Effect of Photoluminophore Light-Correcting Coatings and Bacterization by Associative Microorganisms on the Growth and Productivity of Brassica juncea L. Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Objects of Study and Cultivation Conditions
2.2. Analysis of Auxin Content in the Culture Broth of P. putida KT2442 and R. erythropolis X5 Bacteria
2.3. Bacterization of Plants and Analysis of Stability of Their Association with Plants
2.4. Microscopic Analysis of B. juncea L. Colonized Plants
2.5. Analysis of Sugars and Hydroxy Acids in Plants
2.5.1. Samples Preparation
2.5.2. Chromatography
2.6. Analysis of Photosynthetic Activity of Plants
2.7. Statistical Analysis
3. Results
3.1. The Effect of PLC on IAA Content in Bacterial Culture Medium
3.2. Bacterization of Plants by Associative Microorganisms
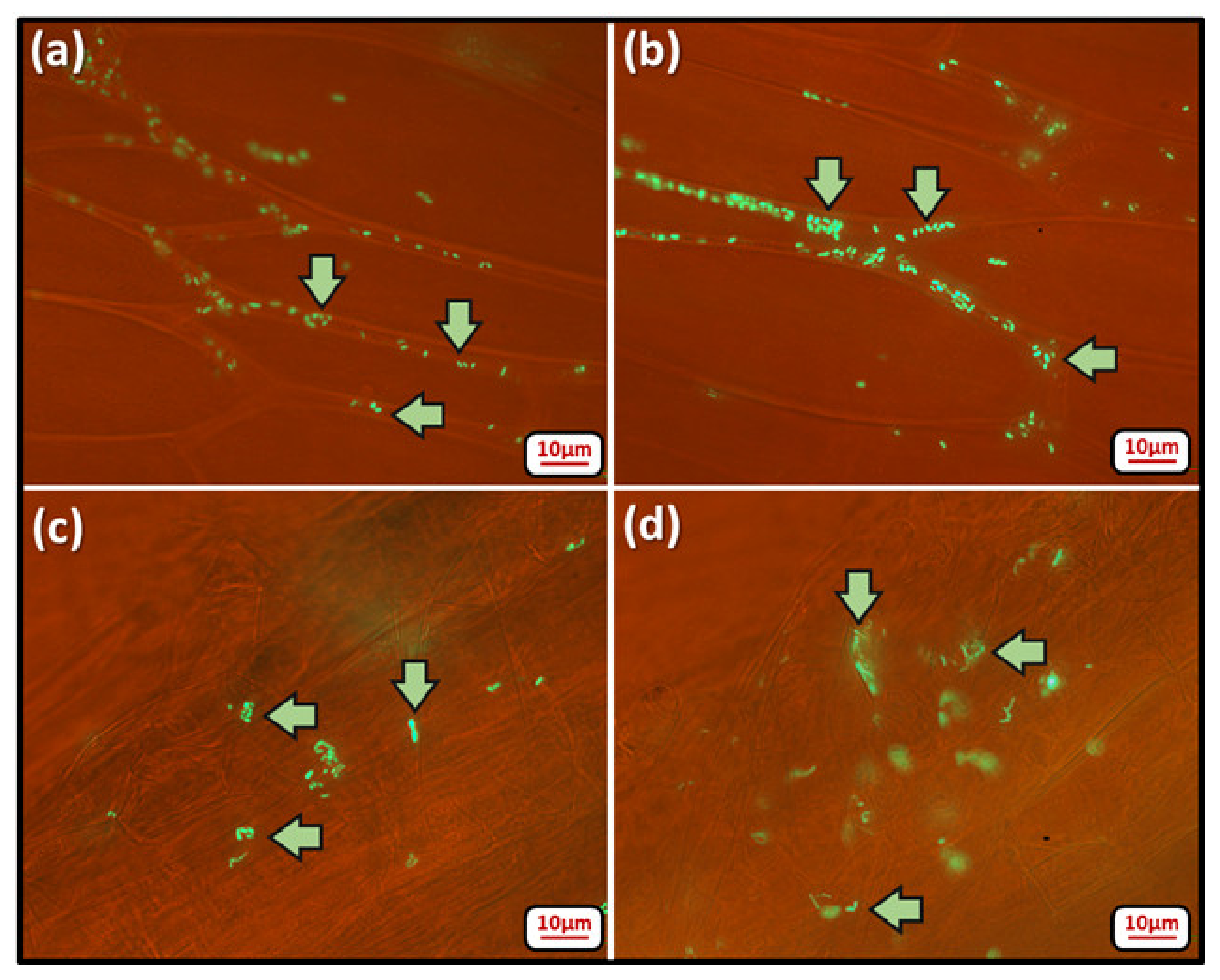
3.3. Microscopic Study of Colonized Plants
3.4. Analysis of the Content of Hydroxy Acids and Sugars in Plant Leaves
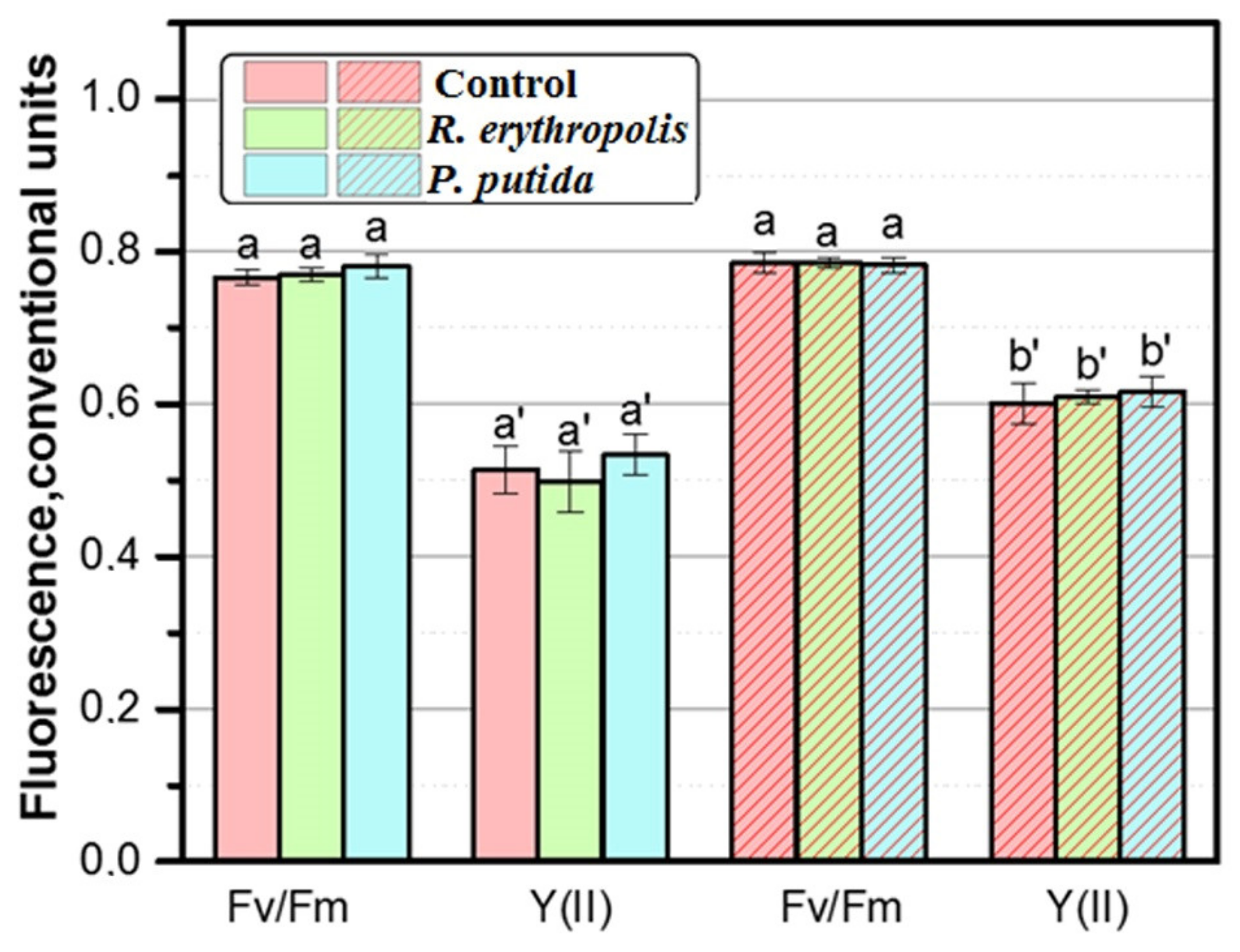
3.5. Analysis of Photosynthetic Apparatus Efficiency in Leaves of B. juncea L. Plants Grown In Vitro
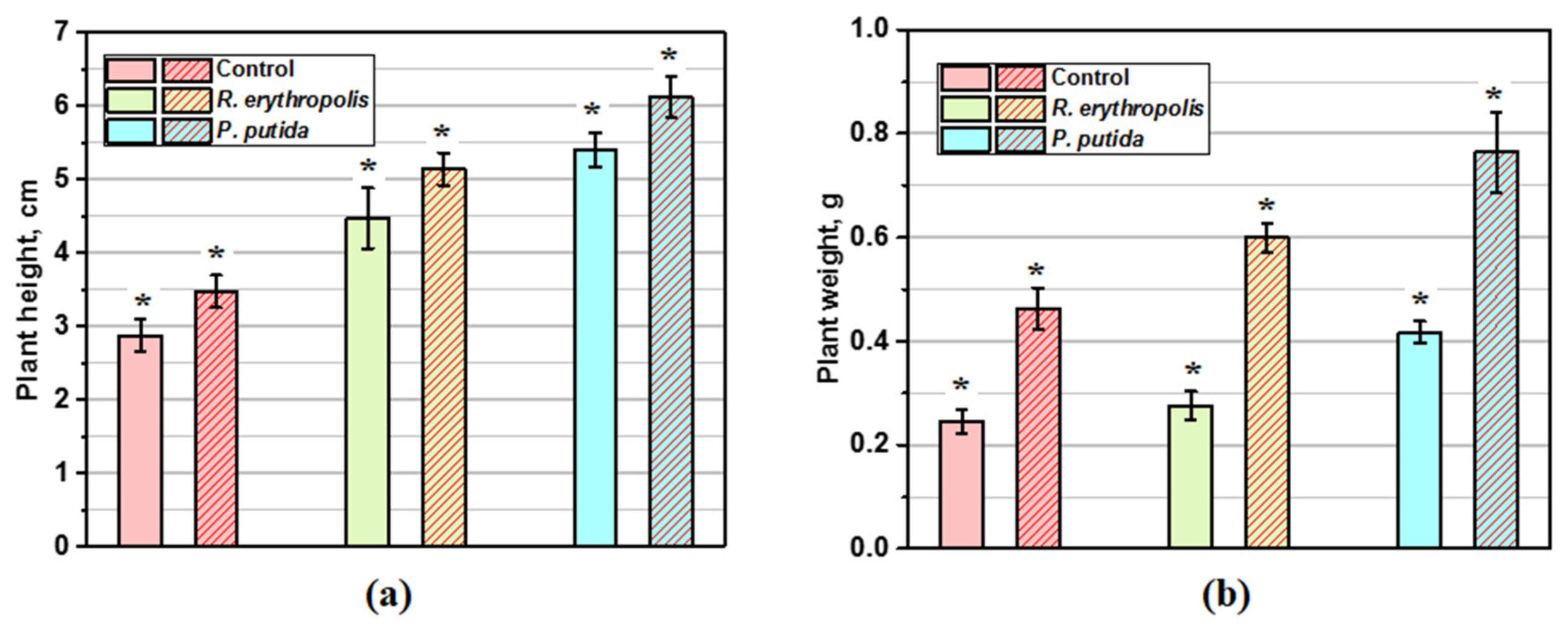
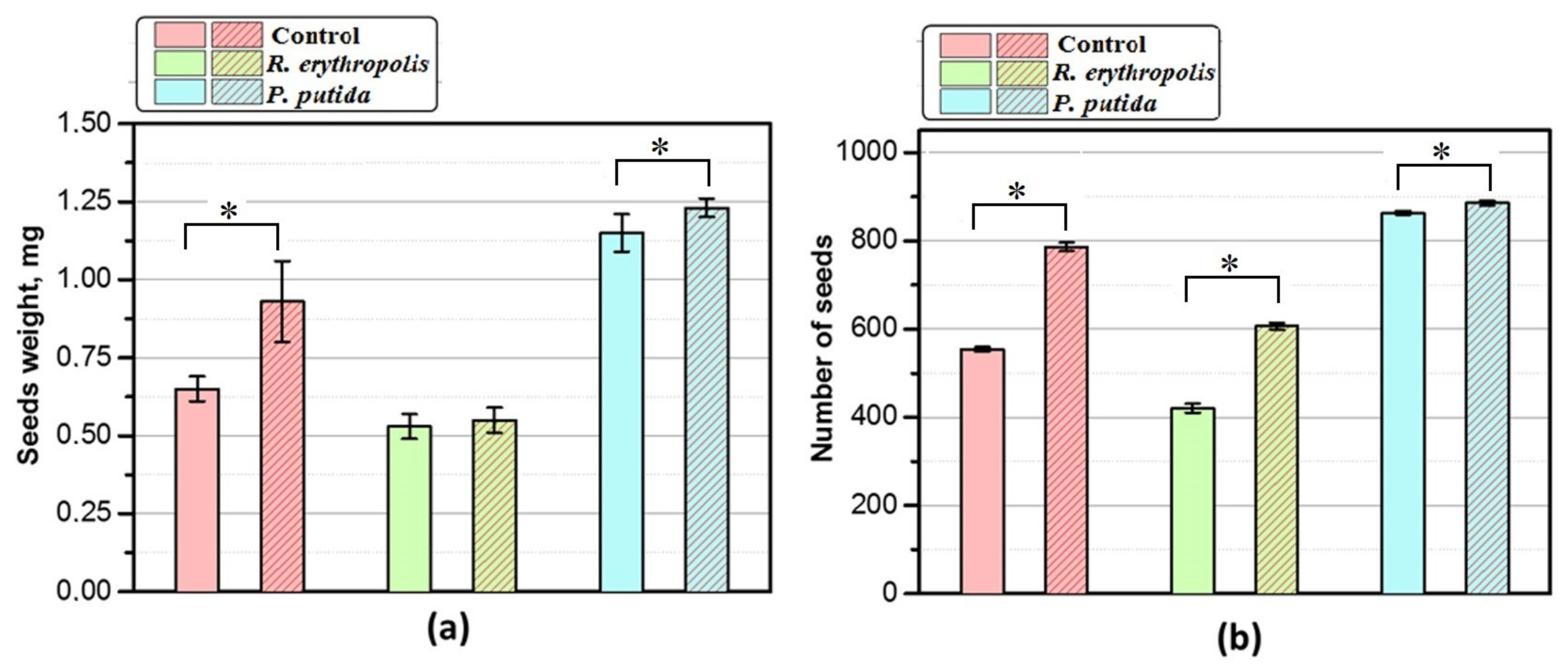
3.6. Plants Productivity Research
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Golovatskaya, I.F.; Raida, V.S.; Leshchuk, R.I.; Karnachuk, R.A.; Minich, A.S.; Bolshakova, S.A.; Prikhodko, S.A.; Usacheva, E.Y.; Burkovskaya, V.T.; Tolstikov, G.A. Physiological and biochemical features of growth and productivity of vegetable crops when growing under light-correctingfilms. Sel’skokhoz. Biol. 2002, 37, 47–51. [Google Scholar]
- Khramov, R.N.; Kreslavski, V.D.; Svidchenko, E.A.; Surin, N.M.; Kosobryukhov, A.A. Influence of photoluminophore-modified agro textile spunbond on growth and photosynthesis of cabbage and lettuce plants. Opt. Express 2019, 28, 31967. [Google Scholar] [CrossRef] [PubMed]
- Khramov, R.; Kosobryukhov, A.; Kreslavski, V.; Balakirev, D.; Khudyakova, A.; Svidchenko, E.; Surin, N.; Ponomarenko, S.; Luponosov, Y. Luminescence of agrotextiles based on red-light-emitting organic luminophore and polypropylene spunbond enhances the growth and photosynthesis of vegetable plants. Front. Plant Sci. 2022, 13, 827679. [Google Scholar] [CrossRef] [PubMed]
