1. Introduction
Industrial workers constantly face health risks at work, one of which is exposure to toxic and harmful gases such as nitrogen dioxide, ammonia (NH
3), hydrogen sulfide (H
2S), methane (CH
4), and carbon monoxide (CO) [
1,
2,
3]. CO is an odorless and colorless gas that can lead to poisoning and explosions; it is a product of the incomplete combustion of organic materials and can be emitted from automobile exhausts and industrial sources [
4,
5]. When exposed to CO gas, hemoglobin in the body’s red blood cells binds with CO gas molecules, which prevents oxygen molecules from being transported to the body’s cells leading to oxygen deprivation [
6,
7]. The NIOSH recommended exposure limit for CO gas is 35 ppm or 40 mg/m
3 for an 8-h time-weighted average and 1200 ppm for the immediately dangerous to life or health value [
8]. Therefore, early detection of CO gas in trace amounts is crucial in preventing adverse effects [
2].
One of the methods for detecting toxic and harmful gases is utilizing a gas sensor, which is preferred over other methods due to its fast response-recovery time, sensitivity, and repeatability [
9]. An ideal gas sensor should have the best sensitivity to its target gas, which depends on the surface-to-volume ratio and the depletion layer width. A high specific surface area provides a large number of areas and channels that enhance interaction between the nanostructure and the test gas hence increasing sensitivity [
10,
11]. Meanwhile, the depletion layer width depends on the band gaps of the sensor material and the intrinsic nature of specific gases.
ZnO is an
n-type semiconductor material, and this feature makes it suitable for detecting oxidizing and reducing gases [
12,
13]. ZnO gas sensors use different mechanisms to sense different types of gases. When ZnO is exposed to oxidizing gases, gas molecules interact directly with the chemisorbed oxygen species on its surface, which causes the adsorbates to become reduced. On the other hand, reducing gases interact with the adsorbed oxygen species on ZnO’s surface and cause a decrease in the sensor’s electrical resistance, as well as decreasing the width of the depletion layer width due to the transfer of free electrons from the oxygen species on the sensor to the conduction band [
13,
14,
15]. Although the ZnO sensor has its apparent advantages, there are still existing limitations in terms of its high working temperature of 200–400 °C and wide band gap of 3.37 eV [
16,
17].
Compared to ZnO, lead sulfide (PbS) is not commonly used as a gas sensor material. The PbS nanoparticle is commonly used in IR detectors, Pb
2+ ion-selective sensors, and phosphor materials for luminescent display devices [
18,
19,
20]. Although PbS colloidal quantum dots showed a promising response as gas sensor material, PbS nanoparticles on their own showed a low response to test gases such as NH
3, NO
2, SO
2, H
2S, and NO [
21,
22]. There are also existing studies in which PbS sensors decorated with other materials, such as Ag, exhibited improved sensing performance compared to pure PbS nanoparticle sensors [
23].
Changes in particle morphology affect surface reactivity and electrical properties [
24,
25]. For the detection of CO gas, it has been proven that Cr-doped ZnO sensors show a lower band gap than pure ZnO due to the increase of electrons in the conduction band, which leads to an increase in the conductivity of the sensor. Copper and cobalt doping has been shown to improve the sensitivity of ZnO sensors to hydrogen disulfide gas and ammonia gas, respectively [
13,
25].
