Introduction of SGLT2 Inhibitors and Variations in Other Disease-Modifying Drugs in Heart Failure Patients: A Single-Centre Real-World Experience
Abstract
:1. Introduction
2. Materials and Methods
3. Results
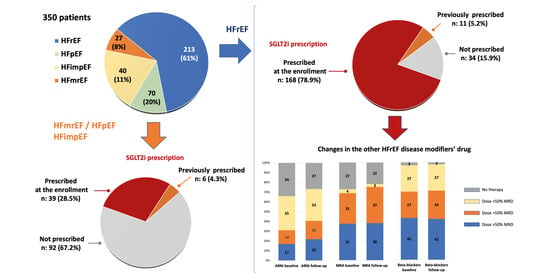
3.1. SGLT2 Inhibitor Therapy
3.2. Changes in Patients with SGLT2 Inhibitor Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; EMPEROR-Reduced Trial Investigators; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; EMPEROR-Preserved Trial Investigators; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; EMPEROR-Preserved Trial Investigators; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Langkilde, A.M.; DELIVER Trial Committees and Investigators; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Vardeny, O.; Fang, J.C.; Desai, A.S.; Jhund, P.S.; Claggett, B.; Vaduganathan, M.; de Boer, R.A.; Hernandez, A.F.; Lam, C.S.P.; Inzucchi, S.E.; et al. Dapagliflozin in heart failure with improved ejection fraction: A prespecified analysis of the DELIVER trial. Nat. Med. 2022, 28, 2504–2511. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; ESC Scientific Document Group; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Iacoviello, M.; Palazzuoli, A.; Gronda, E. Recent advances in pharmacological treatment of heart failure. Eur. J. Clin. Investig. 2021, 51, e13624. [Google Scholar] [CrossRef]
- De Marzo, V.; Savarese, G.; Tricarico, L.; Hassan, S.; Iacoviello, M.; Porto, I.; Ameri, P. Network meta-analysis of medical therapy efficacy in more than 90,000 patients with heart failure and reduced ejection fraction. J. Intern. Med. 2022, 292, 333–349. [Google Scholar] [CrossRef]
- Palmiero, G.; Cesaro, A.; Vetrano, E.; Pafundi, P.C.; Galiero, R.; Caturano, A.; Moscarella, E.; Gragnano, F.; Salvatore, T.; Rinaldi, L.; et al. Impact of SGLT2 Inhibitors on Heart Failure: From Pathophysiology to Clinical Effects. Int. J. Mol. Sci. 2021, 22, 5863. [Google Scholar] [CrossRef]
- Gronda, E.; Vanoli, E.; Iacoviello, M.; Caldarola, P.; Gabrielli, D.; Tavazzi, L. The Benefit of Sodium-Glucose Co-Transporter Inhibition in Heart Failure: The Role of the Kidney. Int. J. Mol. Sci. 2022, 23, 11987. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Wheeler, D.C.; et al. DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2022, 388, 117–127. [Google Scholar]
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Critical Reanalysis of the Mechanisms Underlying the Cardiorenal Benefits of SGLT2 Inhibitors and Reaffirmation of the Nutrient Deprivation Signaling/Autophagy Hypothesis. Circulation 2022, 146, 1383–1405. [Google Scholar] [CrossRef]
- Ferrannini, E.; Baldi, S.; Frascerra, S.; Astiarraga, B.; Heise, T.; Bizzotto, R.; Mari, A.; Pieber, T.R.; Muscelli, E. Faculty Opinions recommendation of Shift to Fatty Substrate Utilization in Response to Sodium-Glucose Cotransporter 2 Inhibition in Subjects Without Diabetes and Patients With Type 2 Diabetes. Diabetes 2016, 65, 1090–1095. [Google Scholar] [CrossRef]
