Bullous Pemphigoid Occurring after Stopping Imatinib Therapy of CML: Is a Continuation of Post-Treatment Follow-Up Needed?
Abstract
:1. Introduction
2. Case Study
- ∗
- Densely packed marrow.
- ∗
- Myeloid hyperplasia with complete range of maturation.
- ∗
- The biopsy findings were most consistent with CML.
- ∗
- Cytogenetic analysis showed a chromosomal translocation with the karyotype: 46,XY,t(9;22)(q34;q11), known as the Philadelphia Chromosome.
- ∗
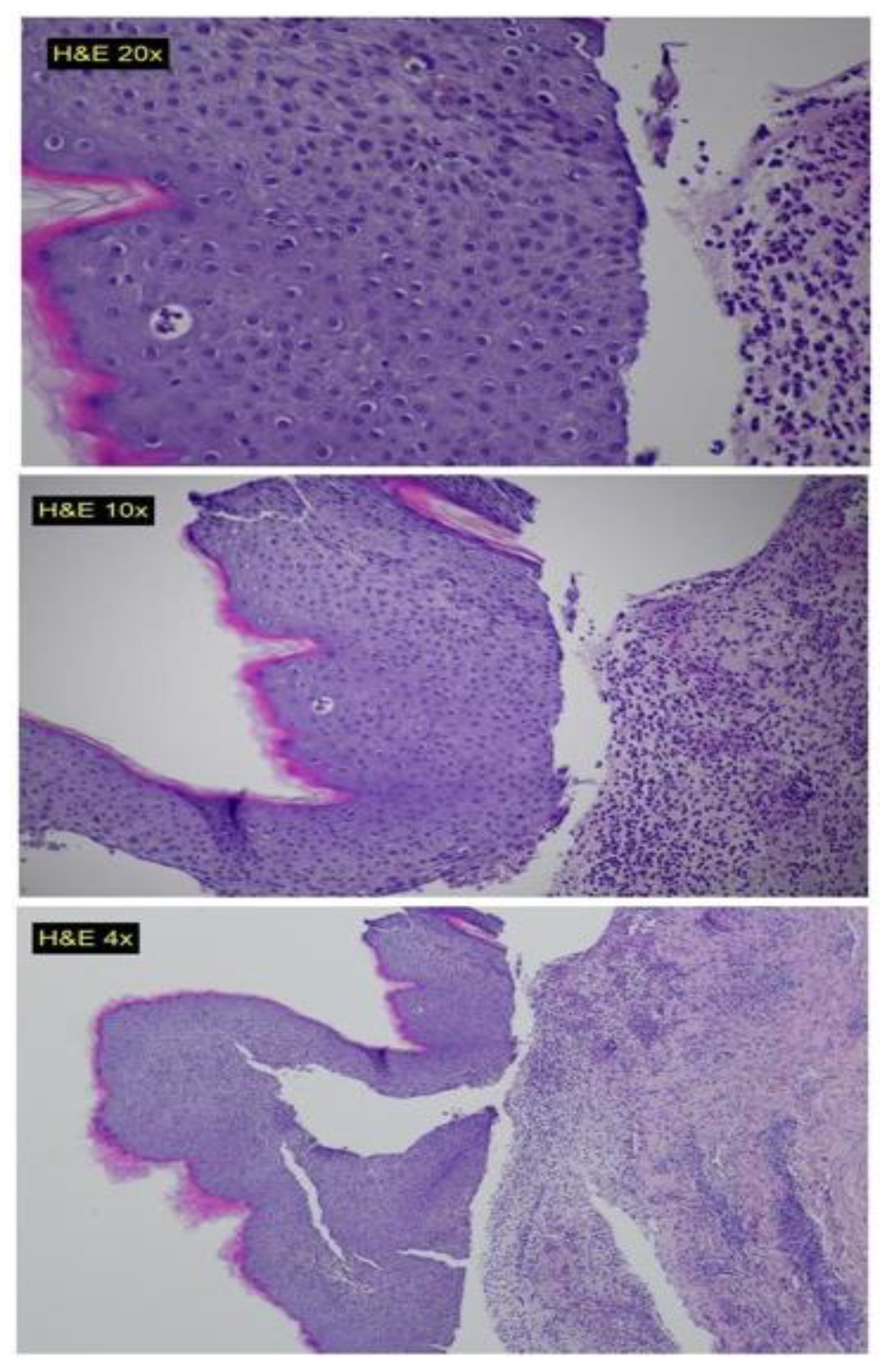
- Sub-epidermal blisters.
