase TiO2 Thin Films: Theory and Experiment. ACS Appl. Mater. Interfaces 2020, 12, 15348–15361. [Google Scholar] [CrossRef]
- Su, K.; Tan, L.; Liu, X.M.; Cui, Z.D.; Zheng, Y.F.; Li, B.; Han, Y.; Li, Z.Y.; Zhu, S.L.; Liang, Y.Q.; et al. Rapid Photo-Sonotherapy for Clinical Treatment of Bacterial Infected Bone Implants by Creating Oxygen Deficiency Using Sulfur Doping. Acs Nano 2020, 14, 2077–2089. [Google Scholar] [CrossRef]
- Xia, P.F.; Cao, S.W.; Zhu, B.C.; Liu, M.J.; Shi, M.S.; Yu, J.G.; Zhang, Y.F. Designing a 0D/2D S-Scheme Heterojunction over Polymeric Carbon Nitride for Visible-Light Photocatalytic Inactivation of Bacteria. Angew. Chem. Int. Ed. 2020, 59, 5218–5225. [Google Scholar] [CrossRef]
- Babudurai, M.; Nwakanma, O.; Romero-Nuñez, A.; Manisekaran, R.; Subramaniam, V.; Castaneda, H.; Jantrania, A. Mechanical activation of TiO2/Fe2O3 nanocomposite for arsenic adsorption: Effect of ball-to-powder ratio and milling time. J. Nanostruct. Chem. 2021, 11, 619–632. [Google Scholar] [CrossRef]
- Cao, F.; Li, Y.; Tang, C.; Qian, X.; Bian, Z. Fast synthesis of anatase TiO2 single crystals by a facile solid-state method. Res. Chem. Intermed. 2016, 42, 5975–5981. [Google Scholar] [CrossRef]
- Hu, J.W.; Geng, X.Z.; Duan, Y.F.; Zhao, W.M.; Zhu, M.Q.; Ren, S.J. Effect of Mechanical-Chemical Modification Process on Mercury Removal of Bromine Modified Fly Ash. Energy Fuels 2020, 34, 9829–9839. [Google Scholar] [CrossRef]
- Do, J.L.; Friscic, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Al-Dossari, M.; Singh, J.; Rawat, M.; Kordy, M.G.M.; Shaban, M. A Review on Green Synthesis of TiO2 NPs: Photocatalysis and Antimicrobial Applications. Polymers 2022, 14, 1444. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Bhattarai, A.; Yadav, A.K.; Adhikari, J.; Singh, M.; Giri, B. Green Synthesis of Silver Nanoparticles Using Tea Leaves from Three Different Elevations. Chemistryselect 2020, 5, 4239–4246. [Google Scholar] [CrossRef]
- Syahin Firdaus Aziz Zamri, M.; Sapawe, N. Effect of pH on Phenol Degradation Using Green Synthesized Titanium Dioxide Nanoparticles. Mater. Today Proc. 2019, 19, 1321–1326. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, S.; Kumar, S.; Singh, J.; Rawat, M. Eco-friendly Approach: Synthesis of Novel Green TiO2 Nanoparticles for Degradation of Reactive Green 19 Dye and Replacement of Chemical Synthesized TiO2. J. Clust. Sci. 2021, 32, 1191–1204. [Google Scholar] [CrossRef]
- Rostami-Vartooni, A.; Nasrollahzadeh, M.; Salavati-Niasari, M.; Atarod, M. Photocatalytic degradation of azo dyes by titanium dioxide supported silver nanoparticles prepared by a green method using Carpobrotus acinaciformis extract. J. Alloys Compd. 2016, 689, 15–20. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef]
- Suriyaraj, S.P.; Selvakumar, R. Room temperature biosynthesis of crystalline TiO2 nanoparticles using Bacillus licheniformis and studies on the effect of calcination on phase structure and optical properties. RSC Adv. 2014, 4, 39619–39624. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Roopan, S.M.; Kirthi, A.V.; Venkatesan, J.; Kim, S.K.; Iyappan, M.; Siva, C. Biological approach to synthesize TiO2 nanoparticles using Aeromonas hydrophila and its antibacterial activity. Spectrochim. Acta A 2013, 107, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, P.; Maruthamuthu, S.; Rajagopal, G. Bio-mediated synthesis of TiO2 nanoparticles and its photocatalytic effect on aquatic biofilm. J. Photochem. Photobiol. B Biol. 2012, 110, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H.M. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Saini, R.; Kumar, P. Green synthesis of TiO2 nanoparticles using Tinospora cordifolia plant extract & its potential application for photocatalysis and antibacterial activity. Inorg. Chem. Commun. 2023, 156, 111221. [Google Scholar]
- Nabi, G.; Majid, A.; Riaz, A.; Alharbi, T.; Kamran, M.A.; Al-Habardi, M. Green synthesis of spherical TiO2 nanoparticles using Citrus Limetta extract: Excellent photocatalytic water decontamination agent for RhB dye. Inorg. Chem. Commun. 2021, 129, 108618. [Google Scholar] [CrossRef]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef]
- Anbumani, D.; Dhandapani, K.V.; Manoharan, J.; Babujanarthanam, R.; Bashir, A.K.H.; Muthusamy, K.; Alfarhan, A.; Kanimozhi, K. Green synthesis and antimicrobial efficacy of titanium dioxide nanoparticles using Luffa acutangula leaf extract. J. King Saud Univ. Sci. 2022, 34, 101896. [Google Scholar] [CrossRef]