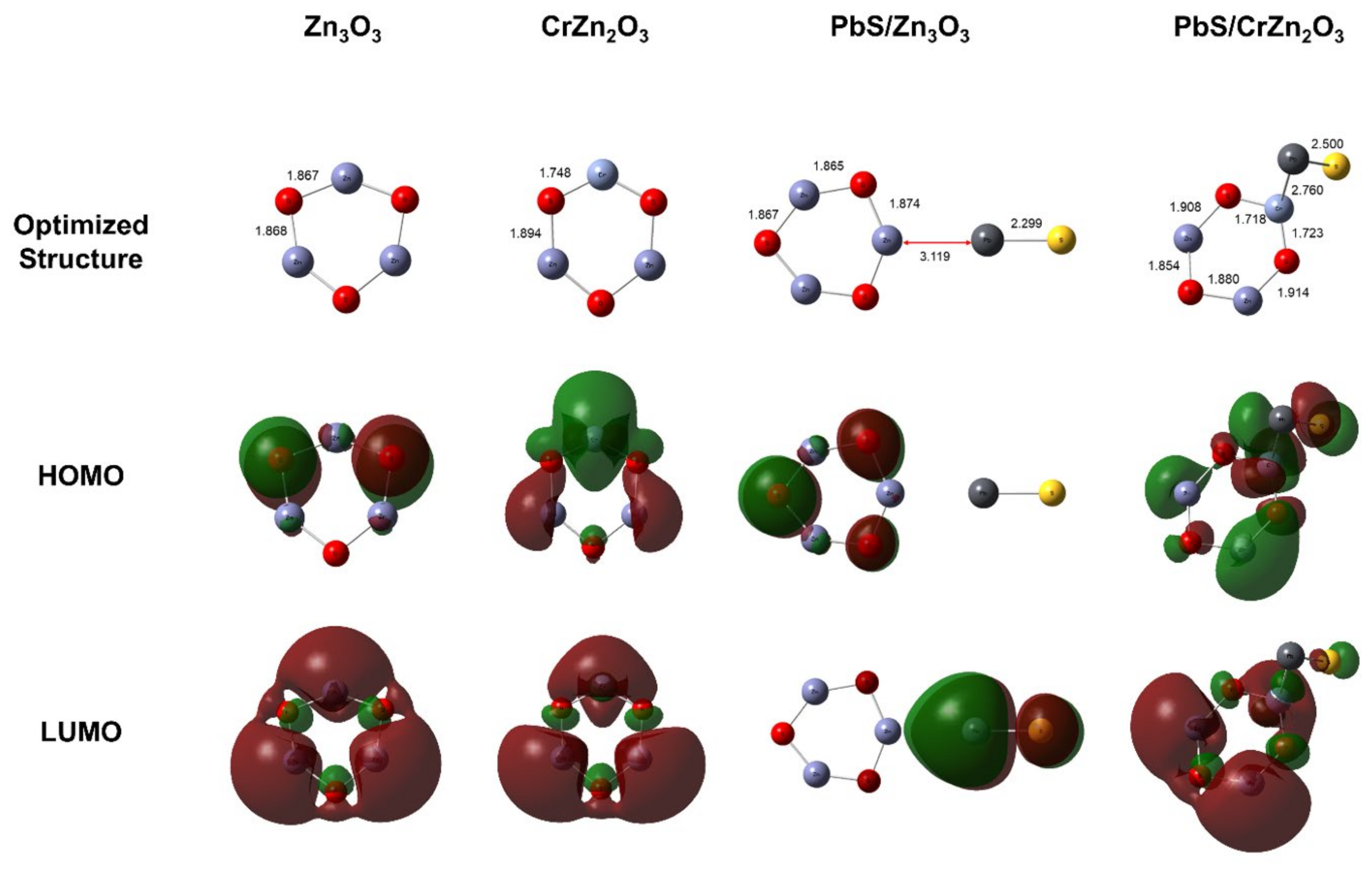
In order to study and optimize the chemical structure and reactivity of the research materials, density-functional theory (DFT) computations were performed to obtain the theoretical calculation data for the study. DFT is a type of electronic structure calculation that is implemented to calculate the electronic structure of atoms, molecules, and solids with an intention to understand the material properties of the fundamental laws of quantum mechanics [
26,
27,
28]. The DFT simulation method is widely applied in materials science, and consequently for semiconducting materials, as it is frequently used to calculate the electronic structure of atoms, molecules, and solids which makes it possible to calculate the band gap difference of the starting materials after loading to fabricate the nanocomposites [
28]. It utilizes theoretical and computational approaches to predict experimental results precisely. Therefore, the DFT simulation results can be corroborated or refuted with experimental data in this study.
This study aims to improve the sensitivity of ZnO and PbS nanocomposites for sensing CO gas. DFT calculations were performed to gain valuable insights, such as the band gap energies of the synthesized nanocomposites. The PbS-ZnO composite was synthesized by mixing it with varying amounts of ZnO and doping it with Cr. The nanocomposites were characterized using X-ray diffractometer (XRD), scanning electron microscopy (SEM), UV–Vis spectroscopy, and Brunauer–Emmett–Teller (BET) analyses. Sensor data such as sensitivity and response-recovery times were calculated. The experimental data were then compared with theoretical data obtained by DFT calculations.