- Shoji, S.; Saito, H.; Jitsuyama, Y.; Tomita, K.; Haoyang, O.; Sakurai, Y.; Okazaki, Y.; Aikawa, K.; Sasaki, K.; Fushimi, K.; et al. Plant growth acceleration using a transparent Eu3+-painted UV-to-red conversion film. Sci. Rep. 2022, 12, 17155. [Google Scholar] [CrossRef]
- Raida, V.S.; Ivanitskii, A.S.; Bushkov, A.V.; Andrienko, O.S.; Tolstikov, G.A. Investigation of peculiarities in conversion of the UV and visible sunlight by light transforming films with europium luminophores. Atmos. Ocean. Opt. 2003, 16, 1029–1034. [Google Scholar]
- Huang, X.; Han, S.; Huang, W.; Liu, X. Enhancing solar cell efficiency: The search for luminescent materials as spectral converters. Chem. Soc. Rev. 2013, 42, 173–201. [Google Scholar] [CrossRef] [PubMed]
- Pashkovskiy, P.; Ivanov, Y.; Ivanova, A.; Kreslavski, V.D.; Vereshchagin, M.; Tatarkina, P.; Kuznetsov, V.V.; Allakhverdiev, S.I. Influence of light of different spectral compositions on growth parameters, photosynthetic pigment contents and gene expression in scots pine plantlets. Int. J. Mol. Sci. 2023, 24, 2063. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Los, D.A.; Schmitt, F.J.; Zharmukhamedov, S.K.; Kuznetsov, V.V.; Allakhverdiev, S.I. The impact of the phytochromes on photosynthetic processes. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 400–408. [Google Scholar] [CrossRef]
- Minich, A.S.; Minich, I.B.; Zelen’chukova, N.S.; Karnachuk, R.A.; Golovatskaya, I.F.; Efimova, M.V.; Raida, V.S. The role of low intensity red luminescent radiation in the control of Arabidopsis thaliana morphogenesis and hormonal balance. Russ. J. Plant Physiol. 2006, 53, 762–767. [Google Scholar] [CrossRef]
- He, W.; Chen, S.; Ling, D.; Gan, L.; Xu, Z.G. Effects of red and blue light on potato leaf senescence and tuber-seed yield. Russ. J. Plant Physiol. 2022, 69, 118. [Google Scholar] [CrossRef]
- Xu, J.M.; Liu, Y.; Liu, M.X.; Xu, Z.G. Proteomic, physiological, and anatomical analyses reveal the effects of red, blue, and white light on the growth of potato plantlets under in vitro culture. Rus. J. Plant Physiol. 2022, 69, 139. [Google Scholar] [CrossRef]
