Provenance Identification of Leaves and Nuts of Bertholletia excelsa Bonpl by Near-Infrared Spectroscopy and Color Parameters for Sustainable Extraction
Abstract
:1. Introduction
2. Material and Methods
2.1. Leaf Samples and Characterization
2.2. Brazil Nut Samples
2.3. Soil Samples
2.4. Color Parameters
2.5. NIR Spectra
2.6. Means Tests
2.7. Principal Component Analysis (PCA)
2.8. Classification Models
3. Results
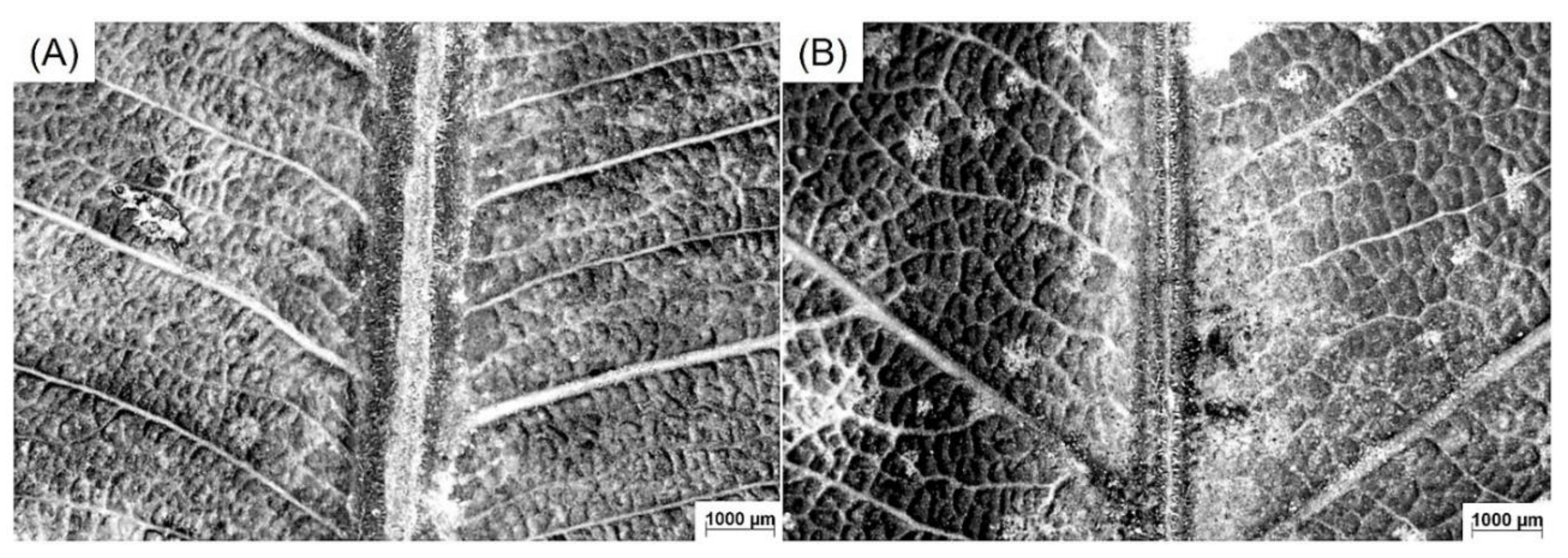
3.1. Leaf Morphology
3.2. Soil Composition
3.3. Color Data and Visible Spectroscopy
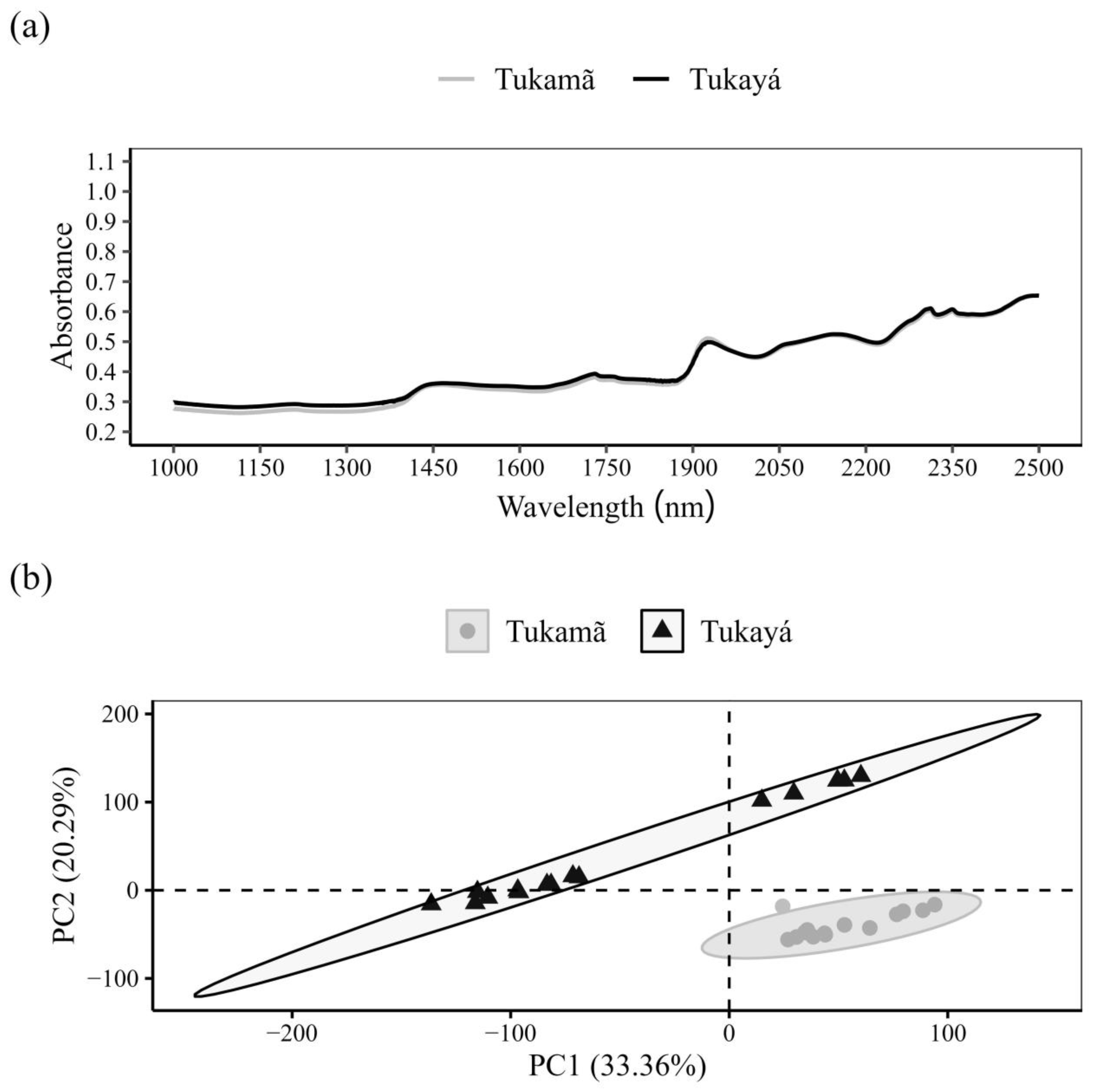
3.4. NIR Spectroscopy–Leaves
3.5. NIR Spectroscopy–Nuts
3.6. Classification Tests
4. Discussion
4.1. Leaf Morphology and Soil Composition
4.2. Color Data and Visible Spectroscopy
4.3. NIR Spectroscopy
4.4. Classification Tests
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scoles, R.; Gribel, R.; Klein, G.N. Crescimento e sobrevivência de castanheira (Bertholletia excelsa Bonpl.) em diferentes condições ambientais na região do rio Trombetas, Oriximiná, Pará. Bol. Mus. Para. Emílio Goeldi Ciências Nat. 2011, 6, 273–293. [Google Scholar] [CrossRef]
- IBGE/SIDRA. Produção da Extração vegetal e da Silvicultura. 2020. Available online: http://www.sidra.ibge.gov.br (accessed on 29 May 2020).