2. Materials and Methods
2.1. Chemical Reagents
All chemical reagents used for the nanofilm synthesis were obtained from Merck. Zinc acetate dihydrate nanopowders (Zn(CH3COO)2∙2H2O), cetyltrimethylammonium bromide powder (CTAB), methanol solution, chromium nitrate nonahydrate (Cr(NO3)3∙9H2O), lead (II) nitrate (Pb(NO3)2), 3-mercaptopropionic acid (3-MPA), sodium sulfide nonahydrate (Na2S∙9H2O), and α-terpineol (C10H18O) were analytical grade and were used without any modifications prior to the synthesis process. The Au-IDE substrates (DRP-G-IDEAU10) were obtained from the Metrohm. Hydrogen peroxide (30%) and ammonia (25%) were used as cleaning agents for the Au-IDE substrate.
2.2. DFT Computational Methods
The geometry optimization and electronic structure calculations of this study were performed using the DFT framework. All DFT calculations were performed using the Gaussian 16 software package, and GaussView 06 was used for the molecular visualization [
29,
30]. Becke’s three-parameter of Lee-Yang-Parr (B3LYP) hybrid functional was considered for the exchange-correlation effects at the ground-state energy level [
31,
32]. It includes a net neutral charge and single spin multiplicity for structural optimization. All the atoms were assigned with LANL2DZ (Los Alamos National Laboratory 2 Double-Zeta) basis sets [
33,
34,
35]. The band gap with respect to the vacuum level was calculated through the difference between the highest occupied molecular orbital (HOMO) and the lower unoccupied molecular orbital (LUMO) represented as follows [
36,
37]:
where
Eg,
EHOMO, and
ELUMO are the band gap, HOMO energy, and LUMO energy, respectively.
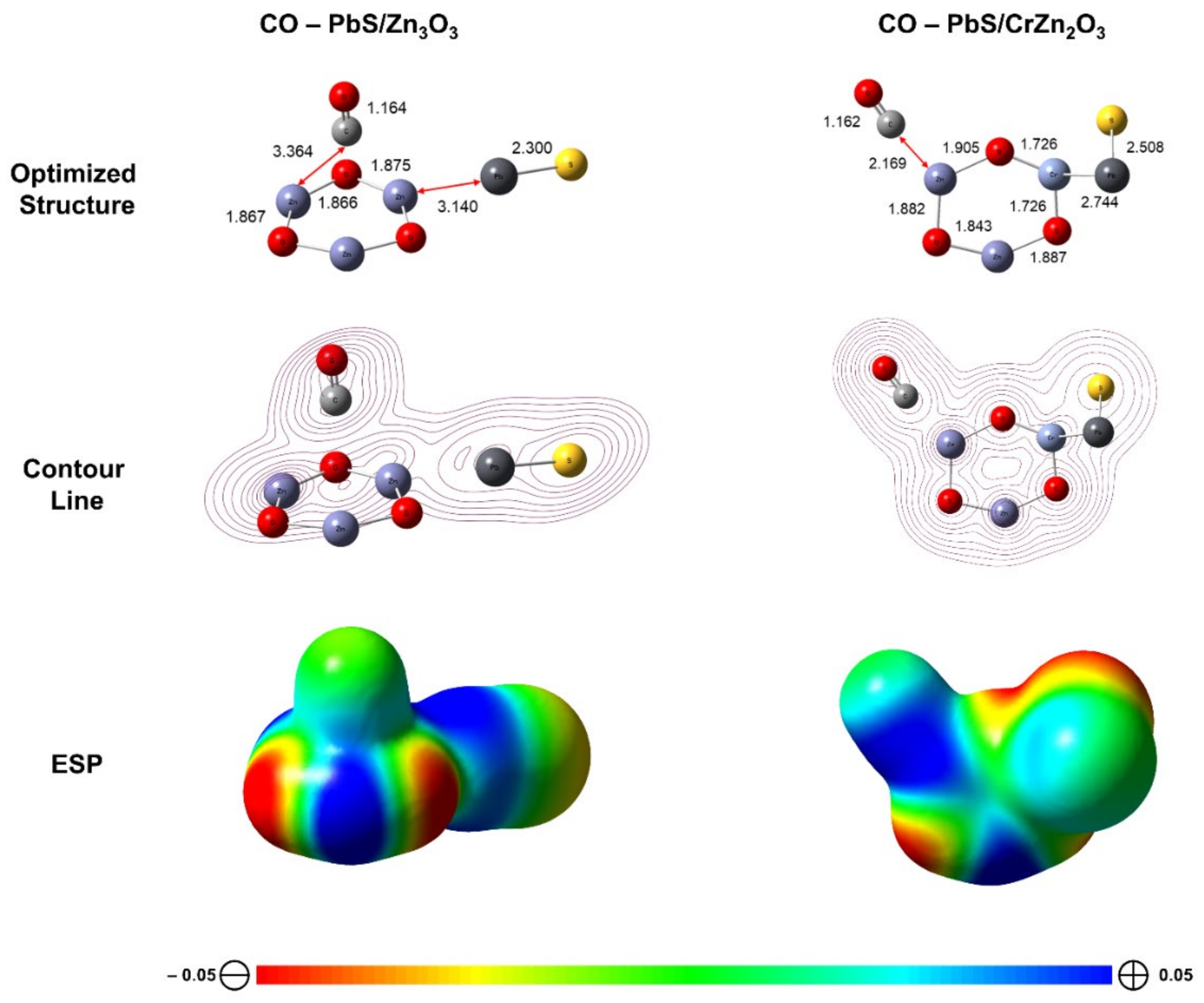
The adsorption energy (
Eads) of CO molecules on the surface was calculated as follows [
38]:
where
Etotal (surface/gas),
Etotal (surface), and
Etotal (gas) are respect to the total energy of the surface with CO gas, isolated surface, and isolated gas. The reaction indicates exothermic as the
Eads is negative.
The contour line and electrostatic potential (ESP) surfaces of CO adsorption upon the surfaces were conducted using the Cubegen utility within the Gaussian 16 package [
29].
2.3. Synthesis of Pure ZnO and CrZnO Nanoparticles
