Passability of Potamodromous Species through a Fish Lift at a Large Hydropower Plant (Touvedo, Portugal)
Abstract
:1. Introduction
2. Materials and Methods
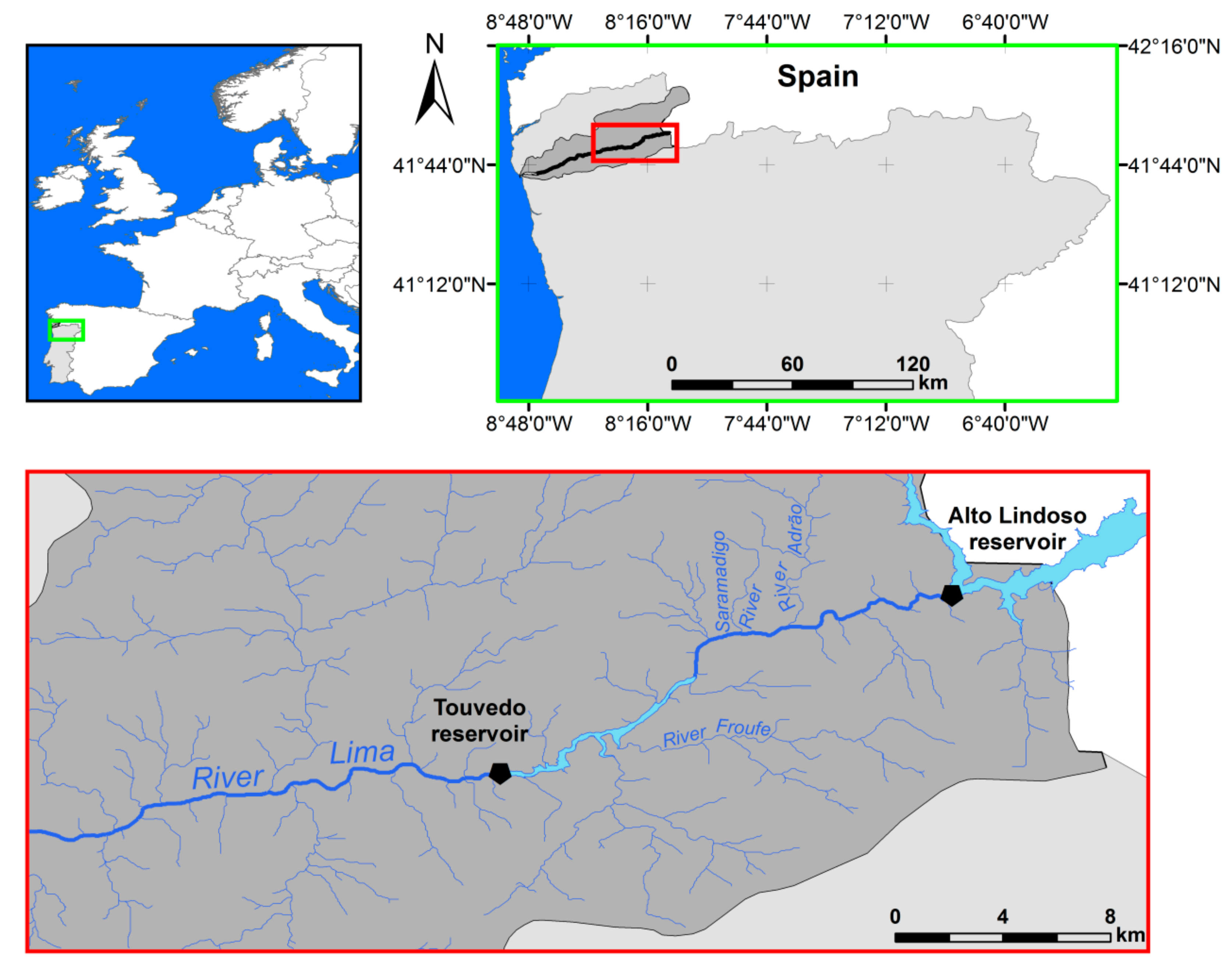
2.1. Study Area
2.2. Fish Passage through the Lift
2.3. Fish Catches Downstream
2.4. Data Analyses
3. Results
3.1. Seasonal Fish Counts in the Lift
3.2. Daily Patterns of Fish Passage
3.3. Effects of Environmental Variables on Fish Passability
3.4. Fish Passage in Relation to Peak-Flow Magnitudes
3.5. Fish Lift Selectivity
3.6. Fish Lift Efficacy
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2005, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.; Liermann, C.R.A.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.; Segurado, P.; Santos, J.M.; Pinheiro, P.; Ferreira, M.T. Does longitudinal connectivity loss affect the distribution of freshwater fish? Ecol. Eng. 2012, 48, 70–78. [Google Scholar] [CrossRef]
- Fullerton, A.H.; Burnett, K.M.; Steel, E.A.; Flitcroft, R.L.; Pess, G.R.; Feist, B.E.; Torgersen, C.E.; Miller, D.J.; Sanderson, B.L. Hydrological connectivity for riverine fish: Measurement challenges and research opportunities. Freshw. Biol. 2010, 55, 2215–2237. [Google Scholar] [CrossRef] [Green Version]
- Maceda-Veiga, A. Towards the conservation of freshwater fish: Iberian Rivers as an example of threats and management practices. Rev. Fish Biol. Fish. 2012, 23, 1–22. [Google Scholar] [CrossRef]
- Boavida, I.; Santos, J.M.; Ferreira, T.; Pinheiro, A. Barbel habitat alterations due to hydropeaking. J. Hydro-Environ. Res. 2015, 9, 237–247. [Google Scholar] [CrossRef]
- Hayes, D.; Moreira, M.; Boavida, I.; Haslauer, M.; Unfer, G.; Zeiringer, B.; Greimel, F.; Auer, S.; Ferreira, T.; Schmutz, S. Life Stage-Specific Hydropeaking Flow Rules. Sustainability 2019, 11, 1547. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.M.; Ferreira, M.T.; Pinheiro, A.N.; Bochechas, J.H. A simple method for assessing minimum flows in regulated rivers: The case of sea lamprey reproduction. Aquat. Conserv. 2004, 14, 481–489. [Google Scholar] [CrossRef]
- Santos, J.M.; Godinho, F.; Ferreira, M.T.; Cortes, R. The organisation of fish assemblages in the regulated Lima basin, Northern Portugal. Limnologica 2004, 34, 224–235. [Google Scholar] [CrossRef] [Green Version]
- Bruder, A.; Tonolla, D.; Schweizer, S.P.; Vollenweider, S.; Langhans, S.D.; Wüest, A. A conceptual framework for hydropeaking mitigation. Sci. Total Environ. 2016, 568, 1204–1212. [Google Scholar] [CrossRef]
- Capra, H.; Plichard, L.; Bergé, J.; Pella, H.; Ovidio, M.; McNeil, E.; Lamouroux, N. Fish habitat selection in a large hydropeaking river: Strong individual and temporal variations revealed by telemetry. Sci. Total Environ. 2017, 578, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Enders, E.C.; Watkinson, D.A.; Ghamry, H.; Mills, K.H.; Franzin, W.G. Fish age and size distributions and species composition in a large, hydropeaking Prairie River. River Res. Appl. 2017, 33, 1246–1256. [Google Scholar] [CrossRef]
