Influence of Temperature, Relative Humidity and Protein Content on the Growth and Development of Larvae of the Lesser Mealworm, Alphitobius diaperinus (Panzer)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Bioassays Series I: Effect of Temperature and Relative Humidity
2.3. Bioassays Series II: Effect of Protein Content in the Diet
2.4. Statistical Analysis
3. Results
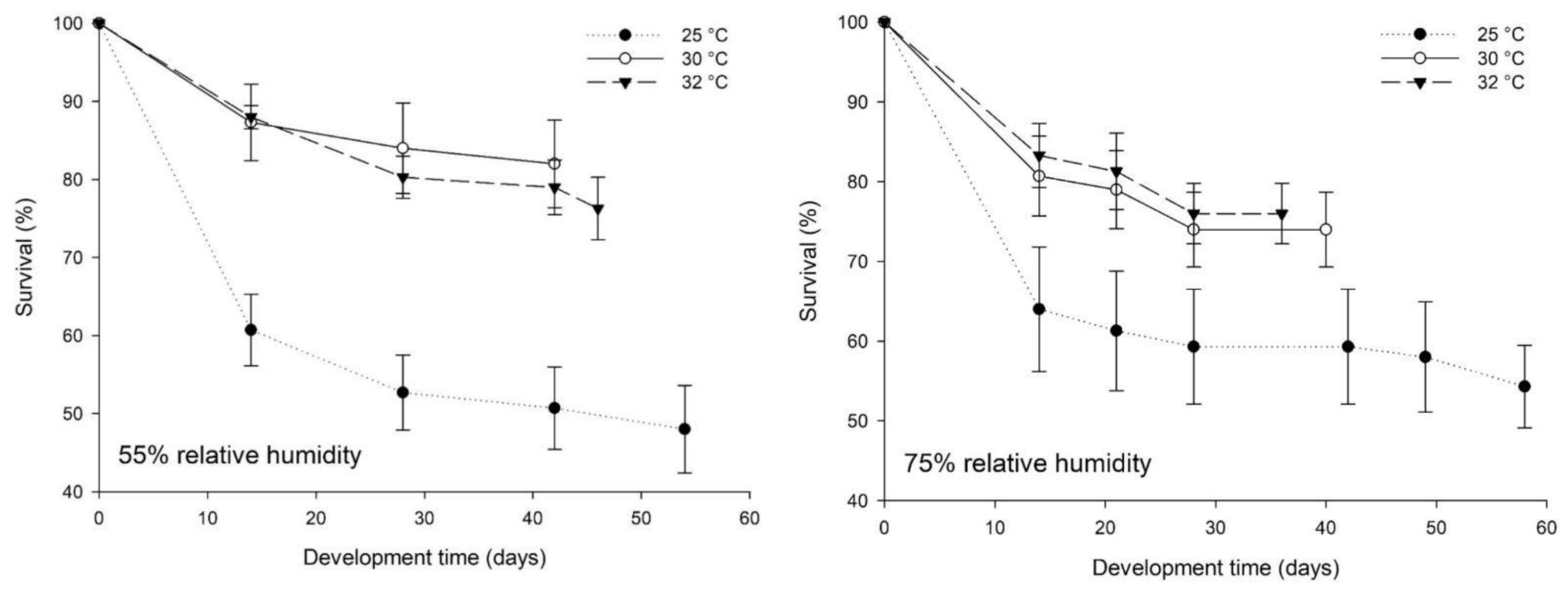
3.1. Bioassays Series I: Effect of Temperature and Relative Humidity
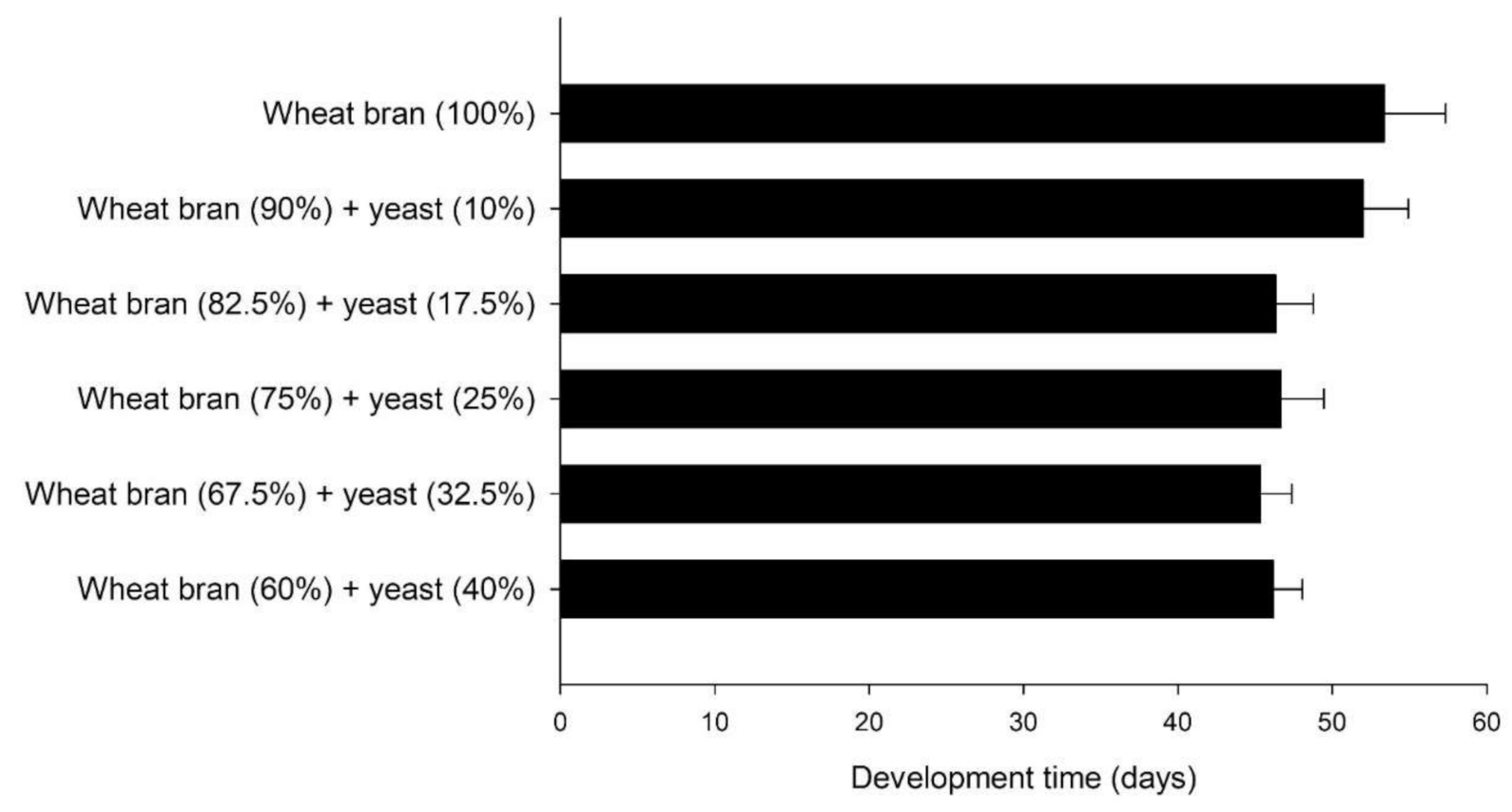
3.2. Bioassays Series II: Effect of Protein Content in the Diet
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jongema, Y. List of Edible Insect Species of the World; Laboratory of Entomology: Wageningen, The Netherlands, 2017. [Google Scholar]
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schluter, O.K. Nutritional Composition and Safety Aspects of Edible Insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Gasco, L.; Biancarosa, I.; Liland, N.S. From Waste to Feed: A Review of Recent Knowledge on Insects as Producers of Protein and Fat for Animal Feeds. Curr. Opin. Green Sustain. Chem. 2020, 23, 67–79. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Athanassiou, C.G. Insects as Food and Feed: If You Can’t Beat Them, Eat Them!—To the Magnificent Seven and Beyond. J. Insect Sci. 2021, 21, 1–5. [Google Scholar] [CrossRef] [PubMed]
- EU Commission Regulation 2017/893 of 24 May 2017 amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as Regards the Provisions on Processed Animal Protein. Available online: https://eur–lex.europa.eu/legal–content/EN/TXT/PDF/?uri=CELEX:32017R0893&from=EN (accessed on 5 October 2021).