Sol–gel synthesis was performed for the synthesis of pure and Cr-doped ZnO nanoparticles. Zinc acetate dihydrate (1.54 g) was dissolved in 1 mL 0.5 M CTAB solution and 14 mL methanol solution. The mixture was stirred at 60 °C for 2 h to obtain a clear alcosol. The pH of the solution was maintained at pH 6. The resulting alcosol was aged at room temperature for 48 h and then dried at 70 °C overnight to obtain a white powder. The white powder was centrifuged with a mixture of n-hexane (6 mL) and absolute ethanol (14 mL) solutions five times at 4000 rpm for 10 min to remove the impurities. The residual white powder was then dried in the oven at 100 °C for 24 h to obtain ZnO, which is also white in appearance. For Cr-doped ZnO, 0.5 wt% Cr(NO
3)
3∙9H
2O was added to the zinc precursor and methanol mixture solution for the alcosol formation. For this study, only 0.5 wt% CrZnO was considered as it was found that specific weight percentage doping resulted in enhanced CO sensing performance in a previous study [
39].
2.4. Synthesis of PbS Nanoparticles
The PbS nanoparticle was synthesized at room temperature. In a three-neck round-bottom flask, 100 mL of 0.1 M Pb(NO3)2 and 1.74 mL 3-MPA were mixed and subjected to constant stirring at room temperature under a nitrogen atmosphere. This was followed by dropwise addition of 100 mL of 0.1 M Na2S∙9H2O to the mixture with constant stirring for 6 h under a nitrogen atmosphere until the mixture turned black. The mixture was washed with a mixture of absolute ethanol (20 mL) and ultrapure water (30 mL) (Milli-Q® Ultrapure Water Systems, Milford, CT, USA) by centrifuging at 4000 rpm for 10 min. The resulting black powder was obtained after drying the reaction product overnight in the oven at 60 °C.
2.5. Synthesis of PbS-Loaded Pure ZnO and CrZnO Nanocomposite
Ex-situ synthesis of PbS-ZnO and PbS-CrZnO was performed using a suspension mixing method. Varying wt% of PbS were weighed to get 0.25, 0.5, 1.0, 1.5, 2.0, and 5 wt% ZnO and CrZnO nanocomposites. In this study, only the 1.5 wt% result is reported for each PbS-ZnO and PbS-CrZnO. These materials were then mixed in excess absolute ethanol solution at room temperature for 7–8 h under constant, vigorous stirring. The resulting suspension was precipitated by centrifuging at 4000 rpm for 10 min and then dried in the oven at 90 °C for 2 h.