- Schmutz, S.; Bakken, T.H.; Friedrich, T.; Greimel, F.; Harby, A.; Jungwirth, M.; Melcher, A.; Unfer, G.; Zeiringer, B. Response of Fish Communities to Hydrological and Morphological Alterations in Hydropeaking Rivers of Austria. River Res. Appl. 2014, 31, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Baras, E.; Lucas, M. Impacts of man’s modifications of river hydrology on the migration of freshwater fishes: A mechanistic perspective. Ecohydrol. Hydrobiol. 2001, 1, 291–304. [Google Scholar]
- Benitez, J.P.; Matondo, B.N.; Dierckx, A.; Ovidio, M. An overview of potamodromous fish upstream movements in medium-sized rivers, by means of fish passes monitoring. Aquat. Ecol. 2015, 49, 481–497. [Google Scholar] [CrossRef]
- Benitez, J.P.; Dierckx, A.; Matondo, B.N.; Rollin, X.; Ovidio, M. Movement behaviours of potamodromous fish within a large anthropised river after the reestablishment of the longitudinal connectivity. Fish. Res. 2018, 207, 140–149. [Google Scholar] [CrossRef]
- Roscoe, D.W.; Hinch, S.G. Effectiveness monitoring of fish passage facilities: Historical trends, geographic patterns and future directions. Fish Fish. 2010, 11, 12–33. [Google Scholar] [CrossRef]
- Clay, C.H. Design of Fishways and Other Fish Facilities, 2nd ed.; Lewis Publishers: Boca Raton, FL, USA, 1995; 256p. [Google Scholar]
- Food and Agriculture Organization (FAO); Deutsche Vereinigung für Wasserwirtschaft, Abwasser und Abfall (DVWK). Fish Passes—Design, Dimensions and Monitoring; FAO: Rome, Italy, 2002; 119p. [Google Scholar]
- Santos, J.M.; Silva, A.; Katopodis, C.; Pinheiro, P.; Pinheiro, A.; Bochechas, J.; Ferreira, M.T. Ecohydraulics of pool-type fishways: Getting past the barriers. Ecol. Eng. 2012, 48, 38–50. [Google Scholar] [CrossRef]
- Larinier, M. Biological factors to be taken into account in the design of fishways, the concept of obstruction to upstream migration. Bull. Fr. Pêche Piscic. 2002, 364, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Allan, J.D.; Castillo, M.M. Stream Ecology—Structure and Function of Running Waters; Springer: Dordrecht, The Netherlands, 2007; 436p. [Google Scholar] [CrossRef]
- Croze, O.; Bau, F.; Delmouly, L. Efficiency of a fish lift for returning Atlantic salmon at a large-scale hydroelectric complex in France. Fish. Manag. Ecol. 2008, 15, 467–476. [Google Scholar] [CrossRef]
- Schletterer, M.; Reindl, R.; Thonhauser, S. Options for re-establishing river continuity, with an emphasis on the special solution “fish lift”: Examples from Austria. Rev. Eletrônica Gestão Tecnol. Ambient. 2016, 4, 109. [Google Scholar] [CrossRef] [Green Version]