- Fathi, A.; Vickneson, K.; Singh, J.S. SGLT2-inhibitors; more than just glycosuria and diuresis. Heart Fail. Rev. 2021, 26, 623–642. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Zinman, B.; Fitchett, D.; Wanner, C.; Ferrannini, E.; Schumacher, M.; Schmoor, C.; Ohneberg, K.; Johansen, O.E.; George, J.T.; et al. How Does Empagliflozin Reduce Cardiovascular Mortality? Insights From a Mediation Analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2018, 41, 356–363. [Google Scholar] [CrossRef]
- Stolfo, D.; Lund, L.H.; Benson, L.; Lindberg, F.; Ferrannini, G.; Dahlström, U.; Sinagra, G.; Rosano, G.M.; Savarese, G. Real-world use of sodium–glucose cotransporter 2 inhibitors in patients with heart failure and reduced ejection fraction: Data from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 2023. epub ahead of print. [Google Scholar] [CrossRef]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomised, trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. 2021, 27, 387–413. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022, 79, 1757–1780. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Claggett, B.L.; Jhund, P.S.; Cunningham, J.W.; Ferreira, J.P.; Zannad, F.; Packer, M.; Fonarow, G.C.; McMurray, J.J.V.; Solomon, S.D. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: A comparative analysis of three randomised controlled trials. Lancet 2020, 396, 121–128. [Google Scholar] [CrossRef]
- D’Amario, D.; Rodolico, D.; Rosano, G.M.; Dahlström, U.; Crea, F.; Lund, L.H.; Savarese, G. Association between dosing and combination use of medications and outcomes in heart failure with reduced ejection fraction: Data from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 2022, 24, 871–884. [Google Scholar] [CrossRef]
- Vardeny, O.; Claggett, B.; Kachadourian, J.; Desai, A.S.; Packer, M.; Rouleau, J.; Zile, M.R.; Swedberg, K.; Lefkowitz, M.; Shi, V.; et al. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: The PARADIGM-HF trial. Eur. J. Heart Fail. 2019, 21, 337–341. [Google Scholar] [CrossRef]
- Delanaye, P.; Scheen, A.J. The diuretic effects of SGLT2 inhibitors: A comprehensive review of their specificities and their role in renal protection. Diabetes Metab. 2021, 47, 101285. [Google Scholar] [CrossRef]
- ter Maaten, J.M. Unravelling the effect of sacubitril/valsartan on loop diuretic dosing. Eur. J. Heart Fail. 2019, 21, 342–344. [Google Scholar] [CrossRef]
- Belančić, A.; Klobučar, S. Sodium-Glucose Co-Transporter 2 Inhibitors as a Powerful Cardioprotective and Renoprotective Tool: Overview of Clinical Trials and Mechanisms. Diabetology 2023, 4, 251–258. [Google Scholar] [CrossRef]