- Ahmad, W.; Jaiswal, K.K.; Soni, S. Green synthesis of titanium dioxide (TiO2) nanoparticles by using Mentha arvensis leaves extract and its antimicrobial properties. Inorg. Nano-Met. Chem. 2020, 50, 1032–1038. [Google Scholar] [CrossRef]
- Ansari, A.; Siddiqui, V.U.; Rehman, W.U.; Akram, M.K.; Siddiqi, W.A.; Alosaimi, A.M.; Hussein, M.A.; Rafatullah, M. Green Synthesis of TiO2 Nanoparticles Using Acorus calamus Leaf Extract and Evaluating Its Photocatalytic and In Vitro Antimicrobial Activity. Catalysts 2022, 12, 181. [Google Scholar] [CrossRef]
- Singh, S.; Maurya, I.C.; Tiwari, A.; Srivastava, P.; Bahadur, L. Green synthesis of TiO2 nanoparticles using Citrus limon juice extract as a bio-capping agent for enhanced performance of dye-sensitized solar cells. Surf. Interfaces 2022, 28, 101652. [Google Scholar] [CrossRef]
- Rahmawati, T.; Butburee, T.; Sangkhun, W.; Wutikhun, T.; Padchasri, J.; Kidkhunthod, P.; Phromma, S.; Eksangsri, T.; Kangwansupamonkon, W.; Leeladee, P.; et al. Green synthesis of Ag-TiO2 nanoparticles using turmeric extract and its enhanced photocatalytic activity under visible light. Colloid. Surf. A 2023, 665, 131206. [Google Scholar] [CrossRef]
- Panneerselvam, A.; Velayutham, J.; Ramasamy, S. Green synthesis of TiO2 nanoparticles prepared from Phyllanthus niruri leaf extract for dye adsorption and their isotherm and kinetic studies. IET Nanobiotechnol. 2021, 15, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, V.D.; Eed, E.M.; Elfasakhany, A.; Badruddin, I.A.; Kamangar, S.; Brindhadevi, K. Green synthesis of titanium dioxide nanoparticles using Laurus nobilis (bay leaf): Antioxidant and antimicrobial activities. Appl. Nanosci. 2021, 13, 1477–1484. [Google Scholar] [CrossRef]
- Taran, M.; Rad, M.; Alavi, M. Biosynthesis of TiO2 and ZnO nanoparticles by Halomonas elongata IBRC-M 10214 in different conditions of medium. Bioimpacts 2018, 8, 81–89. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K. Biosynthesis of metal and oxide nanoparticles using Lactobacilli from yoghurt and probiotic spore tablets. Biotechnol. J. 2010, 5, 285–291. [Google Scholar] [CrossRef]
- Kirthi, A.V.; Rahuman, A.A.; Rajakumar, G.; Marimuthu, S.; Santhoshkumar, T.; Jayaseelan, C.; Elango, G.; Zahir, A.A.; Kamaraj, C.; Bagavan, A. Biosynthesis of titanium dioxide nanoparticles using bacterium Bacillus subtilis. Mater. Lett. 2011, 65, 2745–2747. [Google Scholar] [CrossRef]
- Kulal, D.; Shetty Kodialbail, V. Visible light mediated photocatalytic dye degradation using Ag2O/AgO-TiO2 nanocomposite synthesized by extracellular bacterial mediated synthesis-An eco-friendly approach for pollution abatement. J. Environ. Chem. Eng. 2021, 9, 105389. [Google Scholar] [CrossRef]
- Bansal, V.; Poddar, P.; Ahmad, A.; Sastry, M. Room-temperature biosynthesis of ferroelectric barium titanate nanoparticles. Journal of the American Chem. Soc. 2006, 128, 11958–11963. [Google Scholar] [CrossRef]
- Cantera, J.F.; Wang, J.; Carrea, S.P.P.; Chen, L.; Salmones, J.; González, J. A microwave-ultrasound assisted synthesis of defective TiO2 and WO3/TiO2 nanoparticles for ultralow sulfur diesel production. Mater. Lett. 2024, 360, 136030. [Google Scholar] [CrossRef]
- Giram, D.; Das, A.; Bhanvase, B. Comparative study of ZnO-TiO2 nanocomposites synthesized by ultrasound and conventional methods for the degradation of methylene blue dye. Indian J. Chem. Technol. 2023, 30, 693–704. [Google Scholar]
- Sivaprakash, V.; Narayanan, R. Anodic synthesis of TiO2 nanotubes by step-up voltages. J. Compos. Mater. 2021, 55, 3775–3784. [Google Scholar] [CrossRef]
- Pillai, K. Single crystalline rutile TiO2 nanorods synthesis by onestep catalyst-free vapor transport method. Solid State Commun. 2021, 333, 114342. [Google Scholar] [CrossRef]
- Kang, S.; Choi, J.; Park, G.Y.; Kim, H.R.; Hwang, J. A novel and facile synthesis of Ag-doped TiO2 nanofiber for airborne virus/bacteria inactivation and VOC elimination under visible light. Appl. Surf. Sci. 2022, 599, 153930. [Google Scholar] [CrossRef]
- Cho, S.; Yim, G.; Park, J.T.; Jang, H. Surfactant-free one-pot synthesis of Au-TiO2 core-shell nanostars by inter-cation redox reaction for photoelectrochemical water splitting. Energy Convers. Manag. 2022, 252, 115038. [Google Scholar] [CrossRef]