2.6. Materials Characterization
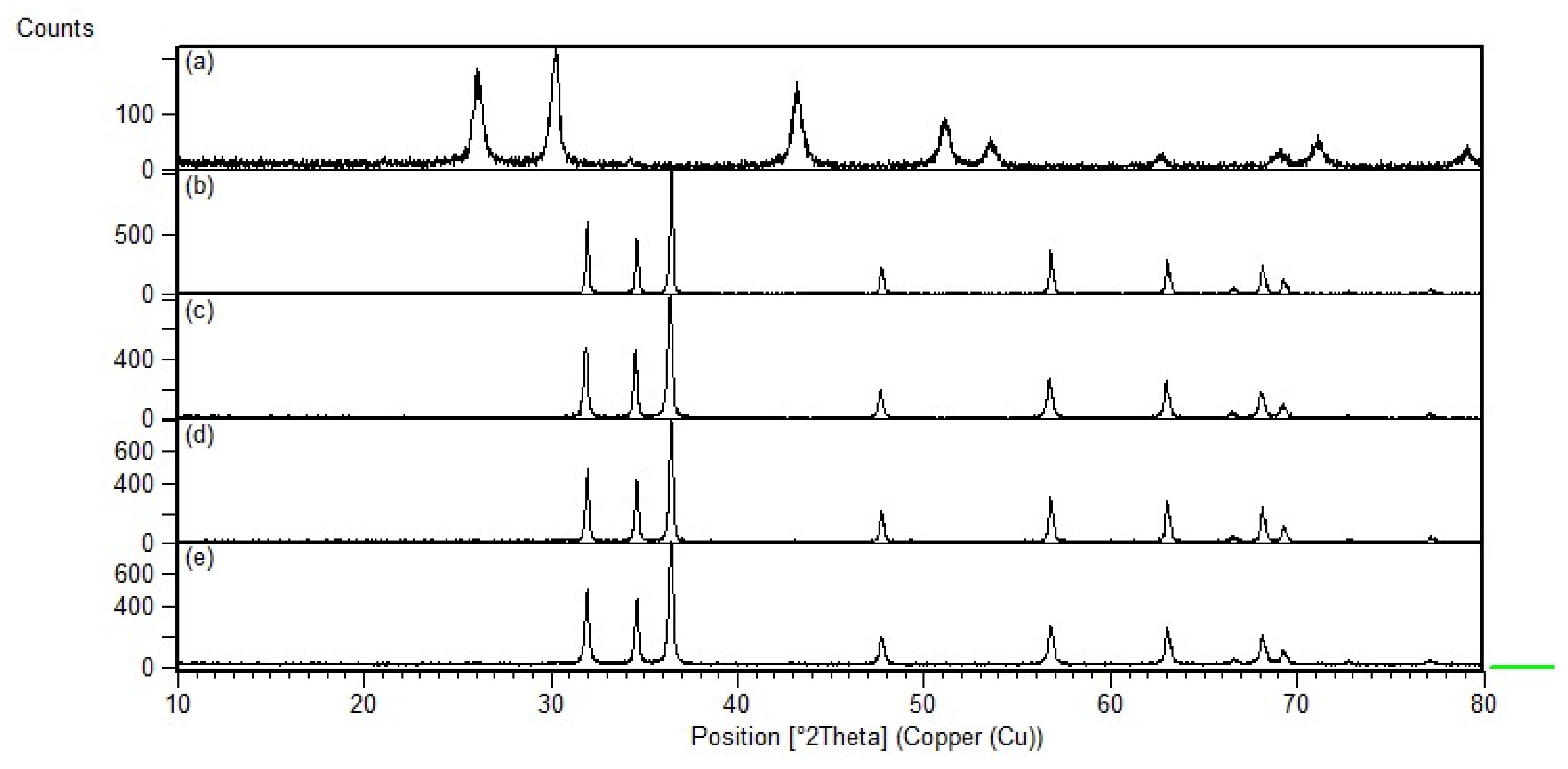
The synthesized nanocomposites’ crystallinity was confirmed using XRD (Shimadzu XRD-7000) at 10–80° at a rate of 2°/min. Crystallite sizes were also analyzed from the XRD data using the Scherrer equation:
where
D is the crystallite size (nm),
K is the shape factor, which is equal to 0.94 for spherical nanoparticles, λ is the wavelength of X-ray radiation of CuKα1 (λ is 0.154056 nm),
β is the full width half maximum intensity (FWHM), and
θ is the Bragg angle.