- ∗
- Interstitial and perivascular mixed cell inflammatory infiltrate composed of numerous neutrophils, eosinophils and mono-nuclear cells in the upper-dermis.
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quintás-Cardama, A.; Cortes, J.E. Chronic Myeloid Leukemia: Diagnosis and Treatment. Mayo Clin. Proc. 2006, 81, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Hehlmann, R. Chronic Myeloid Leukemia in 2020. HemaSphere 2020, 4, e468. [Google Scholar] [CrossRef]
- Amir, M.; Javed, S. A Review on the Therapeutic Role of TKIs in Case of CML in Combination with Epigenetic Drugs. Front. Genet. 2021, 12, 742802. [Google Scholar] [CrossRef]
- Bennour, A.; Saad, A.; Sennana, H. Chronic myeloid leukemia: Relevance of cytogenetic and molecular assays. Crit. Rev. Oncol./Hematol. 2016, 97, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Reddy, E.P.; Aggarwal, A.K. The Ins and Outs of Bcr-Abl Inhibition. Genes Cancer 2012, 3, 447–454. [Google Scholar] [CrossRef]
- Thompson, P.A.; Kantarjian, H.; Cortes, J.E. Diagnosis and Treatment of Chronic Myeloid Leukemia (CML) in 2015. Mayo Clin. Proc. 2015, 90, 1440–1454. [Google Scholar] [CrossRef] [PubMed]
- Chrobák, L.; Voglová, J. Imatinib Mesylate (STI 571)—A New Oral Target Therapy for Chronic Myelogenous Leukemia (CML). Acta Med. 2019, 46, 85–89. [Google Scholar] [CrossRef]
- Sood, A.; Sinha, P.; Raman, D.; Sinha, A. Imatinib-induced IgA Pemphigus: Subcorneal Pustular Dermatosis Type. Indian Dermatol. Online J. 2018, 9, 331–333. [Google Scholar] [CrossRef]
- Valeyrie, L.; Bastuji-Garin, S.; Revuz, J.; Bachot, N.; Wechsler, J.; Berthaud, P.; Tulliez, M.; Giraudier, S. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukemias: A prospective study of 54 patients. J. Am. Acad. Dermatol. 2003, 48, 201–206. [Google Scholar] [CrossRef]
- Kershenovich, R.; Hodak, E.; Mimouni, D. Diagnosis and classification of pemphigus and bullous pemphigoid. Autoimmun. Rev. 2014, 13, 477–481. [Google Scholar] [CrossRef]
- Shalata, W.; Weissmann, S.; Itzhaki Gabay, S.; Sheva, K.; Abu Saleh, O.; Jama, A.A.; Yakobson, A.; Rouvinov, K. A Retrospective, Single-Institution Experience of Bullous Pemphigoid as an Adverse Effect of Immune Checkpoint Inhibitors. Cancers 2022, 14, 5451. [Google Scholar] [CrossRef] [PubMed]
- Etienne, G.; Guilhot, J.; Rea, D.; Rigal-Huguet, F.; Nicolini, F.; Charbonnier, A.; Guerci-Bresler, A.; Legros, L.; Varet, B.; Gardembas, M.; et al. Long-Term Follow-Up of the French Stop Imatinib (STIM1) Study in Patients with Chronic Myeloid Leukemia. J. Clin. Oncol. 2017, 35, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Druker, B.J.; Talpaz, M.; Resta, D.J.; Peng, B.; Buchdunger, E.; Ford, J.M.; Lydon, N.B.; Kantarjian, H.; Capdeville, R.; Ohno-Jones, S.; et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001, 344, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Larson, R.A.; Guilhot, F.; Radich, J.P.; Branford, S.; Hughes, T.P.; Baccarani, M.; Deininger, M.W.; Cervantes, F.; Fujihara, S.; et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N. Engl. J. Med. 2017, 376, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Puttini, M.; Coluccia, A.M.; Boschelli, F.; Cleris, L.; Marchesi, E.; Donella-Deana, A.; Ahmed, S.; Redaelli, S.; Piazza, R.; Magistroni, V.; et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res. 2006, 66, 11314–11322. [Google Scholar] [CrossRef] [PubMed]