- Travade, F.; Larinier, M. Fish locks and fish lifts. Bull. Fr. Pêche Piscic. 2002, 364, 102–118. [Google Scholar] [CrossRef]
- Noonan, M.J.; Grant, J.W.A.; Jackson, C.D. A quantitative assessment of fish passage efficiency. Fish Fish. 2011, 13, 450–464. [Google Scholar] [CrossRef]
- Pompeu, P.D.S.; Martinez, C.B. Efficiency and selectivity of a trap and truck fish passage system in Brazil. Neotrop. Ichthyol. 2007, 5, 169–176. [Google Scholar] [CrossRef]
- Larinier, M.; Chanseau, M.; Bau, F.; Croze, O. The use of radio telemetry for optimizing fish pass design. In Aquatic Telemetry: Advances and Applications, Proceedings of the Fifth Conference on Fish Telemetry held in Ustica, Italy, 9–13 June 2003; FAO/COISPA: Rome, Italy, 2005. [Google Scholar]
- Santos, J.M.; Rivaes, R.; Oliveira, J.; Ferreira, T. Improving yellow eel upstream movements with fish lifts. J. Ecohydraulics 2016, 1, 50–61. [Google Scholar] [CrossRef]
- Santos, J.M.; Ferreira, M.T.; Godinho, F.N.; Bochechas, J. Performance of fish lift recently built at the Touvedo Dam on the Lima River, Portugal. J. Appl. Ichthyol. 2002, 18, 118–123. [Google Scholar] [CrossRef]
- Morán-López, R.; Uceda Tolosa, O. Image techniques in turbid rivers: A ten-year assessment of cyprinid stocks composition and size. Fish. Res. 2017, 195, 186–193. [Google Scholar] [CrossRef]
- Benitez, J.P.; Ovidio, M. The influence of environmental factors on the upstream movements of rheophilic cyprinids according to their position in a river basin. Ecol. Freshw. Fish 2017, 27, 660–671. [Google Scholar] [CrossRef]
- Katopodis, C.; Williams, J.G. The development of fish passage research in a historical context. Ecol. Eng. 2012, 48, 8–18. [Google Scholar] [CrossRef]
- Nieminen, E.; Hyytiäinen, K.; Lindroos, M. Economic and policy considerations regarding hydropower and migratory fish. Fish Fish. 2016, 18, 54–78. [Google Scholar] [CrossRef] [Green Version]
- Boavida, I.; Jesus, J.B.; Pereira, V.; Santos, C.; Lopes, M.; Cortes, R.M.V. Fulfilling spawning flow requirements for potamodromous cyprinids in a restored river segment. Sci. Total Environ. 2018, 635, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.T.; Sousa, L.; Santos, J.M.; Reino, L.; Oliveira, J.; Almeida, P.R.; Cortes, R.V. Regional and local environmental correlates of native Iberian fish fauna. Ecol. Freshw. Fish 2007, 16, 504–514. [Google Scholar] [CrossRef]
- Lucas, M.C.; Mercer, T.; Peirson, G.; Frear, P.A. Seasonal movements of coarse fish in lowland rivers and their relevance to fisheries management. In Management and Ecology of River Fisheries; Cowx, I.G., Ed.; Fishing New Books: Oxford, UK, 2000; pp. 87–100. [Google Scholar]
- Lucas, M.C.; Frear, P.A. Effects of a flow-gauging weir on the migratory behaviour of adult barbel, a riverine cyprinid. J. Fish Biol. 1997, 50, 382–396. [Google Scholar] [CrossRef]