- Garcia-Munoz, P.; Zussblatt, N.P.; Pliego, G.; Zazo, J.A.; Fresno, F.; Chmelka, B.F.; Casas, J.A. Evaluation of photoassisted treatments for norfloxacin removal in water using mesoporous Fe2O3-TiO2 materials. J. Environ. Manag. 2019, 238, 243–250. [Google Scholar] [CrossRef]
- Hu, Y.; Cai, K.Y.; Luo, Z.; Xu, D.W.; Xie, D.C.; Huang, Y.R.; Yang, W.H.; Liu, P. TiO2 nanotubes as drug nanoreservoirs for the regulation of mobility and differentiation of mesenchymal stem cells. Acta Biomater. 2012, 8, 439–448. [Google Scholar] [CrossRef]
- Wen, J.Q.; Li, X.; Liu, W.; Fang, Y.P.; Xie, J.; Xu, Y.H. Photocatalysis fundamentals and surface modification of TiO2 nanomaterials. Chin. J. Catal. 2015, 36, 2049–2070. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Liu, P. Biocompatible triplex Ag@SiO2@mTiO2 core-shell nanoparticles for simultaneous fluorescence-SERS bimodal imaging and drug delivery. Chemistry 2012, 18, 5935–5943. [Google Scholar] [CrossRef]
- Monreal-Pérez, P.; Isasi, J.R.; González-Benito, J.; Olmos, D.; González-Gaitano, G. Cyclodextrin-Grafted TiO2 Nanoparticles: Synthesis, Complexation Capacity, and Dispersion in Polymeric Matrices. Nanomaterials 2018, 8, 642. [Google Scholar] [CrossRef]
- Khoshnood, N.; Zamanian, A.; Massoudi, A. Tailoring in vitro drug delivery properties of titania nanotubes functionalized with (3-Glycidoxypropyl) trimethoxysilane. Mater. Chem. Phys. 2017, 193, 290–297. [Google Scholar] [CrossRef]
- Qiao, Y.T.; Wan, J.Q.; Zhou, L.Q.; Ma, W.; Yang, Y.Y.; Luo, W.X.; Yu, Z.Q.; Wang, H.X. Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1527. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.Z.; Zhang, M.Q.; Ruan, W.H. Surface modification of nanoscale fillers for improving properties of polymer nanocomposites: A review. Mater. Sci. Technol. 2006, 22, 787–796. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, S.; Imran, M.; Sohail, M.; Shah, S.W.A.; de Matas, M. PEGylation: A promising strategy to overcome challenges to cancer-targeted nanomedicines: A review of challenges to clinical transition and promising resolution. Drug Deliv. Transl. Res. 2019, 9, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Gamucci, O.; Bertero, A.; Gagliardi, M.; Bardi, G. Biomedical Nanoparticles: Overview of Their Surface Immune-Compatibility. Coatings 2014, 4, 139–159. [Google Scholar] [CrossRef]
- Selli, D.; Motta, S.; Di Valentin, C. Impact of surface curvature, grafting density and solvent type on the PEGylation of titanium dioxide nanoparticles. J. Colloid. Interf. Sci. 2019, 555, 519–531. [Google Scholar] [CrossRef]
- Sun, X.Y.; Wang, H.D.; Lai, Z.Y.; Zhang, Z.H.; Zhang, Z.C.; Hang, J.Z.; Shi, L.Y. High stability, high solid content, low viscosity titanium dioxide dispersion. J. Coat. Technol. Res. 2024, 1–10. [Google Scholar] [CrossRef]
- Xu, L.A.; Xu, X.L.; Xu, Y.J.; Huang, M.Y.; Li, Y.L. Fabrication and immediate release characterization of UV responded oregano essential oil loaded microcapsules by chitosan-decorated titanium dioxide. Food Chem. 2023, 400, 133965. [Google Scholar] [CrossRef]
- Yousefi, E.; Javadpour, S.; Ansari, M.; Eslami, H. Sonodynamic therapy of cancer using a novel TiO2-based nanoparticles. Mater. Technol. 2021, 36, 521–528. [Google Scholar]
- Wu, L.Z.; Wu, X.; Wu, L.Z.; Chen, D.D.; Zhang, T.; Zheng, H.; Xiao, X.F. Polydopamine-Modified Titanium Dioxide Nanotube Arrays Doped with Calcium as a Sustained Drug Delivery System. ACS Omega 2024, 9, 4949–4956. [Google Scholar] [CrossRef]
- Matiyani, M.; Rana, A.; Karki, N.; Garwal, K.; Pal, M.; Sahoo, N.G. Development of multi-functionalized graphene oxide based nanocarrier for the delivery of poorly water soluble anticancer drugs. J. Drug Deliv. Sci. Technol. 2023, 83, 104412. [Google Scholar] [CrossRef]
- Cheng, F.; Sajedin, S.M.; Kelly, S.M.; Lee, A.F.; Kornherr, A. UV-stable paper coated with APTES-modified P25 TiO2 nanoparticles. Carbohydr. Polym. 2014, 114, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Khabibullin, A.; Bhangaonkar, K.; Mahoney, C.; Lu, Z.; Schmitt, M.; Sekizkardes, A.K.; Bockstaller, M.R.; Matyjaszewski, K. Grafting PMMA Brushes from α-Alumina Nanoparticles via SI-ATRP. Acs Appl. Mater. Interfaces 2016, 8, 5458–5465. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.; Kandasamy, G.; Upadhyay, R.K.; Bhattacharya, G.; Banerjee, D.; Maity, D.; Deshusses, M.A.; Roy, S.S. Terephthalic acid capped iron oxide nanoparticles for sensitive electrochemical detection of heavy metal ions in water. J. Electroanal. Chem. 2017, 788, 91–98. [Google Scholar] [CrossRef]