- Vinay, K.; Yanamandra, U.; Dogra, S.; Handa, S.; Suri, V.; Kumari, S.; Khadwal, A.; Prakash, G.; Lad, D.; Varma, S.; et al. Long-term mucocutaneous adverse effects of imatinib in Indian chronic myeloid leukemia patients. Int. J. Dermatol. 2018, 57, 332–338. [Google Scholar] [CrossRef]
- Tanvetyanon, T.; Nand, S. Overcoming recurrent cutaneous reactions from imatinib using once-weekly dosing. Ann. Pharmacother. 2003, 37, 1818–1820. [Google Scholar] [CrossRef]
- Hwang, J.-E.; Yoon, J.-Y.; Bae, W.-K.; Shim, H.-J.; Cho, S.-H.; Chung, I.-J. Imatinib induced severe skin reactions and neutropenia in a patient with gastrointestinal stromal tumor. BMC Cancer 2010, 10, 438. [Google Scholar] [CrossRef]
- Larson, R.A.; Druker, B.J.; Guilhot, F.; O’Brien, S.G.; Riviere, G.J.; Krahnke, T.; Gathmann, I.; Wang, Y. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: A subanalysis of the IRIS study. Blood 2008, 111, 4022–4028. [Google Scholar] [CrossRef]
- Deininger, M.W.; O’Brien, S.G.; Ford, J.M.; Druker, B.J. Practical management of patients with chronic myeloid leukemia receiving imatinib. J. Clin. Oncol. 2003, 21, 1637–1647. [Google Scholar] [CrossRef]
- Hsiao, L.-T.; Chung, H.-M.; Lin, J.-T.; Chiou, T.-J.; Liu, J.-H.; Fan, F.S.; Wang, W.-S.; Yen, C.-C.; Chen, P.-M. Stevens-Johnson syndrome after treatment with STI571: A case report. Br. J. Haematol. 2002, 117, 620–622. [Google Scholar] [CrossRef]
- Scott, L.C.; White, J.D.; Reid, R.; Cowie, F. Management of Skin Toxicity Related to the Use of Imatinib Mesylate (STI571, Glivectrade mark) for Advanced Stage Gastrointestinal Stromal Tumours. Sarcoma 2005, 9, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Mughal, T.I.; Schrieber, A. Principal long-term adverse effects of imatinib in patients with chronic myeloid leukemia in chronic phase. Biologics 2010, 4, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.; Micallef-Eynaud, P.; Douglas, W.S.; Murphy, J.A.; Hay, I.; Holyoake, T.L.; Drummond, M.W. A spectrum of skin reactions caused by the tyrosine kinase inhibitor imatinib mesylate (sti 571, glivec®). Br. J. Haematol. 2003, 120, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Aleem, A. Hypopigmentation of the skin due to imatinib mesylate in patients with chronic myeloid leukemia. Hematol./Oncol. Stem Cell Ther. 2009, 2, 358–361. [Google Scholar] [CrossRef]
- Pretel-Irazabal, M.; Tuneu-Valls, A.; Ormaechea-Pérez, N. Adverse skin effects of imatinib, a tyrosine kinase inhibitor. Actas Dermo-Sifiliogr. 2014, 105, 655–662. [Google Scholar] [CrossRef]