- Doadrio, I.; Perea, S.; Garzón-Heydt, P.; González, J.L. Ictiofauna Continental Española. Bases Para Su Seguimiento; D.G. Medio Natural y Política Forestal; MARM: Madrid, Spain, 2011; 616p.
- Rodriguez-Ruiz, A.; Granado-Lorencio, C. Spawning period and migration of three species of cyprinids in a stream with Mediterranean regimen (SW Spain). J. Fish Biol. 1992, 41, 545–556. [Google Scholar] [CrossRef]
- Santos, J.M.; Ferreira, M.T.; Godinho, F.N.; Bochechas, J. Efficacy of a nature-like bypass channel in a Portuguese lowland river. J. Appl. Ichthyol. 2005, 21, 381–388. [Google Scholar] [CrossRef]
- Ovidio, M.; Baras, E.; Goffaux, D.; Giroux, F.; Philippart, J.C. Seasonal variations of activity pattern of brown (Salmo trutta) in a small stream, as determined by radio-telemetry. Hydrobiologia 2002, 470, 195–202. [Google Scholar] [CrossRef]
- García-Vega, A.; Sanz-Ronda, F.J.; Fuentes-Pérez, J.F. Seasonal and daily upstream movements of brown trout Salmo trutta in an Iberian regulated river. Knowl. Manag. Aquat. Ecosyst. 2017, 418, 9. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.M.; Oliveira, J.M.; Rivaes, R.; Pizarro, R.A.; Ferreira, M.T.; Pádua, J.; Marin, C. Plano de Ação para a Otimização do Ascensor de Peixes do Aproveitamento Hidroelétrico de Touvedo; Relatório Final; Instituto Superior de Agronomia da Universidade de Lisboa e EDP Labelec: Lisboa, Portugal, 2014; 142p. [Google Scholar]
- Jonsson, N. Influence of water flow, water temperature and light on fish migration in rivers. Nordic J. Freshw. Res. 1991, 66, 20–35. [Google Scholar]
- Bunt, C.M.; Castro-Santos, T.; Haro, A. Performance of fish passage structures at upstream barriers to migration. River Res. Appl. 2012, 28, 457–478. [Google Scholar] [CrossRef]
- Larinier, M. Fish passage experience at small-scale hydro-electric power plants in France. Hydrobiologia 2008, 609, 97–108. [Google Scholar] [CrossRef]
- Laine, A.; Jokivirta, T.; Katopodis, C. Atlantic salmon, Salmo salar L., and sea trout, Salmo trutta L., passage in a regulated northern river—Fishway efficiency, fish entrance and environmental factors. Fish. Manag. Ecol. 2002, 9, 65–77. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; 498p. [Google Scholar] [CrossRef]
- Plichard, L.; Capra, H.; Mons, R.; Pella, H.; Lamouroux, N. Comparing electrofishing and snorkelling for characterizing fish assemblages over time and space. Can. J. Fish. Aquat. Sci. 2017, 74, 75–86. [Google Scholar] [CrossRef]
- Santos, J.M.; Pinheiro, P.J.; Ferreira, M.T.; Bochechas, J. Monitoring fish passes using infrared beaming: A case study in an Iberian river. J. Appl. Ichthyol. 2008, 24, 26–30. [Google Scholar] [CrossRef]
- De Leeuw, J.J.; Winter, H.V. Migration of rheophilic fish in the large lowland rivers Meuse and Rhine, the Netherlands. Fish. Manag. Ecol. 2008, 15, 409–415. [Google Scholar] [CrossRef]