- EU Commission Regulation 2021/1372 of 17 August 2021 amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as Regards the Prohibition to Feed Non-Ruminant Farmed Animals, Other Than fur Animals, with Protein Derived from Animals. Available online: https://eur–lex.europa.eu/legal–content/EN/TXT/PDF/?uri=CELEX:32021R1372&from=EN (accessed on 5 October 2021).
- European Food Safety Authority (EFSA); NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens); Turck, D.J.; Castenmiller, S.; De Henauw, K.I.; Hirsch-Ernst, J.; Kearney, A.; Maciuk, I.; Mangelsdorf, H.J.; McArdle, A.; et al. Scientific Opinion on the Safety of Dried Yellow Mealworm (Tenebrio molitor larva) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, 6343. [Google Scholar]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Athanassiou, C.G. The Lesser Mealworm Alphitobius diaperinus: A Noxious Pest or a Promising Nutrient Source? Rev. Aquac. 2019, 11, 1418–1437. [Google Scholar] [CrossRef]
- Adámková, A.; Kouřimská, L.; Borkovcová, M.; Kulma, M.; Mlček, J. Nutritional Values of Edible Coleoptera (Tenebrio molitor, Zophobas morio and Alphitobius diaperinus) Reared in the Czech Republic. Potravinarstvo 2016, 10, 663–671. [Google Scholar]
- Ricciardi, C.; Baviera, C. Role of Carbohydrates and Proteins in Maximizing Productivity in Alphitobius diaperinus (Coleoptera: Tenebrionidae). Redia XCIX 2016, 99, 97–105. [Google Scholar]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; Van Huis, A.; Van Boekel, M.A.J.S. Extraction and Characterization of Protein Fractions from Five Insect Species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Van Broekhoven, S.; Oonincx, D.G.A.B.; Van Huis, A.; Van Loon, J.J.A. Growth Performance and Feed Conversion Efficiency of Three Edible Mealworm Species (Coleoptera: Tenebrionidae) on Diets Composed of Organic Byproducts. J. Insec. Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chung, T.H.; Park, H.C.; Shin, M.J.; Park, I.G.; Choi, I.H. Effects of Diet Composition on Growth Performance and Feed Conversion Efficiency in Alphitobius diaperinus Larvae. J. Entomol. Acarol. Res. 2019, 51, 7761. [Google Scholar] [CrossRef]
- Soetemans, L.; Gianotten, N.; Bastiaens, L. Agri-Food Side-Stream Inclusion in the diet of Alphitobius diaperinus. Part 2: Impact on Larvae Composition. Insects 2020, 11, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianotten, N.; Soetemans, L.; Bastiaens, L. Agri-Food Side-Stream Inclusions in the Diet of Alphitobius diaperinus Part 1: Impact on Larvae Growth Performance Parameters. Insects 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumbos, C.I.; Bliamplias, D.; Gourgouta, M.; Michail, V.; Athanassiou, C.G. Rearing Tenebrio molitor and Alphitobius diaperinus Larvae on Seed Cleaning Process Byproducts. Insects 2021, 12, 293. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Guadalupe Rojas, M.; Shapiro-IIan, D.I.; Tedders, W.L. Use of Nutrient Self-Selection as a Diet Refining Tool in Tenebrio molitor (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2013, 48, 206–221. [Google Scholar] [CrossRef]
- Rho, M.S.; Lee, K.P. Geometric Analysis of Nutrient Balancing in the Mealworm Beetle, Tenebrio molitor L. (Coleoptera: Tenebrionidae). J. Insect Physiol. 2014, 71, 37–45. [Google Scholar] [CrossRef]