- Jia, X.Q.; Ma, J.P.; Xia, F.; Xu, Y.M.; Gao, J.; Xu, J. Carboxylic acid-modified metal oxide catalyst for selectivity-tunable aerobic ammoxidation. Nat. Commun. 2018, 9, 933. [Google Scholar] [CrossRef]
- Pal, S.; Taurino, A.; Catalano, M.; Licciulli, A. Block Copolymer and Cellulose Templated Mesoporous TiO2-SiO2 Nanocomposite as Superior Photocatalyst. Catalysts 2022, 12, 770. [Google Scholar] [CrossRef]
- Joseph, C.G.; Taufiq-Yap, Y.H.; Letshmanan, E.; Vijayan, V. Heterogeneous Photocatalytic Chlorination of Methylene Blue Using a Newly Synthesized TiO2-SiO2 Photocatalyst. Catalysts 2022, 12, 156. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.H.; Xiong, X.; Wu, X.P.; Xie, Z.A.; Liu, Z.H. Synthesis of hollow TiO2@SiO2 spheres via a recycling template method for solar heat protection coating. Ceram. Int. 2021, 47, 2678–2685. [Google Scholar] [CrossRef]
- Shi, J.; Chen, Z.; Wang, B.; Wang, L.; Lu, T.; Zhang, Z. Reactive Oxygen Species-Manipulated Drug Release from a Smart Envelope-Type Mesoporous Titanium Nanovehicle for Tumor Sonodynamic-Chemotherapy. ACS Appl. Mater. Interfaces 2015, 7, 28554–28565. [Google Scholar] [CrossRef]
- Meng, L.; Liu, Z.H.; Lan, C.W.; Xu, N. In-Situ Fabricating Ag Nanoparticles on TiO2 for Unprecedented High Catalytic Activity of 4-Nitrophenol Reduction. Catal. Lett. 2022, 152, 912–920. [Google Scholar] [CrossRef]
- Motamedisade, A.; Johnston, M.R.; Alotaibi, A.E.H.; Andersson, G.A. Au9 nanocluster adsorption and agglomeration control through sulfur modification of mesoporous TiO2. Phys. Chem. Chem. Phys. 2024, 26, 9500–9509. [Google Scholar] [CrossRef]
- Mirzaei, M.; Zarch, M.B.; Darroudi, M.; Sayyadi, K.; Keshavarz, S.T.; Sayyadi, J.; Fallah, A.; Maleki, H. Silica Mesoporous Structures: Effective Nanocarriers in Drug Delivery and Nanocatalysts. Appl. Sci. 2020, 10, 7533. [Google Scholar] [CrossRef]
- Mabrouk, M.; Moaness, M.; Beherei, H.H. Fabrication of mesoporous zirconia and titania nanomaterials for bone regeneration and drug delivery applications. J. Drug Deliv. Sci. Technol. 2022, 78, 103957. [Google Scholar] [CrossRef]
- Liu, D.; Bi, Y.-G. Controllable fabrication of hollow TiO2 spheres as sustained release drug carriers. Adv. Powder Technol. 2019, 30, 2169–2177. [Google Scholar] [CrossRef]
- Mai, Z.; Chen, J.; Cao, Q.; Hu, Y.; Dong, X.; Zhang, H.; Huang, W.; Zhou, W. Rational design of hollow mesoporous titania nanoparticles loaded with curcumin for UV-controlled release and targeted drug delivery. Nanotechnology 2021, 32, 205604. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.R.; Wang, Y.Y.; Zhang, P.; Alomar, T.S.; Wang, G.Q.; Gao, Y.A.; Liu, M.; AlMasoud, N.; El-Bahy, Z.M.; Ren, J.N.; et al. Targeted synthesis of hollow titania microspheres with sustained release behaviors of 1,2-benzisothiazolin-3-one (BIT) for good marine antifouling performance. Adv. Compos. Hybrid Mater. 2023, 6, 151. [Google Scholar] [CrossRef]
- Ye, J.; Liu, W.; Cai, J.; Chen, S.; Zhao, X.; Zhou, H.; Qi, L. Nanoporous anatase TiO2 mesocrystals: Additive-free synthesis, remarkable crystalline-phase stability, and improved lithium insertion behavior. J. Am. Chem. Soc. 2011, 133, 933–940. [Google Scholar] [CrossRef]
- Pakdel, E.; Zhao, H.; Wang, J.; Tang, B.; Varley, R.J.; Wang, X. Superhydrophobic and photocatalytic self-cleaning cotton fabric using flower-like N-doped TiO2/PDMS coating. Cellulose 2021, 28, 8807–8820. [Google Scholar] [CrossRef]
- Wang, H.-X.; Li, X.-X.; Tang, L. Effects of surfactants on the morphology and properties of TiO2. Appl. Phys. A 2020, 126, 448. [Google Scholar] [CrossRef]
- Pakdel, E.; Wang, J.; Allardyce, B.J.; Rajkhowa, R.; Wang, X. Functionality of nano and 3D-microhierarchical TiO2 particles as coagulants for sericin extraction from the silk degumming wastewater. Sep. Purif. Technol. 2016, 170, 92–101. [Google Scholar] [CrossRef]
- Chao, C.S.; Liu, K.H.; Tung, W.L.; Chen, S.Y.; Liu, D.M.; Chang, Y.P. Bioactive TiO2 ultrathin film with worm-like mesoporosity for controlled drug delivery. Microporous Mesoporous Mater. 2012, 152, 58–63. [Google Scholar] [CrossRef]