- Ovidio, M.; Capra, H.; Philippart, J.C. Field protocol for assessing small obstacles to migration of brown trout Salmo trutta, and European grayling Thymallus thymallus: A contribution to the management of free movement in rivers. Fish. Manag. Ecol. 2007, 14, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Granado-Lorencio, C. Fish species ecology in Spanish freshwater ecosystems. Limnetica 1992, 8, 255–261. [Google Scholar]
- Flodmark, L.E.W.; Vollestad, L.A.; Forseth, T. Performance of juvenile brown trout exposed to fluctuating water level and temperature. J. Fish Biol. 2004, 65, 460–470. [Google Scholar] [CrossRef]
- Alexandre, C.M.; Quintella, B.R.; Ferreira, A.F.; Romão, F.A.; Almeida, P.R. Swimming performance and ecomorphology of the Iberian barbel Luciobarbus bocagei (Steindachner, 1864) on permanent and temporary rivers. Ecol. Freshw. Fish 2014, 23, 244–258. [Google Scholar] [CrossRef]
- Vilizzi, L.; Copp, G.H. An analysis of 0+ barbel (Barbus barbus) response to discharge fluctuations in a flume. River Res. Appl. 2005, 21, 421–438. [Google Scholar] [CrossRef]
- Webb, J.; Hawkins, A.D. The movement and spawning behaviour of adult salmon in the Girnock Burn, a tributary of the Aberdeenshire Dee, 1986. In Scotish Fisheries Research Report; Department of Agriculture and Fisheries for Scotland: Edinburgh, Scotland, 1989; Volume 40, 41p. [Google Scholar]
- Fraser, D.F.; Gilliam, J.F.; Akkara, J.T.; Albanese, B.W.; Snider, S.B. Night feeding by guppies under predator release: Effects on growth and daytime courtship. Ecology 2004, 85, 312–319. [Google Scholar] [CrossRef]
- Lobón-Cerviá, J.; Rincón, P.A. Environmental determinants of recruitment and their influence on the population dynamics of stream-living brown trout Salmo trutta. Oikos 2004, 105, 641–646. [Google Scholar] [CrossRef]
- Piecuch, J.; Lojkasek, B.; Lusk, S.; Marek, T. Spawning migration of brown trout, Salmo trutta in the Morávka reservoir. Folia Zool. 2007, 56, 201–212. [Google Scholar]
- Santos, J.M.; Reino, L.; Porto, M.; Oliveira, J.; Pinheiro, P.; Almeida, P.R.; Cortes, R.; Ferreira, M.T. Complex size-dependent habitat associations in potamodromous fishspecies. Aquat. Sci. 2010, 73, 233–245. [Google Scholar] [CrossRef]
- Romão, F.; Quintella, B.R.; Pereira, T.J.; Almeida, P.R. Swimming performance of two Iberian cyprinids: The Tagus nase Pseudochondrostoma polylepis (Steindachner, 1864) and the bordallo Squalius carolitertii (Doadrio, 1988). J. Appl. Ichthyol. 2012, 28, 26–30. [Google Scholar] [CrossRef]
- Plaut, I. Critical swimming speed: Its ecological relevance. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 131, 41–50. [Google Scholar] [CrossRef]
- Silva, A.T.; Santos, J.M.; Ferreira, M.T.; Pinheiro, A.N.; Katopodis, C. Effects of water velocity and turbulence on the behaviour of Iberian barbel (Luciobarbus bocagei, Steindachner 1864) in an experimental pool-type fishway. River Res. Appl. 2011, 27, 360–373. [Google Scholar] [CrossRef]