- Hughes, A.; Clarson, J.; Tang, C.; Vidovic, L.; White, D.L.; Hughes, T.P.; Yong, A.S.M. CML patients with deep molecular responses to TKI have restored immune effectors and decreased PD-1 and immune suppressors. Blood 2017, 129, 1166–1176. [Google Scholar] [CrossRef]
- Jackson, S.R.; Koestenbauer, J.; Carroll, A.P.; Oo, T.H.; Chou, S.; Indrajit, B. Paraneoplastic bullous pemphigoid—A sign of clear cell renal carcinoma. Urol. Case Rep. 2020, 30, 101119. [Google Scholar] [CrossRef]
- Patsatsi, A.; Murrell, D.F. Bruton Tyrosine Kinase Inhibition and Its Role as an Emerging Treatment in Pemphigus. Front. Med. 2021, 8, 708071. [Google Scholar] [CrossRef]
- Murrell, D.F.; Patsatsi, A.; Stavropoulos, P.; Baum, S.; Zeeli, T.; Kern, J.S.; Roussaki-Schulze, A.V.; Sinclair, R.; Bassukas, I.D.; Thomas, D.; et al. Proof of concept for the clinical effects of oral rilzabrutinib, the first Bruton tyrosine kinase inhibitor for pemphigus vulgaris: The phase II BELIEVE study. Br. J. Dermatol. 2021, 185, 745–755. [Google Scholar] [CrossRef]
| Parameters [Normal Range (Units)] | Results |
|---|---|
| White blood cells [4.8 to 10.8 (103 cells/μL)] | 75 |
| Eosinophil’s [1 to 3%] | 5.3% |
| Basophils [0 to 1.5%] | 6.8% |
| Blasts [%] | 0.73 |
| Platelets [130 to 400 (103/μL)] | 173 |
| Hemoglobin [14 to 18 (g/dL)] | 8.9 |
| Lactate dehydrogenase [150 to 480 (U/L)] | 1373 |
| Alanine aminotransferase [0 to 41 (U/L)] | 156 |
| Gamma-glutamyl transferase [0 to 49 (U/L)] | 295 |
| Aspartate aminotransferase [0 to 37 (U/L)] | 76 |
| Total Bilirubin [0 to 1.1 (mg/dL)] | 3.4 |
| IgG | positive + 2 shining—linearly within the region of the basal membrane |
| IgA | positive + 1 shining—linearly within the region of the basal membrane |
| IgM | negative |
| C3 | Positive + 4 shining—linearly within the region of the basal membrane |
| Fibrinogen | Positive + 2 shining—linearly within the region of the basal membrane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakobson, A.; Neime, A.E.; Abu Saleh, O.; Al Athamen, K.; Shalata, W. Bullous Pemphigoid Occurring after Stopping Imatinib Therapy of CML: Is a Continuation of Post-Treatment Follow-Up Needed? Clin. Pract. 2023, 13, 1082-1089. https://doi.org/10.3390/clinpract13050096
Yakobson A, Neime AE, Abu Saleh O, Al Athamen K, Shalata W. Bullous Pemphigoid Occurring after Stopping Imatinib Therapy of CML: Is a Continuation of Post-Treatment Follow-Up Needed? Clinics and Practice. 2023; 13(5):1082-1089. https://doi.org/10.3390/clinpract13050096
Chicago/Turabian StyleYakobson, Alexander, Ala Eddin Neime, Omar Abu Saleh, Kayed Al Athamen, and Walid Shalata. 2023. "Bullous Pemphigoid Occurring after Stopping Imatinib Therapy of CML: Is a Continuation of Post-Treatment Follow-Up Needed?" Clinics and Practice 13, no. 5: 1082-1089. https://doi.org/10.3390/clinpract13050096
APA StyleYakobson, A., Neime, A. E., Abu Saleh, O., Al Athamen, K., & Shalata, W. (2023). Bullous Pemphigoid Occurring after Stopping Imatinib Therapy of CML: Is a Continuation of Post-Treatment Follow-Up Needed? Clinics and Practice, 13(5), 1082-1089. https://doi.org/10.3390/clinpract13050096