- Lu, C.H.; Liu, Y.C.; Hsu, Y.T.; Wang, H.L.; Der Chung, J. Enhancing flower stalk emergence in Phalaenopsis by red light supplementation. Am. J. Plant Sci. 2016, 7, 639–648. [Google Scholar] [CrossRef]
- Novoplansky, A.; Sachs, T.; Cohen, D.; Bar, R.; Bodenheimer, J.; Reisfeld, R. Increasing plant productivity by changing the solar spectrum. Sol. Energy Mater. 1990, 21, 17–23. [Google Scholar] [CrossRef]
- Hemming, S.; van Os, E.A.; Hemming, J.; Dieleman, J.A. The effect of new developed fluorescent greenhouse films on the growth of Fragaria × ananassa “Elsanta”. Eur. J. Hortic. Sci. 2006, 71, 145–154. [Google Scholar]
- Schettini, E.; de Salvador, F.R.; Scarascia-Mugnozza, G.; Vox, G. Radiometric properties of photoselective and photoluminescent greenhouse plastic films and their effects on peach and cherry tree growth. J. Hortic. Sci. Biotechnol. 2011, 86, 79–83. [Google Scholar] [CrossRef]
- Yalçın, R.A.; Ertürk, H. Improving crop production in solar illuminated vertical farms using fluorescence coatings. Biosyst. Eng. 2020, 193, 25–36. [Google Scholar] [CrossRef]
- Kang, J.H.; Yoon, H.I.; Lee, J.M.; Kim, J.P.; Son, J.E. Electron transport and photosynthetic performance in Fragaria × ananassa Duch. acclimated to the solar spectrum modified by a spectrum conversion film. Photosynth. Res. 2021, 151, 31–46. [Google Scholar] [CrossRef]
- Sánchez-Lanuza, M.B.; Menéndez-Velázquez, A.; Peñas-Sanjuan, A.; Navas-Martos, F.J.; Lillo-Bravo, I.; Delgado-Sánchez, J.M. Advanced photonic thin films for solar irradiation tuneability oriented to greenhouse applications. Materials 2021, 14, 2357. [Google Scholar] [CrossRef]
- Castronuovo, D.; Candido, V.; Margiotta, S.; Manera, C.; Miccolis, V.; Basile, M.; D’Addabbo, T. Potential of a corn-starch based biodegradable plastic film for soil solarization. Acta Hortic. 2005, 698, 201–205. [Google Scholar] [CrossRef]
- Tsia, H.Y.; Tsen, W.C.; Shu, Y.C.; Chuang, F.S.; Chen, C.C. Compatibility and characteristics of poly (butylene succinate) and propylene-coethylene copolymer blend. Polym. Test. 2009, 28, 875–885. [Google Scholar] [CrossRef]
- Balakrishna, A.N.; Lakshmipathy, R.; Bagyaraj, D.J.; Ashwin, R. Effect of soil solarization on native AM fungi and microbial biomass. Agric. Res. 2015, 4, 196–201. [Google Scholar] [CrossRef]