The surface morphology and elemental analyses of the gas sensing composites were obtained using an FE-SEM (JEOL JSM-7600F) spectrometer. An accelerating voltage of 10 kV with a working distance of 9–16 mm was maintained during the analysis. UV–Vis diffuse reflectance spectra (DRS) were also obtained using an Agilent Cary 5000 UV–Vis-NIR spectrophotometer, and the band gaps were calculated from the DRS data using the Tauc plot method [
40]. The Tauc plot method is obtained using the Kubelka–Munk function (Equation (4)) where a graph of (
F(
r)
hv)
2 against photon energy (eV) is plotted, and the
x-axis extrapolated value is the material band gap based on the following calculations:
where
k is the molar absorption coefficient,
R is the reflectance data from the UV–Vis data, and
s is the scattering factor.
The material surface area and pore sizes were determined using a BET analyzer (Micromeritics ASAP 2020 BET analyzer, Norcross, GA, USA) at 300 °C under a nitrogen gas environment.
2.7. Gas Sensor Fabrication
Initial cleaning was performed for the gold interdigitated (Au-IDE) substrates prior to the deposition of the gas-sensing nanocomposites. Briefly, 100 mL of distilled water was mixed with 10 mL hydrogen peroxide in a 250 mL beaker. The substrates were placed inside the mixture with the gold side facing up. The beaker was then heated on a hot plate at 80 °C for 15 min or until bubbles formed on the gold electrode’s surface. Ammonia solution (2 mL) was added to the beaker and heated up for another 30 min until effervescence was observed. The heat was turned off so the substrates could be removed from the cleaning solution with a tweezer. The substrates were rinsed with distilled water then carefully dried with a paper towel followed by drying in an oven preheated to 60 °C for 30 min.