- Seyed-Talebi, S.M.; Kazeminezhad, I.; Motamedi, H. TiO2 hollow spheres as a novel antibiotic carrier for the direct delivery of gentamicin. Ceram. Int. 2018, 44, 13457–13462. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, C.L.; Chen, B.A.; Wang, X.M. Daunorubicin-TiO2 nanocomposites as a “smart” pH-responsive drug delivery system. Int. J. Nanomed. 2012, 7, 235–242. [Google Scholar] [CrossRef]
- Malekimusavi, H.; Ghaemi, A.; Masoudi, G.; Chogan, F.; Rashedi, H.; Yazdian, F.; Omidi, M.; Javadi, S.; Haghiralsadat, B.F.; Teimouri, M.; et al. Graphene oxide-l-arginine nanogel: A pH-sensitive fluorouracil nanocarrier. Biotechnol. Appl. Biochem. 2019, 66, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Yuan, L.L.; Yao, C.J.; Fang, J.; Wu, M.H. Cytotoxicity Evaluation of pH-Controlled Antitumor Drug Release System of Titanium Dioxide Nanotubes. J. Nanosci. Nanotechnol. 2015, 15, 4143–4148. [Google Scholar] [CrossRef]
- Jia, H.Y.; Kerr, L.L. Kinetics of Drug Release from Drug Carrier of Polymer/TiO2 Nanotubes Composite-pH Dependent Study. J. Appl. Polym. Sci. 2015, 132, 41570. [Google Scholar] [CrossRef]
- Pour, M.M.; Moghbeli, M.R.; Larijani, B.; Javar, H.A. pH-Sensitive mesoporous bisphosphonate-based TiO2 nanoparticles utilized for controlled drug delivery of dexamethasone. Chem. Pap. 2022, 76, 439–451. [Google Scholar] [CrossRef]
- Han, J.; Jang, E.K.; Ki, M.R.; Son, R.G.; Kim, S.; Choe, Y.; Pack, S.P.; Chung, S. pH-responsive phototherapeutic poly(acrylic acid)-calcium phosphate passivated TiO2 nanoparticle-based drug delivery system for cancer treatment applications. J. Ind. Eng. Chem. 2022, 112, 258–270. [Google Scholar] [CrossRef]
- Yan, S.J.; Shi, H.C.; Song, L.J.; Wang, X.H.; Liu, L.; Luan, S.F.; Yang, Y.M.; Yin, J.H. Nonleaching Bacteria-Responsive Antibacterial Surface Based on a Unique Hierarchical. Acs Appl. Mater. Interfaces 2016, 8, 24471–24481. [Google Scholar] [CrossRef]
- Chen, J.; Shi, X.; Zhu, Y.; Chen, Y.; Gao, M.; Gao, H.; Liu, L.; Wang, L.; Mao, C.; Wang, Y. On-demand storage and release of antimicrobial peptides using Pandora’s box-like nanotubes gated with a bacterial infection-responsive polymer. Theranostics 2020, 10, 109–122. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Yazdian, F.; Koulivand, A.; Rahmani, E. Green synthesized polyvinylpyrrolidone/titanium dioxide hydrogel nanocomposite modified with agarose macromolecules for sustained and pH-responsive release of anticancer drug. Int. J. Biol. Macromol. 2023, 240, 124345. [Google Scholar] [CrossRef]
- Zhang, H.J.; Ji, Y.D.; Chen, Q.Q.; Zhu, X.; Zhang, X.G.; Tan, Z.Y.; Tian, Q.Q.; Yang, X.B.; Zhang, Z.Z. Invitro and invivo chemo-phototherapy of magnetic TiO2 drug delivery system formed by pH-sensitive coordination bond. J. Biomater. Appl. 2016, 31, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Bagwasi, S.; Tian, B.Z.; Zhang, J.L.; Nasir, M. Synthesis, characterization and application of bismuth and boron Co-doped TiO2: A visible light active photocatalyst. Chem. Eng. J. 2013, 217, 108–118. [Google Scholar] [CrossRef]
- Low, W.; Boonamnuayvitaya, V. Enhancing the photocatalytic activity of TiO2 co-doping of graphene-Fe3+ ions for formaldehyde removal. J. Environ. Manag. 2013, 127, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Al-Nemrawi, N.K.; Alshraiedeh, N.A.H.; Zayed, A.L.; Altaani, B.M. Low Molecular Weight Chitosan-Coated PLGA Nanoparticles for Pulmonary Delivery of Tobramycin for Cystic Fibrosis. Pharmaceuticals 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Al-Nemrawi, N.K.; Alsharif, S.S.M.; Alzoubi, K.H.; Alkhatib, R.Q. Preparation and characterization of insulin chitosan-nanoparticles loaded in buccal films. Pharm. Dev. Technol. 2019, 24, 967–974. [Google Scholar] [CrossRef]
- Al-Nemrawi, N.; Hameedat, F.; Al-Husein, B.; Nimrawi, S. Photolytic Controlled Release Formulation of Methotrexate Loaded in Chitosan/TiO2 Nanoparticles for Breast Cancer. Pharmaceuticals 2022, 15, 149. [Google Scholar] [CrossRef]
- Sargazi, S.; Er, S.; Sacide Gelen, S.; Rahdar, A.; Bilal, M.; Arshad, R.; Ajalli, N.; Farhan Ali Khan, M.; Pandey, S. Application of titanium dioxide nanoparticles in photothermal and photodynamic therapy of cancer: An updated and comprehensive review. J. Drug Deliv. Sci. Technol. 2022, 75, 103605. [Google Scholar] [CrossRef]
- Shen, Y.; Xie, C.; Xiao, X. Black phosphorus-incorporated titanium dioxide nanotube arrays for near-infrared–triggered drug delivery. J. Drug Deliv. Sci. Technol. 2022, 72, 103400. [Google Scholar] [CrossRef]