- Fernández-Bayo, J.D.; Achmona, Y.; Harrold, D.R.; Claypool, J.T.; Simmons, B.A.; Singer, S.W.; Dahlquist-Willard, R.M.; Stapleton, J.J.; Vander Gheynst, J.S.; Simmons, C.W. Comparison of soil biosolarization with mesophilic and thermophilic solid digestates on soil microbial quantity and diversity. Appl. Soil Ecol. 2017, 119, 183–191. [Google Scholar] [CrossRef]
- Di Mola, I.; Ventorino, V.; Cozzolino, E.; Ottaiano, L.; Romano, I.; Duri, L.G.; Pepe, O.; Mori, M. Biodegradable mulching vs traditional polyethylene film for sustainable solarization: Chemical properties and microbial community response to soil management. Appl. Soil Ecol. 2021, 163, 103921. [Google Scholar] [CrossRef]
- Moreno, M.M.; Moreno, A.; Mancebo, I. Comparison of different mulch materials in a tomato (Solanum lycopersicum L.) crop. Span. J. Agric. Res. 2009, 7, 454–464. [Google Scholar] [CrossRef]
- Waterer, D. Evaluation of biodegradable mulches for production of warm-season vegetable crops. Can. J. Plant Sci. 2010, 90, 737–743. [Google Scholar] [CrossRef]
- Costa, R.; Saraiva, A.; Carvalho, L.; Duarte, E. The use of biodegradable mulch films on strawberry crop in Portugal. Sci. Hortic. 2014, 173, 65–70. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Duri, L.G.; Riccardi, R.; Spigno, P.; Leone, V.; Mori, M. The effect of novel biodegradable films on agronomic performance of zucchini squash grown under open-field and greenhouse conditions. Aust. J. Crop Sci. 2019, 13, 1810. [Google Scholar] [CrossRef]
- Cozzolino, E.; Giordano, M.; Fiorentino, N.; El-Nakhel, C.; Pannico, A.; Di Mola, I.; Mori, M.; Kyriacou, M.C.; Cola, G.; Rouphael, Y. Appraisal of biodegradable mulching films and vegetal-derived biostimulant application as eco-sustainable practices for enhancing lettuce crop performance and nutritive value. Agronomy 2020, 10, 427. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, H.; Jin, T.; Liu, H.; Men, J.; Cai, G.; Cernava, T.; Duan, G.; Jin, D. Biodegradable mulch films significantly affected rhizosphere microbial communities and increased peanut yield. Sci. Total Environ. 2023, 871, 162034. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Schroth, M.N.; Miller, T.D. Effects of rhizosphere colonization by plant growth promoting rhizobacteria on potato plant development and yield. Phytopathology 1980, 70, 1078–1082. [Google Scholar] [CrossRef]