- Eyler, S.M.; Welsh, S.A.; Smith, D.R.; Rockey, M.M. Downstream Passage and Impact of Turbine Shutdowns on Survival of Silver American Eels at Five Hydroelectric Dams on the Shenandoah River. Trans. Am. Fish. Soc. 2016, 145, 964–976. [Google Scholar] [CrossRef]
- Song, C.; Omalley, A.; Roy, S.G.; Barber, B.L.; Zydlewski, J.; Mo, W. Managing dams for energy and fish tradeoffs: What does a win-win solution take? Sci. Total Environ. 2019, 669, 833–843. [Google Scholar] [CrossRef]
- Mateus, C.S.; Quintella, B.R.; Almeida, P.R. The critical swimming speed of Iberian barbel Barbus bocagei in relation to size and sex. J. Fish Biol. 2008, 73, 1783–1789. [Google Scholar] [CrossRef]
- Tudorache, C.; Viaene, P.; Blust, R.; Vereecken, H.; De Boek, G. A comparison of swimming capacity and energy use in seven European freshwater fish species. Ecol. Freshw. Fish 2008, 17, 284–291. [Google Scholar] [CrossRef]
- Amaral, S.D.; Branco, P.; da Silva, A.T.; Katopodis, C.; Viseu, T.; Ferreira, M.T.; Pinheiro, A.N.; Santos, J.M. Upstream passage of potamodromous cyprinids over small weirs: The influence of key-hydraulic parameters. J. Ecohydraulics 2016, 1, 79–89. [Google Scholar] [CrossRef]
- Amaral, S.D.; Branco, P.; Romão, F.; Viseu, T.; Ferreira, M.T.; Pinheiro, A.N.; Santos, J.M. The effect of weir crest width and discharge on passage performance of a potamodromous cyprinid. Mar. Freshw. Res. 2018, 69, 1795–1804. [Google Scholar] [CrossRef]
- Cooke, S.J.; Hinch, S.G. Improving the reliability of fishway attraction and passage efficiency estimates to inform fishway engineering, science, and practice. Ecol. Eng. 2013, 58, 123–132. [Google Scholar] [CrossRef]
- Teixeira-de-Mello, F.; Kristensen, E.A.; Meerhoff, M.; González-Bergonzoni, I.; Baattrup-Pedersen, A.; Iglesias, C.; Kristensen, P.B.; Mazzeo, N.; Jeppesen, E. Monitoring fish communities in wadeable lowland streams: Comparing the efficiency of electrofishing methods at contrasting fish assemblages. Environ. Monit. Assess. 2013, 186, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.C.; Baras, E. Methods for studying spatial behaviour of freshwater fishes in the natural environment. Fish Fish. 2000, 1, 283–316. [Google Scholar] [CrossRef]
- Eberstaller, J.; Hinterhofer, M.; Parasiewicz, P. The effectiveness of two nature-like bypass channels in an upland Austrian river. In Fish Migration and Fish Bypasses; Jungwirth, M., Schmutz, S., Weiss, S., Eds.; Fishing News Books: Oxford, UK, 1998; pp. 363–383. [Google Scholar]
- INAG-Instituto da Água. Desenvolvimento de um Indice de Qualidade para a Fauna Piscícola; Ministério da Agricultura, Mar, Ambiente e Ordenamento do Território: Lisbon, Portugal, 2012; 17p.