- Cammack, J.A.; Tomberlin, J.K. The Impact of Diet Protein and Carbohydrate on Select Life-History Traits of the Black Soldier Fly Hermetia illucens (L.) (Diptera: Stratiomyidae). Insects 2017, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of Rearing Substrate on Growth Performance, Waste Reduction Efficiency and Chemical Composition of Black Soldier Fly (Hermetia illucens) Larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef] [PubMed]
- Waldbauer, G.P. The Consumption and Utilization of Food by Insects. Adv. Insect Phys. 1968, 5, 229–288. [Google Scholar]
- Oonincx, D.G.A.B.; Van Broekhoven, S.; Van Huis, A.; Van Loon, J.J.A. Feed Conversion, Survival and Development, and Composition of Four Insect Species on Diets Composed of Food By-Products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [Green Version]
- Zar, H.J. Biostatistical Analysis; Prentice Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Cossins, A.R.; Bowler, K. Temperature Biology of Animals; Chapman and Hall: New York, NY, USA, 1987. [Google Scholar]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of Size and Temperature on Metabolic Rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef] [Green Version]
- Wilson, T.H.; Miner, E.D. Influence of Temperature on Development of the Lesser Mealworm, Alphitobius diaperinus. J. Kansas Entomol. Soc. 1969, 42, 294–303. [Google Scholar]
- Rueda, L.M.; Axtell, R.C. Temperature-Dependent Development and Survival of the Lesser Mealworm, Alphitobius diaperinus. Med. Vet. Entomol. 1996, 10, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Bjørge, J.D.; Overgaard, J.; Maltea, H.; Gianotten, N.; Heckmann, L. Role of Temperature on Growth and Metabolic Rate in the Tenebrionid Beetles Alphitobius diaperinus and Tenebrio molitor. J. Insect Physiol. 2018, 107, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Axtell, R.C. Biology and economic importance of the darkling beetle in poultry houses. In Proceedings of the North Carolina State University Poultry Supervisors’ Short Course, Raleigh, NC, USA, 19 April 1994; pp. 8–17. [Google Scholar]
- Davis, G.R.F. Essential Dietary Amino Acids for Growth of Larvae of the Yellow Mealworm, Tenebrio molitor L. J. Nutr. 1975, 105, 1071–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraenkel, G. The Nutrition of the Mealworm, Tenebrio molitor L. (Tenebrionidae, Coleoptera). Physiol. Zool. 1950, 23, 92–108. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Oonincx, D.G.A.B.; Karapanagiotidis, I.T.; Vrontaki, M.; Gourgouta, M.; Asimaki, A.; Mente, E.; Athanassiou, C.G. Agricultural Byproducts from Greece as Feed for Yellow Mealworm Larvae: Circular Economy at Local Level. J. Insects Food Feed. 2021, in press. [Google Scholar] [CrossRef]
- Cohen, A.C. Insect Diets: Science and Technology; CRC Press: London, UK, 2003. [Google Scholar]
- Murray, D.R.P. The Importance of Water in the Normal Growth of Larvae of Tenebrio molitor. Entomol. Exp. Appl. 1968, 11, 149–168. [Google Scholar] [CrossRef]
- Phaff, H.J.; Miller, M.W.; Mark, E. The Life Yeasts, 2nd ed.; Harvard University Press: Cambridge, CA, USA; London, UK, 1978; p. 341. [Google Scholar]
- Sacakli, P.; Koksal, B.H.; Ergun, A.; Ozsoy, B. Usage of Brewer’s Yeast (Saccharomyces cerevisiae) as a Replacement of Vitamin and Trace Mineral Premix in Broiler Diets. Rev. Méd. Vét. 2013, 64, 39–44. [Google Scholar]
- All About Feed: Yeast Probiotics to Feed Insects. Available online: https://www.allaboutfeed.net/all–about/new–proteins/yeast–probiotics–to–feed–insects (accessed on 6 August 2021).