The nanofilm pastes were prepared by grinding 0.2 g of the nanopowder with α-terpineol solution using an agate mortar until it formed a paste. The paste was then deposited onto the Au-IDE via the doctor blading method and then dried in a preheated oven at 60 °C for an hour. The nanofilm was then calcined at 400 °C for 2 h at the ramping rate of 2 °C/min. The synthesized nanofilms were stored in an oven at 100 °C overnight before performing the gas sensing analyses.
2.8. Gas Sensor Setup
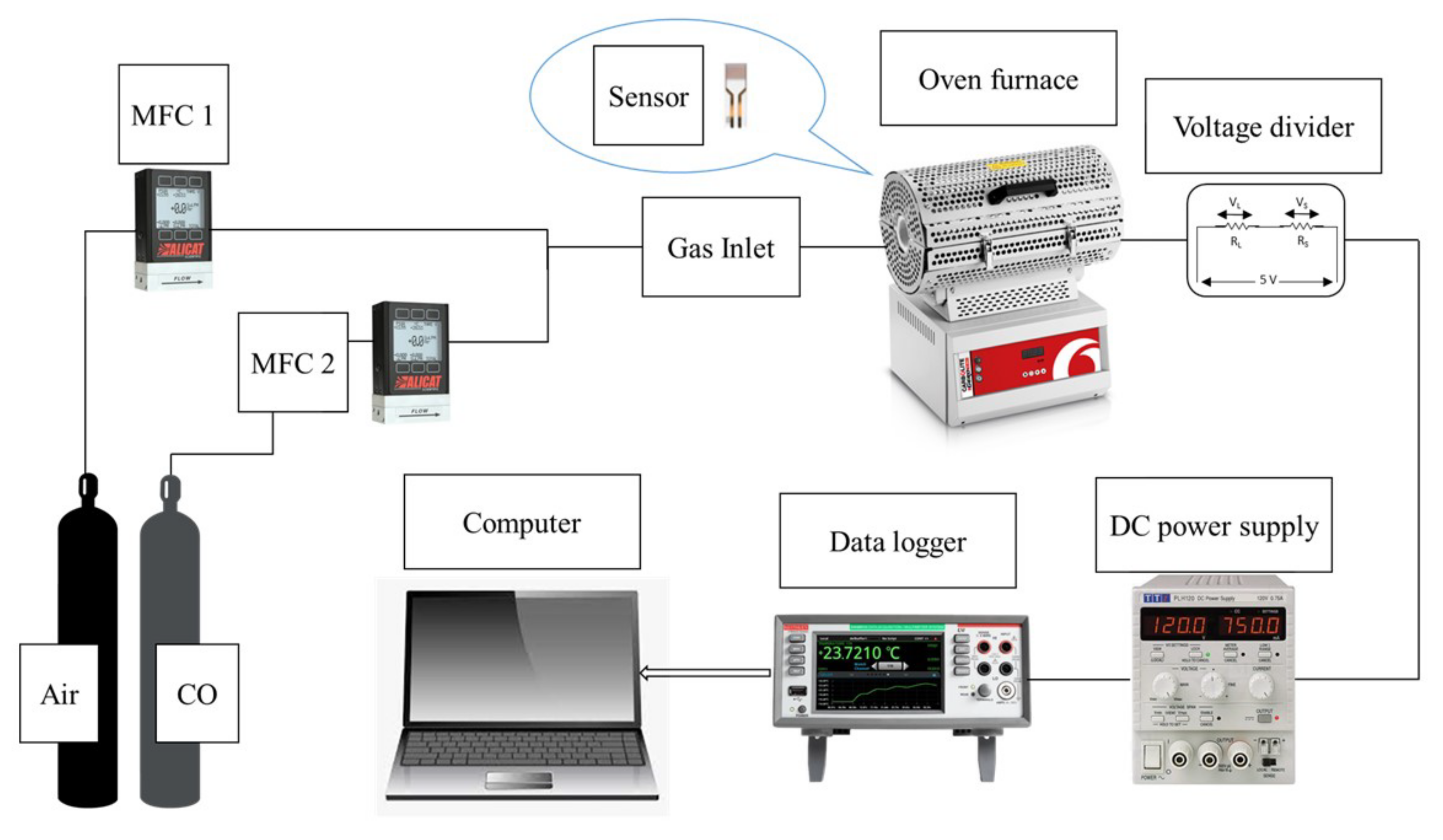
The gas sensing setup is a modified system consisting of a Carbolite Gero tube furnace (30–3000 °C), Alicat Scientific and Horiba mass flow controllers (MFC), a Keithley Data Acquisition and Logging Multimeter System (DAQ6510), and a power supply (TTi PLH120) connected with platinum wires (ϕ 0.3 mm). The test and reference gas were 1000 ppmv CO and compressed air, respectively. A customized quartz tube and ceramic substrate holder were used. The gas sensor setup is shown in
Figure 1.
2.9. Gas Sensing Procedure
The nanofilm-deposited substrate was inserted into customized holders connected with platinum wires (ϕ 0.3 mm), which were secured in a quartz tube. They were then inserted into an oven furnace, which was heated to 300 °C for 90 min until the temperature stabilized. Compressed air was first allowed into the tube to set the reference environment before test gas was inserted for the gas sensing procedure. The gas flow was controlled to obtain CO gas concentrations of 100, 200, and 300 ppmv. The sensing data was recorded using the Keithley data acquisition system, which was then used to calculate gas sensing sensitivity as well as response-recovery time. The sensitivity of the sensor was calculated using the following:
where
Rg is the resistance while exposed to the test gas and
Ra is the resistance when exposed to air [
39,
41]. Response time is the time taken by the sensor to reach 90% of its maximum response value when subjected to the test gas, and recovery time is the time taken by the sensor to obtain 10% of the maximum response value [
42].