- Welbaum, G.; Sturz, A.V.; Dong, Z.; Nowak, J. Managing soil microorganisms to improve productivity of agroecosystems. Crit. Rev. Plant Sci. 2004, 23, 175–193. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. The role of bacterial indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef] [PubMed]
- Svarovskaya, L.I.; Altunina, L.K.; Filatov, D.A. Biodegradation of petroleum hydrocarbons by soil microflora activated with photoluminescent films. Pet. Chem. 2007, 47, 219–224. [Google Scholar] [CrossRef]
- Svarovskaya, L.I.; Altunina, L.K.; Filatov, D.A. Activation of indigenous micorflora in oil-contaminated soils using photoluminescent films. Appl. Biochem. Microbiol. 2008, 44, 585–589. [Google Scholar] [CrossRef]
- Filatov, D.A.; Svarovskaya, L.I.; Ovsyannikova, V.S.; Altunina, L.K. The effect of film-corrected light on oxygenase activity of microorganisms of the genus Pseudomonas. Microbiology 2011, 80, 158–163. [Google Scholar] [CrossRef]
- Smalla, K.; Heuer, H.; Götz, A.; Niemeyer, D.; Krögerrecklenfort, E.; Tiedze, E. Exogenous isolation of antibiotic resistance plasmids from piggery manure slurries reveals a high prevalence and diversity of IncQ-like plasmids. Appl. Environ. Microbiol. 2000, 66, 4854–4862. [Google Scholar] [CrossRef]
- Habe, H.; Omori, T. Genetics of polycyclic aromatic hydrocarbon metabolism in diverse aerobic bacteria. Biosci. Biotechnol. Biochem. 2003, 67, 225–243. [Google Scholar] [CrossRef]
- Delegan, Y.; Valentovich, L.; Petrikov, K.; Vetrova, A.; Akhremchuk, A.; Akimov, V. Complete genome sequence of Rhodococcus erythropolis X5, a psychrotrophic hydrocarbon-degrading biosurfactant-producing bacterium. Microbiol. Resour. Announc. 2019, 8, e01234-19. [Google Scholar] [CrossRef]
- Abashcheva, M.A.; Horavidi, E.X.; Akatova, E.V.; Nechaeva, I.A.; Babkina, E.E.; Ponamoreva, O.N. Influence of physico-chemical environmental factors on the surface properties of biosurfactant produced by the strain Rhodococcus erythropolis X5. Izv. TulGU 2023, 4, 28–37. [Google Scholar] [CrossRef]