- Kapelios, C.J.; Laroche, C.; Crespo-Leiro, M.G.; Anker, S.D.; Coats, A.J.; Díaz-Molina, B.; Filippatos, G.; Lainscak, M.; Maggioni, A.P.; Heart Failure Long-Term Registry Investigators Group; et al. Association between loop diuretic dose changes and outcomes in chronic heart failure: Observations from the ESC-EORP Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2020, 22, 1424–1437. [Google Scholar] [CrossRef]


| All Patients | LVEF < 40% (HfrEF) | LVEF 41–49% (HfmrEF or HfimpEF) | LVEF ≥ 50% HfpEF or HfimpEF) | p | |
|---|---|---|---|---|---|
| Number | 350 | 213 | 59 | 78 | |
| Age (years) | 66 ± 12 | 65 ± 12 | 66 ± 11 | 67 ± 13 | 0.448 |
| Males, (%) | 280 (80) | 182 (85) | 48 (81) | 50 (64) | <0.001 |
| De novo HF, n (%) | 16 (5) | 13 (6) | 3 (5) | 0 (0) | 0.086 |
| HfimpEF, n (%) | 70 (20) | - | 32 ((54) | 38 (49) | - |
| Ischemic aetiology, n (%) | 147 (42) | 105 (49) | 22 (37) | 20 (26) | 0.001 |
| Diabetes mellitus, n (%) | 126 (36) | 75 (35) | 22 (37) | 29 (37) | 0.929 |
| Arterial hypertension, n (%) | 230 (66) | 128 (60) | 46 (78) | 56 (72) | 0.019 |
| Atrial fibrillation, n, (%) | 48 (14) | 27 (13) | 7 (12) | 14 (18) | 0.462 |
| NYHA class I, n (%) II, n (%) III, n (%) | 27 (7.7) 193 (55.1) 130 (37.2) | 10 (4.7) 124 (58.2) 79 (37.1) | 4 (6.8) 31 (52.5) 27 (34) | 13 (16.7) 38 (48.7) 27 (34.6) | 0.017 |
| SAP (mm Hg) | 124 ± 19 | 121 ± 18 | 127 ± 22 | 132 ± 18 | 0.043 |
| Heart rate (beats/minute) | 68 ± 12 | 68 ± 12 | 67 ± 9 | 70 ± 16 | 0.356 |
| LVEF (%) | 39 ± 9 | 32 ± 6 | 45 ± 2 | 53 ± 3 | <0.001 |
| Creatinine (mg/dl) | 1.30 ± 0.8 | 1.30 ± 0.6 | 1.17 ± 0.6 | 1.39 ± 1.2 | 0.038 |
| GFR-EPI (mL/min/1.73 m2) | 62.5 ± 24.7 | 61 ± 21 | 71 ± 25 | 61 ± 28 | 0.053 |
| Concomitant therapy at the enrollment | |||||
| ARNi, n (%) | 179 (51) | 139 (51) | 23 (39) | 17 (22) | <0.001 |
| Sacubitril/valsartan dose (mg/die) | 191 ± 134 | 191 ± 124 | 254 ± 137 | 276 ± 139 | 0.006 |
| ACE-I, n (%) | 66 (19) | 33 (15) | 15 (25) | 20 (26) | 0.067 |
| Enalapril equivalent dose (mg/die) | 9.9 ± 6.7 | 9.1 ± 6.7 | 9.6 ± 6.3 | 11.6 ± 7.1 | 0.449 |
| ARB, n (%) | 52 (15) | 20 (9) | 10 (17) | 22 (28) | <0.001 |
| Valsartan equivalent dose (mg/die) | 139 ± 103 | 53 ± 49 | 73 ± 55 | 109 ± 91 | 0.047 |
| Beta-blockers, n (%) | 330 (94) | 207 (97) | 53 (90) | 70 (90) | 0.015 |
| Bisoprolol equivalent dose (mg/die) | 4.8 ± 3.2 | 4.9 ± 3.3 | 4.3 ± 2.6 | 4.8 ± 3.2 | 0.369 |
| MRA, n (%) | 235 (67) | 145 (68) | 39 (66) | 845 (58) | 0.255 |
| MRA dose | 38.4 ± 30.1 | 39.1 ± 29.2 | 34.8 ± 28.2 | 39.2 ± 33.9 | 0.674 |
| Loop diuretics, n (%) | 250 (71) | 156 (73) | 41 (69) | 53 (68) | 0.633 |
| Furosemide equivalent dose (mg/die) | 71 ± 86 | 49 ± 70 | 55 ± 93 | 55 ± 93 | 0.807 |
| ICD and/or CRT, n (%) | 191 (55) | 139 (65) | 26 (44) | 23 (29) | <0.001 |