- Castro-Santos, T.; Cotel, A.; Webb, P.W. Fishway evaluations for better bioengineering—An integrative approach. Am. Fish. Soc. Symp. 2009, 69, 557–575. [Google Scholar]
- Pelicice, F.M.; Agostinho, A.A. Fish-Passage Facilities as Ecological Traps in Large Neotropical Rivers. Conserv. Biol. 2008, 22, 180–188. [Google Scholar] [CrossRef]





| Variable | ß | F-Test | p-Value | D |
|---|---|---|---|---|
| P. duriense | 1.127 | |||
| Water temperature | 0.466 | 22.425 | <0.001 | |
| Flow variation | 0.228 | 4.894 | 0.029 | |
| Photoperiod | 0.198 | 3.955 | 0.049 | |
| L. bocagei | 1.812 | |||
| Water temperature | 0.167 | 7.138 | 0.008 | |
| Mean daily flow | 0.155 | 7.733 | 0.006 | |
| Acumulated rainfall | 0.276 | 12.818 | <0.001 | |
| S. trutta fario | 1.996 | |||
| Mean daily flow | 0.151 | 3.941 | 0.049 |
| Barbel | Nase | Trout | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Month | Fish Lift | Electr. Catch | Ratio | Fish Lift | Electr. Catch | Ratio | Fish Lift | Electr. Catch | Ratio |
| N Day−1 | N Unit Effort−1 | N Day−1 | N Unit Effort−1 | N Day−1 | N Unit Effort−1 | ||||
| Apr | 0.70 | 0 | (a) | 0.13 | 5 | 0.03 | 0.43 | 5 | 0.09 |
| May | 1.52 | 0 | (a) | 0.42 | 1 | 0.42 | 0.16 | 0 | (a) |
| Jun | 1.36 | 2 | 0.68 | 0.50 | 18 | 0.03 | 0.03 | 1 | 0.03 |
| Jul | 0.77 | 0 | (a) | 1.26 | 13 | 0.10 | 0.06 | 1 | 0.06 |
| Aug | 2.80 | 0 | (a) | 23.30 | 30 | 0.75 | 0.07 | 2 | 0.03 |
| Sep | 3.21 | 1 | 3.10 | 13.76 | 31 | 0.43 | 0.45 | 3 | 0.14 |
| Oct | 4.03 | 12 | 0.34 | 4.03 | 34 | 0.47 | 0.35 | 1 | 0.35 |
| Nov | 1.34 | 1 | 1.30 | 1.34 | 28 | 0.09 | 0.14 | 2 | 0.07 |
| Dec | 0.62 | 0 | (a) | 0.62 | 29 | 0.03 | 0.10 | 9 | 0.01 |
| Mean | 1.82 | 1.78 | 1.36 | 5.04 | 21.00 | 0.26 | 0.20 | 2.67 | 0.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mameri, D.; Rivaes, R.; Oliveira, J.M.; Pádua, J.; Ferreira, M.T.; Santos, J.M. Passability of Potamodromous Species through a Fish Lift at a Large Hydropower Plant (Touvedo, Portugal). Sustainability 2020, 12, 172. https://doi.org/10.3390/su12010172
Mameri D, Rivaes R, Oliveira JM, Pádua J, Ferreira MT, Santos JM. Passability of Potamodromous Species through a Fish Lift at a Large Hydropower Plant (Touvedo, Portugal). Sustainability. 2020; 12(1):172. https://doi.org/10.3390/su12010172
Chicago/Turabian StyleMameri, Daniel, Rui Rivaes, João M. Oliveira, João Pádua, Maria T. Ferreira, and José M. Santos. 2020. "Passability of Potamodromous Species through a Fish Lift at a Large Hydropower Plant (Touvedo, Portugal)" Sustainability 12, no. 1: 172. https://doi.org/10.3390/su12010172
APA StyleMameri, D., Rivaes, R., Oliveira, J. M., Pádua, J., Ferreira, M. T., & Santos, J. M. (2020). Passability of Potamodromous Species through a Fish Lift at a Large Hydropower Plant (Touvedo, Portugal). Sustainability, 12(1), 172. https://doi.org/10.3390/su12010172