- Zakharchenko, N.S.; Anisimov, S.; Dyadishchev, I.V.; Ponomarenko, S.A.; Robert Khramov, R.N. Effect of light-converting coatings on the growth of Sarepta mustard (Brassica juncea L.) plants colonized by associative microorganisms. BIO Web Conf. 2022, 42, 01024. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. Revised medium for rapid growth and bioassays with tobacco cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.E.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Lab. Press: Cold Spring Harbor, NY, USA, 1989; 1626p. [Google Scholar]
- Luponosov, Y.N.; Solodukhin, A.N.; Balakirev, D.O.; Surin, N.M.; Svidchenko, E.A.; Pisarev, S.A.; Fedorov, Y.V.; Ponomarenko, S.A. Triphenylamine-based luminophores with different side and central aromatic blocks: Synthesis, thermal, photophysical and photochemical properties. Dyes Pigments 2020, 179, 108397. [Google Scholar] [CrossRef]
- Balakirev, D.O.; Solodukhin, A.N.; Peregudova, S.M.; Svidchenko, E.A.; Surin, N.M.; Fedorov, Y.V.; Ponomarenko, S.A.; Luponosov, Y.N. Luminescent push-pull triphenylamine-based molecules end-capped with various electron-withdrawing groups: Synthesis and properties. Dyes Pigments 2023, 208, 110777. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S.L.; Herschberger, N.; Kim, S.H.; Doty, S.L. Diazotrophic endophytes of poplar and willow for growth promotion of rice plants in nitrogen-limited conditions. Crop Sci. 2015, 55, 1765–1772. [Google Scholar] [CrossRef]
- Knapp, D.R. Handbook of Analytical Derivatization Reactions; Wiley-InterScience Publication: Hoboken, NJ, USA, 1979; 768p. [Google Scholar]
- Genty, B.; Harbinson, J.; Cailly, A.L.; Rizza, F. Fate of excitation at PS II in leaves: The non-photochemical side. In Proceedings of the Third BBSRC Robert Hill Symposium on Photosynthesis, University of Sheffield, Department of Molecular Biology and Biotechnology, Western Bank, Sheffield, UK, 31 March–3 April 1996. Abstract no. P28 31. [Google Scholar]
- Tsavkelova, E.A.; Cherdyntseva, T.A.; Botina, S.G.; Netrusov, A.I. Bacteria associated with orchid roots and microbial production of auxin. Microbiol. Res. 2007, 162, 69–76. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Evseeva, N.V.; Tkachenko, O.V.; Burygin, G.L.; Vysotskaya, L.B.; Akhtyamova, Z.A.; Kudoyarova, G.R. Rhizobacteria inoculation effects on phytohormone status of potato microclones cultivated in vitro under osmotic stress. Biomolecules 2020, 10, 1231. [Google Scholar] [CrossRef]