| SGLT2i | |||||
| Before baseline evaluation, n (%) | 17 (5) | 11 (5) | 3 (5) | 3 (4) | |
| After baseline evaluation, n (%) | 207 (59) | 168 (79) | 24 (41) | 15 (19) | <0.001 |
| Patients with HfrEF | Patients with HfmrEF/HfpEF/HfimpEF | |||||
|---|---|---|---|---|---|---|
| Dapa | Empa | p | Dapa | Empa | p | |
| n: 158 | n: 21 | n: 29 | n: 14 | |||
| Age (years) | 64 ± 11 | 68 ± 10 | 0.161 | 66 ± 11 | 69 ± 10 | 0.309 |
| Males (%) | 84 | 95 | 0.461 | 89 | 64 | 0.149 |
| Weight (kg) | 79 ± 16 | 82 ± 23 | 0.583 | 85 ± 14 | 88 ± 19 | 0.607 |
| NYHA class | 234 ± 0.5 | 2.5 ± 0.6 | 0.087 | 2.3 ± 0.7 | 2.6 ± 0.5 | 0.103 |
| SAP (mmHg) | 120 ± 16 | 121 ± 18 | 0.621 | 122 ± 17 | 135 ± 30 | 0.072 |
| Heart rate (bpm) | 68 ± 11 | 68 ± 8 | 0.940 | 67 ± 11 | 70 ± 14 | 0.481 |
| LVEF (%) | 32 ± 6 | 31 ± 6 | 0.601 | 47 ± 5 | 49 ± 4 | 0.109 |
| Creatinine (mg/dl) | 1.28 ± 0.59 | 1.27 ± 0.26 | 0.910 | 1.09 ± 0.33 | 1.31 ± 0.59 | 0.181 |
| GFR-EPI (mL/min/1.73 m2) | 62 ± 21 | 59 ± 18 | 0.611 | 73 ± 25 | 69 ± 29 | 0.181 |
| Concomitant therapy at baseline | ||||||
| ARNi, % | 69 | 57 | 0.276 | 34 | 14 | 0.166 |
| Sacubitril/valsartan dose (mg/die) | 185 ± 122 | 242 ± 143 | 0.141 | 165 ± 131 | 400 ± 0 | - * |
| ACE-I, % | 15 | 14 | 0.974 | 21 | 21 | 0.955 |
| Enalapril equivalent dose (mg/die) | 8.9 ± 6.7 | 10.4 ± 9.4 | 0.731 | 8.0 ± 7.4 | 20 ± 0 | - † |
| ARB % | 54 | 41 | 0.493 | 27 | 29 | 0.946 |
| Valsartan equivalent dose (mg/die) | 54 ± 52 | 41 ± 38 | 0.688 | 150 ± 108 | 140 ± 133 | 0.891 |
| Beta-blockers (%) | 97 | 100 | 0.461 | 100 | 86 | 0.037 |
| Bisoprolol equivalent dose (mg/die) | 5.1 ± 3.4 | 4.2 ± 2.8 | 0.248 | 4.7 ± 3.1 | 5.2 ± 3.7 | 0.627 |
| MRA % | 75 | 57 | 0.090 | 83 | 64 | 0.179 |
| MRA dose (mg/die) | 42 ± 28 | 50 ± 32 | 0.826 | 43 ± 26 | 50 ± 28 | 0.466 |
| Loop diuretics % | 73 | 100 | 0.006 | 66 | 71 | 0.698 |
| Furosemide equivalent dose (mg/die) | 66 ± 77 | 59 ± 57 | 0.707 | 88 ± 114 | 78 ± 72 | 0.804 |
| All Patients with SGLT2i | Baseline | After | p |
|---|---|---|---|
| Weight (kg) | 80.7 ± 16.7 | 79.6 ± 16.4 | 0.002 |
| NYHA class | 2.4 ± 0.6 | 2.3 ± 0.6 | 0.010 |
| SAP (mmHg) | 122 ± 18 | 118 ± 18 | 0.005 |
| Heart rate (bpm) | 68 ± 11 | 67 ± 10 | 0.463 |
| LVEF (%) | 35 ± 8 | 37 ± 9 | <0.001 |
| Creatinine (mg/dl) | 1.26 ± 0.37 | 1.30 ± 0.45 | 0.044 |
| GFR-EPI (mL/min/1.73 m2) | 61 ± 20 | 61 ± 21 | 0.469 |
| Concomitant therapy | |||
| ARNi, % | 61 | 68 | <0.001 |
| Sacubitril/valsartan dose (mg/die) | 178 ± 133 | 207 ± 130 | <0001 |
| ACE-I, % | 14 | 11 | 0.070 |
| Enalapril equivalent dose (mg/die) | 9.3 ± 7.5 | 8.4 ± 6.6 | 0.213 |
| ARB, % | 14 | 12 | 0.343 |
| Valsartan equivalent dose (mg/die) | 72 ± 72 | 72 ± 76 | 1.00 |
| Beta-blockers, % | 97 | 97 | 1.00 |
| Bisoprolol equivalent dose (mg/die) | 5.1 ± 3.3 | 5.3 ± 3.3 | 0.094 |