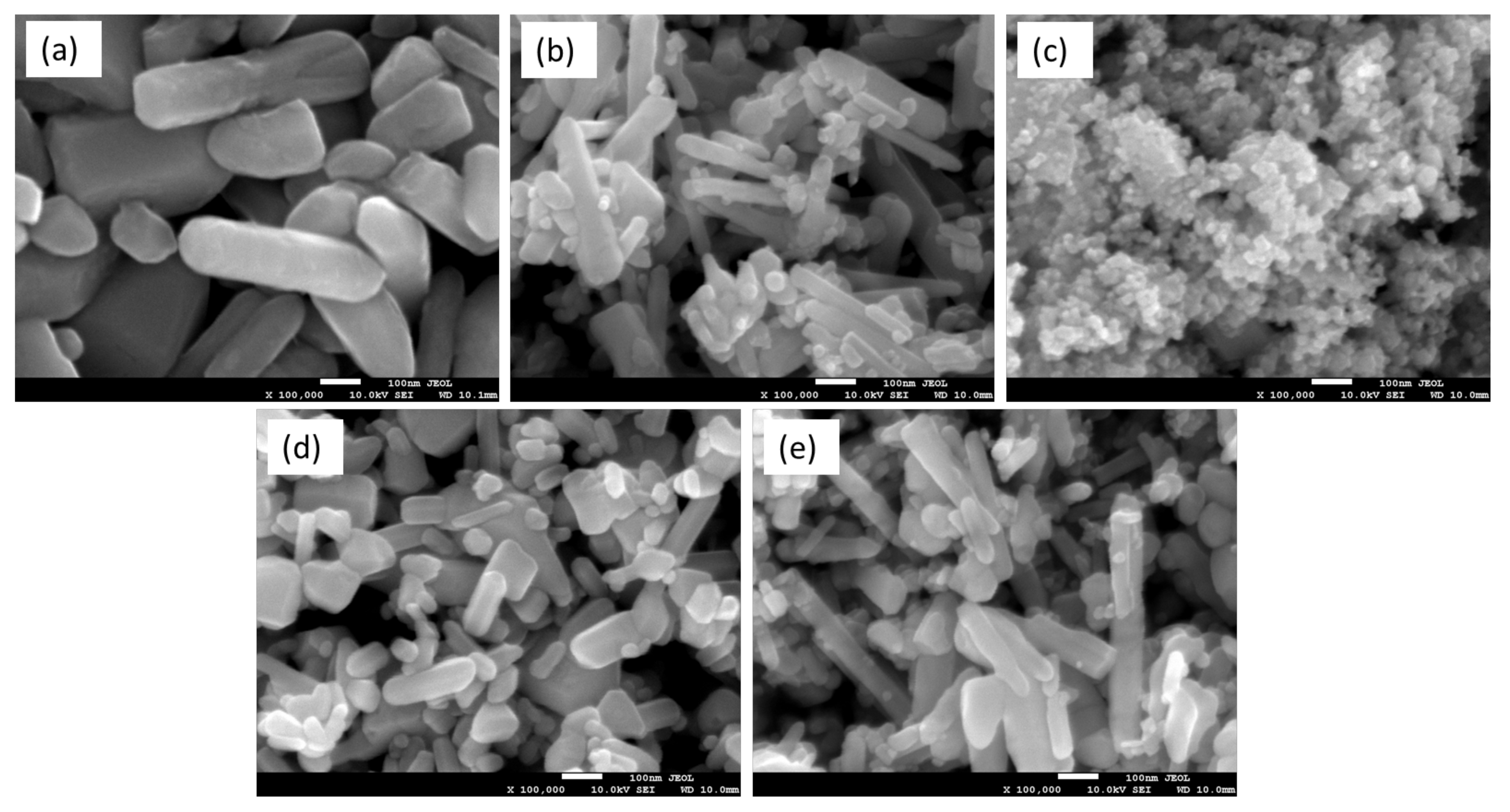
4. Conclusions
PbS-loaded ZnO and CrZnO nanocomposites were successfully synthesized via a sol–gel method and then blended at 1.5 wt%. XRD confirmed the hexagonal wurtzite peaks of ZnO and CrZnO, as well as the PbS after loading to form the nanocomposites, and the Scherrer-calculated crystallite sizes of the PbS-ZnO and PbS-CrZnO nanocomposites were 57.6 nm and 44.3 nm, respectively. SEM images confirmed the morphologies of ZnO, CrZnO, and PbS nanoparticles, as well as the presence of PbS after loading onto the nanocomposites. The DFT calculated that band gaps decreased the loading of PbS onto the pure ZnO (from 3.753 eV to 3.540 eV) and CrZnO materials (from 1.736 eV to 1.518 eV). This trend was supported by the band gaps calculated from UV–Vis data for PbS-ZnO (4.813 eV to 4.795 eV) and PbS-CrZnO (5.068 eV to 3.337 eV). The decrease in band gaps was due to the formation of p-n heterojunctions, which improved electron charge transport and prevents energy loss by suppressing the charge-carrier recombination. BET analysis results showed that 1.5 wt% PbS-CrZnO had the highest surface area and pore size at 18.2 m2/g and 30.0 nm, respectively. The sensing results of the 1.5 wt% PbS-ZnO and 1.5 wt% PbS-CrZnO nanocomposites showed better sensitivity as gas sensors compared to pure ZnO, with PbS-CrZnO having the best sensitivities ranging from 78.5 to 195.2%. This high sensitivity could be attributed to the smaller crystallite sizes, lower band gap, and higher working surface area of the nanocomposite. PbS-CrZnO showed the best response time in the present study, which decreased with increasing CO concentration.