- Kasperbauer, M.J. Phytochrome involvement in regulation of the photosynthetic apparatus and plant adaptation. Plant Physiol. Biochem. 1988, 26, 519–524. [Google Scholar]
- Cao, K.; Yu, J.; Xu, D.; Ai, K.; Bao, E.; Zou, Z. Exposure to lower red to far-red light ratios improve tomato tolerance to salt stress. BMC Plant Biol. 2018, 18, 92. [Google Scholar] [CrossRef]
- Senger, H.; Schmidt, W. Diversity of Photoreceptors. In Photomorphogenesis in Plants, 2nd ed.; Kendrick, R.E., Kronenberg, G.H.M., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 137–158. [Google Scholar] [CrossRef]
- Neff, M.M.; Fankhauser, C.; Chory, J. Light: An indicator of time and place. Genes Dev. 2000, 14, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Devlin, P.F.; Christie, J.M.; Terry, M.J. Many hands make light work. J. Exp. Bot. 2007, 58, 3071–3077. [Google Scholar] [CrossRef] [PubMed]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [CrossRef]
- Demenko, V.I.; Lebedev, V.I. Adaptation of plants obtained in vitro to non-sterile conditions. RSAU–MTAA Bull. 2011, 1, 60–70. [Google Scholar]
- Zakharchenko, N.S.; Furs, O.V.; Rukavtsova, E.B.; Tarlachkov, S.V.; Kosobryukhov, A.A.; Kreslavski, V.D.; Dyachenko, O.V.; Buryanov, Y.I.; Shevchuk, T.V. The Brassica napus L. plants expressing antimicrobial peptide cecropin P1 are safe for colonization by beneficial associative microorganisms. J. Plant Interact. 2021, 16, 432–442. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Martirosyan, Y.T.; Martirosyan, L.Y.; Kosobryukhov, A.A. Dynamic regulation of photosynthetic processes under variable spectral led irradiation of plants. Agric. Biol. 2019, 54, 130–139. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Bunkin, N.F.; Shafeev, G.A.; Astashev, M.E.; Glinushkin, A.P.; Grinberg, M.A.; Vodeneev, V.A. Development and application of photoconversion fluoropolymer films for greenhouses located at high or polar latitudes. J. Photochem. Photobiol. B 2020, 213, 112056. [Google Scholar] [CrossRef]