| MRA, % | 77 | 79 | 0.522 |
| MRA dose (mg/die) | 43 ± 27 | 43 ± 25 | 0.826 |
| Loop diuretics, % | 75 | 69 | 0.014 |
| Furosemide equivalent dose (mg/die) | 69 ± 79 | 63 ± 106 | 0.309 |
| Patients with HFrEF and SGLT2i (n: 178) | Baseline | After | |
| Weight (kg) | 79.5 ± 16.8 | 78.4 ± 16.4 | 0.007 |
| NYHA class | 2.3 ± 0.6 | 2.2 ± 0.6 | 0.004 |
| SAP (mmHg) | 119 ± 16 | 116 ± 16 | 0.019 |
| Heart rate (bpm) | 68 ± 11 | 67 ± 10 | 0.117 |
| LVEF (%) | 32 ± 6 | 35 ± 8 | <0.001 |
| Creatinine (mg/dl) | 1.28 ± 0.35 | 1.30 ± 0.43 | 0.183 |
| GFR-EPI (mL/min/1.73 m2) | 60 ± 18 | 60 ± 21 | 0.798 |
| Concomitant therapy | |||
| ARNi, % | 68 | 77 | <0.001 |
| Sacubitril/valsartan dose (mg/die) | 175 ± 131 | 206 ± 128 | <0001 |
| ACE-I, % | 14 | 9 | 0.027 |
| Enalapril equivalent dose (mg/die) | 7.7 ± 6.4 | 6.7 ± 5.4 | 0.189 |
| ARB, % | 10 | 8 | 0.289 |
| Valsartan equivalent dose (mg/die) | 70 ± 35 | 67 ± 20 | 0.674 |
| Beta-blockers, % | 98 | 98 | 1.00 |
| Bisoprolol equivalent dose (mg/die) | 5.1 ± 3.3 | 5.2 ± 3.2 | 0.240 |
| MRA, % | 76 | 79 | 0.359 |
| MRA dose (mg/die) | 42 ± 28 | 41 ± 24 | 0.651 |
| Loop diuretics, % | 76 | 69 | 0.009 |
| Furosemide equivalent dose (mg/die) | 63 ± 73 | 59 ± 102 | 0.342 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabella, E.; Correale, M.; Alcidi, G.; Pugliese, R.; Ioannoni, S.; Romano, M.; Palmieri, G.; Di Biase, M.; Brunetti, N.D.; Iacoviello, M. Introduction of SGLT2 Inhibitors and Variations in Other Disease-Modifying Drugs in Heart Failure Patients: A Single-Centre Real-World Experience. Clin. Pract. 2023, 13, 1015-1024. https://doi.org/10.3390/clinpract13050090
Tabella E, Correale M, Alcidi G, Pugliese R, Ioannoni S, Romano M, Palmieri G, Di Biase M, Brunetti ND, Iacoviello M. Introduction of SGLT2 Inhibitors and Variations in Other Disease-Modifying Drugs in Heart Failure Patients: A Single-Centre Real-World Experience. Clinics and Practice. 2023; 13(5):1015-1024. https://doi.org/10.3390/clinpract13050090
Chicago/Turabian StyleTabella, Erika, Michele Correale, Gianmarco Alcidi, Rosanna Pugliese, Sara Ioannoni, Matteo Romano, Gianpaolo Palmieri, Matteo Di Biase, Natale Daniele Brunetti, and Massimo Iacoviello. 2023. "Introduction of SGLT2 Inhibitors and Variations in Other Disease-Modifying Drugs in Heart Failure Patients: A Single-Centre Real-World Experience" Clinics and Practice 13, no. 5: 1015-1024. https://doi.org/10.3390/clinpract13050090
APA StyleTabella, E., Correale, M., Alcidi, G., Pugliese, R., Ioannoni, S., Romano, M., Palmieri, G., Di Biase, M., Brunetti, N. D., & Iacoviello, M. (2023). Introduction of SGLT2 Inhibitors and Variations in Other Disease-Modifying Drugs in Heart Failure Patients: A Single-Centre Real-World Experience. Clinics and Practice, 13(5), 1015-1024. https://doi.org/10.3390/clinpract13050090