- Chen, B.; Liang, Y.; Song, Y.; Liang, Y.; Jiao, J.; Bai, H.; Li, Y. Photothermal-Controlled Release of IL-4 in IL-4/PDA-Immobilized Black Titanium Dioxide (TiO2) Nanotubes Surface to Enhance Osseointegration: An In Vivo Study. Materials 2022, 15, 5962. [Google Scholar] [CrossRef]
- Song, Y.Y.; Hildebrand, H.; Schmuki, P. Photoinduced release of active proteins from TiO2 surfaces. Electrochem. Commun. 2009, 11, 1429–1433. [Google Scholar] [CrossRef]
- Kumar, A.; Agarwala, V.; Singh, D. Microwave absorbing behavior of metal dispersed TiO2 nanocomposites. Adv. Powder Technol. 2014, 25, 483–489. [Google Scholar] [CrossRef]
- Liu, J.W.; Che, R.C.; Chen, H.J.; Zhang, F.; Xia, F.; Wu, Q.S.; Wang, M. Microwave Absorption Enhancement of Multifunctional Composite Microspheres with Spinel Fe3O4 Cores and Anatase TiO2 Shells. Small 2012, 8, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Cui, B.; Zhao, W.; Wang, Y.; Chang, Z.; Wang, Y. Glycine-functionalized Fe3O4@TiO2:E3+,Yb3+ nanocarrier for microwave-triggered controllable drug release and study on mechanism of loading release process using microcalorimetry. Expert Opin. Drug Deliv. 2015, 12, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Si, Y.Y.; Di, M.Y.; Tang, D.J.; Meng, L.; Cui, B. A novel microwave stimulus remote-controlled anticancer drug release system based on Janus TiO2−x&mSiO2 nanocarriers. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111968. [Google Scholar]
- Liu, Y.; Hui, Z.; Zhan, Z.; Cui, L.; Liu, X.; Cui, B. “Biped” Janus Fe3O4@nSiO2@TiO2−x&mSiO2 Nanoparticles for Drug Delivery and Microwave-Triggered Drug Release. Nano 2023, 18, 2350057. [Google Scholar]
- Deckers, R.; Rome, C.; Moonen, C.T.W. The role of ultrasound and magnetic resonance in local drug delivery. J. Magn. Reson. Imaging 2008, 27, 400–409. [Google Scholar] [CrossRef]
- Ye, L.Z.; Zhu, X.J.; Liu, Y. Numerical study on dual-frequency ultrasonic enhancing cavitation effect based on bubble dynamic evolution. Ultrason. Sonochem. 2019, 59, 104744. [Google Scholar] [CrossRef]
- Zhou, J.; Frank, M.A.; Yang, Y.; Boccaccini, A.R.; Virtanen, S. A novel local drug delivery system: Superhydrophobic titanium oxide nanotube arrays serve as the drug reservoir and ultrasonication functions as the drug release trigger. Mater. Sci. Eng. C 2018, 82, 277–283. [Google Scholar] [CrossRef]
- Rehman, F.U.; Rauf, M.A.; Ullah, S.; Shaikh, S.; Qambrani, A.; Muhammad, P.; Hanif, S. Ultrasound-activated nano-TiO2 loaded with temozolomide paves the way for resection of chemoresistant glioblastoma multiforme. Cancer Nanotechnol. 2021, 12, 17. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Qian, Y.; Xie, Y.; Sun, Q.; Gao, M.; Li, C. Dual-targeted nanoformulation with Janus structure for synergistic enhancement of sonodynamic therapy and chemotherapy. Chin. Chem. Lett. 2023, 34, 107853. [Google Scholar] [CrossRef]
- Jing, Y.; Zhu, Y.; Yang, X.; Shen, J.; Li, C. Ultrasound-Triggered Smart Drug Release from Multifunctional Core−Shell Capsules One-Step Fabricated by Coaxial Electrospray Method. Langmuir 2010, 27, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, C.; Liu, Y.; Wang, J.; Gao, Y.; Zhang, X.; Jiang, T.; Wang, S. PEGylated mesoporous silica as a redox-responsive drug delivery system for loading thiol-containing drugs. Int. J. Pharm. 2014, 477, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, Z.; Sun, W.; Yang, Y.; Jin, H.; Qiu, L.; Chen, J.; Chen, J. Co-responsive smart cyclodextrin-gated mesoporous silica nanoparticles with ligand-receptor engagement for anti-cancer treatment. Mater. Sci. Eng. C 2019, 103, 109831. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Hu, Y.; Cai, K.; Ding, X.; Zhang, Q.; Li, M.; Ma, X.; Zhang, B.; Zeng, Y.; Li, P.; et al. Intracellular redox-activated anticancer drug delivery by functionalized hollow mesoporous silica nanoreservoirs with tumor specificity. Biomaterials 2014, 35, 7951–7962. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.; Zhao, M.; Lei, Z.; Guo, H.; Tang, Y.; Yan, H. Redox-responsive hollow mesoporous silica nanoparticles constructed via host–guest interactions for controllable drug release. J. Biomater. Sci. Polym. Ed. 2019, 31, 472–490. [Google Scholar] [CrossRef]
- Malekmohammadi, S.; Hadadzadeh, H.; Rezakhani, S.; Amirghofran, Z. Design and Synthesis of Gatekeeper Coated Dendritic Silica/Titania Mesoporous Nanoparticles with Sustained and Controlled Drug Release Properties for Targeted Synergetic Chemo-Sonodynamic Therapy. Acs Biomater. Sci. Eng. 2019, 5, 4405–4415. [Google Scholar] [CrossRef]