- Richard, N.; Rabot, R.; Beutin, C.; van Loon, J.J.A. Live Yeast Probiotic Can Boost Growth Performance of Yellow Meal Worm and Black Soldier Fly Larvae. In Proceedings of the Insectinov3: Insect Production for Human and Animal Nutrition, Paris, France, 26–28 November 2019. [Google Scholar]




| Source | df | FCR | SGR | Total Produced Larval Biomass | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Corrected model | 5 | 7.1 | <0.001 | 7.3 | <0.001 | 7.4 | <0.001 |
| Intercept | 1 | 390.3 | <0.001 | 1464.8 | <0.001 | 711.3 | <0.001 |
| Temperature | 2 | 16.2 | <0.001 | 13.0 | <0.001 | 14.9 | <0.001 |
| Relative humidity | 1 | 0.6 | 0.440 | 5.3 | 0.028 | 1.0 | 0.317 |
| Temperature × Relative humidity | 2 | 1.3 | 0.298 | 2.4 | 0.106 | 3.0 | 0.066 |
| Temperature/ Relative Humidity | Feed Conversion Ratio (FCR) | Specific Growth Rate (SGR, %) | Total Produced Larval Biomass (mg) |
|---|---|---|---|
| 25 °C–55% | 4.3 ± 0.6 a | 8.8 ± 0.3 c | 317.8 ± 37.9 b |
| 30 °C–55% | 2.2 ± 0.2 b | 11.6 ± 1.0 abc | 589.3 ± 58.1 a |
| 32 °C–55% | 2.0 ± 0.2 b | 11.0 ± 0.8 bc | 654.4 ± 55.0 a |
| 25 °C–75% | 3.9 ± 0.5 a | 9.4 ± 0.8 bc | 483.7 ± 58.3 ab |
| 30 °C–75% | 3.1 ± 0.3 ab | 11.9 ± 0.4 ab | 513.0 ± 46.1 ab |
| 32 °C–75% | 2.2 ± 0.1 b | 14.2 ± 0.7 a | 688.8 ± 38.2 a |
| Substrate | Feed Conversion Ratio (FCR) | Specific Growth Rate (SGR, %) | Total Produced Larval Biomass (mg) |
|---|---|---|---|
| Wheat bran (100%) | 3.1 ± 0.7 a | 14.7 ± 0.5 c | 384.4 ± 58.6 b |
| Wheat bran (90%) + yeast (10%) | 2.1 ± 0.2 ab | 15.5 ± 0.7 bc | 491.5 ± 49.4 ab |
| Wheat bran (82.5%) + yeast (17.5%) | 2.2 ± 0.3 ab | 16.8 ± 0.7 abc | 493.8 ± 54.5 ab |
| Wheat bran (75%) + yeast (25%) | 2.3 ± 0.4 ab | 17.5 ± 0.5 ab | 503.3 ± 75.9 ab |
| Wheat bran (67.5%) + yeast (32.5%) | 1.6 ± 0.1 b | 18.5 ± 0.4 a | 660.1 ± 43.7 a |
| Wheat bran (60%) + yeast (40%) | 1.5 ± 0.1 b | 18.3 ± 0.5 a | 683.1 ± 38.3 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsou, K.; Rumbos, C.I.; Baliota, G.V.; Gourgouta, M.; Athanassiou, C.G. Influence of Temperature, Relative Humidity and Protein Content on the Growth and Development of Larvae of the Lesser Mealworm, Alphitobius diaperinus (Panzer). Sustainability 2021, 13, 11087. https://doi.org/10.3390/su131911087
Kotsou K, Rumbos CI, Baliota GV, Gourgouta M, Athanassiou CG. Influence of Temperature, Relative Humidity and Protein Content on the Growth and Development of Larvae of the Lesser Mealworm, Alphitobius diaperinus (Panzer). Sustainability. 2021; 13(19):11087. https://doi.org/10.3390/su131911087
Chicago/Turabian StyleKotsou, Konstantina, Christos I. Rumbos, Georgia V. Baliota, Marina Gourgouta, and Christos G. Athanassiou. 2021. "Influence of Temperature, Relative Humidity and Protein Content on the Growth and Development of Larvae of the Lesser Mealworm, Alphitobius diaperinus (Panzer)" Sustainability 13, no. 19: 11087. https://doi.org/10.3390/su131911087