- Minich, A.S.; Minich, I.B.; Shaitarova, O.V.; Permyakova, N.L.; Zelenchukova, N.S.; Ivanitsky, A.E.; Filatov, D.A.; Ivlev, G.A. Vital activity of Lactuca sativa and soil microorganisms under fluorescent films. Vestn. TGPU 2011, 8, 78–84. [Google Scholar]
- Kusnetsov, S.I.; Leplianin, G.V.; Murinov, Y.I.; Schelokov, R.N.; Karasev, V.E.; Tolstikov, G.A. “Polisvetan”, a high-performance material for cladding greenhouses. Plasticulture 1989, 3, 13–20. [Google Scholar]
- Simakin, A.V.; Ivanyuk, V.V.; Dorokhov, A.S.; Gudkov, S.V. Photoconversion fluoropolymer films for the cultivation of agricultural plants under conditions of insufficient insolation. Appl. Sci. 2020, 10, 8025. [Google Scholar] [CrossRef]
- Kopitar, D.; Marasovic, P.; Jugov, N.; Schwarz, I. Biodegradable nonwoven agrotextile and films. Polymers 2022, 2, 2272. [Google Scholar] [CrossRef]
- Available online: http://www.tnstate.edu/faculty/ablalock/documents/Pot-N-Pot.pdf (accessed on 1 December 2009).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharchenko, N.S.; Rukavtsova, E.B.; Yampolsky, I.V.; Balakirev, D.O.; Dyadishchev, I.V.; Ponomarenko, S.A.; Luponosov, Y.N.; Filonov, A.E.; Mikhailov, P.A.; Zvonarev, A.N.; et al. Effect of Photoluminophore Light-Correcting Coatings and Bacterization by Associative Microorganisms on the Growth and Productivity of Brassica juncea L. Plants. Microbiol. Res. 2024, 15, 1957-1972. https://doi.org/10.3390/microbiolres15040131
Zakharchenko NS, Rukavtsova EB, Yampolsky IV, Balakirev DO, Dyadishchev IV, Ponomarenko SA, Luponosov YN, Filonov AE, Mikhailov PA, Zvonarev AN, et al. Effect of Photoluminophore Light-Correcting Coatings and Bacterization by Associative Microorganisms on the Growth and Productivity of Brassica juncea L. Plants. Microbiology Research. 2024; 15(4):1957-1972. https://doi.org/10.3390/microbiolres15040131
Chicago/Turabian StyleZakharchenko, Natalia S., Elena B. Rukavtsova, Ilia V. Yampolsky, Dmitry O. Balakirev, Ivan V. Dyadishchev, Sergey A. Ponomarenko, Yuriy N. Luponosov, Andrey E. Filonov, Pavel A. Mikhailov, Anton N. Zvonarev, and et al. 2024. "Effect of Photoluminophore Light-Correcting Coatings and Bacterization by Associative Microorganisms on the Growth and Productivity of Brassica juncea L. Plants" Microbiology Research 15, no. 4: 1957-1972. https://doi.org/10.3390/microbiolres15040131