- Hu, X.X.; Hao, X.H.; Wu, Y.; Zhang, J.C.; Zhang, X.N.; Wang, P.C.; Zou, G.Z.; Liang, X.J. Multifunctional hybrid silica nanoparticles for controlled doxorubicin loading and release with thermal and pH dual response. J. Mater. Chem. B 2013, 1, 1109–1118. [Google Scholar] [CrossRef]
- Timin, A.S.; Muslimov, A.R.; Lepik, K.V.; Saprykina, N.N.; Sergeev, V.S.; Afanasyev, B.V.; Vilesov, A.D.; Sukhorukov, G.B. Triple-responsive inorganic-organic hybrid microcapsules as a biocompatible smart platform for the delivery of small molecules. J. Mater. Chem. B 2016, 4, 7270–7282. [Google Scholar] [CrossRef]
- Innocenzi, P.; Malfatti, L. Mesoporous ordered titania films: An advanced platform for photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2024, 58, 100646. [Google Scholar] [CrossRef]
- Yu, S.; Mu, Y.; Zhang, X.; Li, J.; Lee, C.; Wang, H. Molecular mechanisms underlying titanium dioxide nanoparticles (TiO2 NP) induced autophagy in mesenchymal stem cells (MSC). J. Toxicol. Environ. Health Part A 2019, 82, 997–1008. [Google Scholar] [CrossRef]
- Ren, Y.; Feng, X.; Lang, X.; Wang, J.; Du, Z.; Niu, X.; Li, X. Evaluation of Osteogenic Potentials of Titanium Dioxide Nanoparticles with Different Sizes and Shapes. J. Nanomater. 2020, 2020, 8887323. [Google Scholar] [CrossRef]
- Kose, O.; Tomatis, M.; Leclerc, L.; Belblidia, N.-B.; Hochepied, J.-F.; Turci, F.; Pourchez, J.; Forest, V. Impact of the Physicochemical Features of TiO2 Nanoparticles on Their In Vitro Toxicity. Chem. Res. Toxicol. 2020, 33, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, P.H.; Knudsen, K.B.; Štrancar, J.; Umek, P.; Koklič, T.; Garvas, M.; Vanhala, E.; Savukoski, S.; Ding, Y.; Madsen, A.M.; et al. Effects of physicochemical properties of TiO2 nanomaterials for pulmonary inflammation, acute phase response and alveolar proteinosis in intratracheally exposed mice. Toxicol. Appl. Pharmacol. 2020, 386, 114830. [Google Scholar] [CrossRef] [PubMed]
- Padin-Gonzalez, E.; Lancaster, P.; Bottini, M.; Gasco, P.; Tran, L.; Fadeel, B.; Wilkins, T.; Monopoli, M.P. Understanding the Role and Impact of Poly (Ethylene Glycol) (PEG) on Nanoparticle Formulation: Implications for COVID-19 Vaccines. Front. Bioeng. Biotechnol. 2022, 10, 882363. [Google Scholar] [CrossRef]


| Type | Advantages | Disadvantages | Application |
|---|---|---|---|
| TiO2 | Easy surface functionalization, antibacterial properties, photocatalytic degradation properties | Wide band gap, fast hydrolysis rate and lack of biosafety evaluation | Nano-biosensing, medical implantation, drug delivery, and antimicrobial agents |
| SiO2 | Low toxicity, easy surface functionalization | Lack of biosafety evaluation | Bioimaging, drug delivery |
| Fe3O4 | Magnetic, biodegradability | Easy to aggregate and oxidize | Biological separation and detection, magnetic resonance imaging, and magnetic hyperthermia |
| Methods | Classification | Characteristic |
|---|---|---|
| Sol-gel method | - | Simple, fast, economically less expensive, low processing temperature, homogeneity of the produced material |
| Hydrothermal method | - | High purity, good dispersibility, low safety and high energy consumption |
| Template method | Hard template method | Simple operation and various structures |
| Soft template method | ||
| Gas-state method | Physical vapor deposition | High purity, uniform distribution, small particle size and good dispersion, expensive |
| Chemical vapor deposition | High purity, high reaction temperature | |
| Solid-state method | - | Easy to operate, short processing time, impurity, incomplete morphology and inhomogeneous particle size |
| Green synthesis method | - | Naturally adaptable, environmentally friendly and cost-effective |
| S/N | Plant Extract | Reactant | Shape | Average Particle Size (nm) | Application | Ref. |
|---|---|---|---|---|---|---|
| 1 | Tinospora cordifolia | Titanium (IV) isopropoxide | Triangular and irregularly shape | 7–21 | Photocatalysis | [94] |
| 2 | Citrus Limetta | Titanium butoxide, water | Spherical | 80–100 | Photocatalysis | [95] |
| 3 | Syzygium cumini | Titanium-isopropoxide, water | Spherical | 15–22 | Photocatalysis | [96] |
| 4 | Luffa acutangula | Titanium sulfate, water | Aggregated | 10–49 | Antibacterial | [97] |
| 5 | Mentha arvensis | Titanium tetra-isopropoxide, ethanol | Spherical | 20–70 | Antibacterial | [98] |
| 6 | Acorus calamus | Titanium (IV) isopropoxide, water, aqueous ammonia | Spherical and interconnected | 11–30 | Photocatalysis and antibacterial | [99] |
| 7 | Citrus limon | Titanium (IV) butoxide, isopropanol, glacial acetic acid | Spherical | 9–18 | Dye-sensitized solar cells | [100] |
| 8 | Curcuma Longa L. | Methanol, titanium tetra-isopropoxide, water | Spherical, cubic and hexagonal | 20.8–40.1 | Photocatalysis | [101] |
| 9 | Phyllanthus niruri | Titanium isopropoxide, water | Spherical | 20 | Dye adsorption | [102] |
| 10 | Laurus nobilis | Titanium tetra isopropoxide, water | Spherical | 80–120 | Antibacterial and antioxidant | [103] |
| S/N | Bacterial Species | Reactant | Shape | Average Particle Size (nm) | Application | Ref. |
|---|---|---|---|---|---|---|
| 1 | Aeromonas hydrophila | Metatitanic acid (TiO (OH)2) | Spherical | 40–50 | Antibacterial | [104] |
| 2 | Lactobacilli | TiO (OH)2 | Irregular | 10–25 | - | [105] |
| 3 | Bacillus subtilis | TiO (OH)2, water | Spherical | 66–77 | - | [106] |
| 4 | Bacillus subtilis | Ti4+ ions, water | Spherical | 10–30 | Photocatalysis | [92] |
| 5 | Alcaligenes aquatilis | K2TiF6, water, silver nitrate solution | Spherical | 10–90 | Photocatalysis | [107] |
| 6 | Fusarium oxysporum | Aqueous solution, (CH3COO)2Ba, K2TiF6 | Spherical | 10 | - | [108] |
| TiO2-Type | Synthesis Method | Drug | Stimulus | Ref. |
|---|---|---|---|---|
| MTN | Polymer sacrificial method | Amoxicillin | Sustained release | [142] |
| HMTN | Hydrothermal method | Doxorubicin | Sustained release | [143] |
| HMTN | Stöber method | Curcumin | UV | [144] |
| HMTN | Surface-layer-absorption template | 1,2-benzisothiazolin-3-one | Sustained release | [145] |
| plush TiO2 | Hydrothermal method | Doxorubicin | Sustained release | [45] |
| TiO2 film | EISA method | Ibuprofen and vancomycin | Sustained release | [150] |
| HMTN | Sol-gel method | Gentamycin | Sustained release | [151] |
| DNR-TiO2 | - | Daunorubicin | pH | [152] |
| PDA-TNTs | - | Ibuprofen | Sustained release | [22] |
| GPTMS-TNTs | Hydrothermal method | Dexamethasone | Sustained release | [120] |
| TNTs | - | Doxorubicin | pH | [154] |
| TNTs | Two-step anodization method | Methotrexate | pH | [155] |
| MBTNPs | Template method | Dexamethasone | pH | [156] |
| TiO2@PAA-CaP NPs | Hydrothermal method | Doxorubicin | pH | [157] |
| PVP-Ag-TiO2 | - | Doxorubicin | pH | [160] |
| TiO2@Fe3O4-PEI | Sol-gel method | Doxorubicin | pH | [161] |
| CS-NPs | Ionic gelation method | Methotrexate | Light | [166] |
| BP-TNTs | - | Ibuprofen | NIR | [168] |
| BP-TNTs | Electrochemical reduction method | IL-4 | NIR | [169] |
| TiO2 | - | Proteins | UV | [170] |
| Fe3O4@TiO2 | Hydrothermal method | Etoposide | Microwave | [173] |
| TiO2-x&mSiO2 | Sol-gel method | Doxorubicin | Microwave and pH | [174] |
| Fe3O4@nSiO2@TiO2-x&mSiO2 | - | Doxorubicin | Microwave | [175] |
| Superhydrophobic -TNTs | Tetracycline hydrochloride | Electrochemical anoDization method | Ultrasound | [178] |
| TiO2 nanosticks | - | GlioblasToma multiforme | Ultrasound | [179] |
| TiO2-x @NaGdF4 | - | IR780 iodine | Ultrasound | [180] |
| TiO2 Core-Shell Capsules | Coaxial electrospray method | Paclitaxel | Ultrasound | [181] |
| PEI-FA-DSTNs | - | Curcumin | Ultrasound | [186] |
| MTN-CD | Sol-gel method | Docetaxel | Ultrasound | [138] |
| SiO2/TiO2 | Template method | Rh-B | UV, ultrasound and enzymatic treatment | [188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, F.; Zhu, Y.; Wu, T.; Li, C.; Liu, Y.; Wu, X.; Ma, J.; Zhang, K.; Ouyang, H.; Qiu, X.; et al. Titanium Dioxide Nanomaterials: Progress in Synthesis and Application in Drug Delivery. Pharmaceutics 2024, 16, 1214. https://doi.org/10.3390/pharmaceutics16091214
Zuo F, Zhu Y, Wu T, Li C, Liu Y, Wu X, Ma J, Zhang K, Ouyang H, Qiu X, et al. Titanium Dioxide Nanomaterials: Progress in Synthesis and Application in Drug Delivery. Pharmaceutics. 2024; 16(9):1214. https://doi.org/10.3390/pharmaceutics16091214
Chicago/Turabian StyleZuo, Fanjiao, Yameng Zhu, Tiantian Wu, Caixia Li, Yang Liu, Xiwei Wu, Jinyue Ma, Kaili Zhang, Huizi Ouyang, Xilong Qiu, and et al. 2024. "Titanium Dioxide Nanomaterials: Progress in Synthesis and Application in Drug Delivery" Pharmaceutics 16, no. 9: 1214. https://doi.org/10.3390/pharmaceutics16091214