- Homma, A.K.O. Plant extractivism or plantation: What is the best option for the Amazon. Estud. Avançados 2012, 26, 167–186. [Google Scholar] [CrossRef]
- Tonini, H.; Pedrozo, C.Â. Variações anuais na produção de frutos e sementes de Castanheira-do-Brasil (Bertholletia excelsa Bonpl., Lecythidaceae) em florestas nativas de Roraima. Rev. Árvore 2014, 38, 133–144. [Google Scholar] [CrossRef]
- Peres, C.A.; Baider, C.; Zuidema, P.A.; Wadt, L.H.; Kainer, K.A.; Gomes-Silva, D.A.P.; Salomāo, R.P.; Simȯes, L.L.; Franciosi, E.R.N.; Valverde, F.C.; et al. Demographic threats to the sustainability of Brazil not exploitation. Science 2003, 302, 2112–2114. [Google Scholar] [CrossRef] [PubMed]
- Salomão, R.D.P. Densidade, estrutura e distribuição espacial de castanheira-do-brasil (Bertholletia excelsa H. & B.) em dois platôs de floresta ombrófila densa na Amazônia setentrional brasileira. Bol. Do Mus. Para. Emilio Goeldi Ciências Nat. 2009, 4, 11–25. [Google Scholar]
- Sousa, D.G.; De Almeida, S.S.; Do Amaral, D.D. Estrutura de uma população manejada de castanheira (Bertholletia excelsa) na Floresta Nacional de Caxiuanã, Pará. Bol. Do Mus. Para. Emílio Goeldi Ciências Nat. 2014, 9, 353–370. [Google Scholar] [CrossRef]
- AIPHX (Associação Indígena Pyjahyry Xipaia). Relatório gerencial consolidado da executora. In Plano Básico Ambiental—Componente Indígena Programa de Atividades Produtivas; JGP: Sao Paolo, Brazil, 2023. [Google Scholar]
- Durgante, F.M.; Higuchi, N.; Almeida, A.; Vicentini, A. Species spectral signature: Discriminating closely related plant species in the Amazon with near-infrared leaf-spectroscopy. For. Ecol. Manag. 2013, 291, 240–248. [Google Scholar] [CrossRef]
- Lang, C.; Costa, F.R.C.; Camargo, J.L.C.; Durgante, F.M.; Vicentini, A. Near infrared spectroscopy facilitates rapid identification of both young and mature Amazonian tree species. PLoS ONE 2015, 8, 134–521. [Google Scholar] [CrossRef]
- Lang, C.; Almeida, D.R.A.; Costa, F.R.C. Discrimination of taxonomic identity at species, genus and family levels using Fourier Transformed Near-Infrared Spectroscopy (FT-NIR). For. Ecol. Manag. 2017, 406, 219–227. [Google Scholar] [CrossRef]
- Tsuchikawa, S.; Kobori, H. A review of recent application of near infrared spectroscopy to wood science and technology. J. Wood Sci. 2015, 3, 213–220. [Google Scholar] [CrossRef]
- Tigabu, M.; Daneshvar, A.; Jingjing, R.; Wu, P.; Ma, X.; Odén, P.C. Multivariate discriminant analysis of single seed near infrared spectra for sorting dead-filled and viable seeds of three pine species: Does one model fit all species. Forests 2019, 10, 469. [Google Scholar] [CrossRef]
- Farhadi, M.; Tigabu, M.; Pietrzykowski, M.; Danusevičius, D.; Odén, P.C. Application of near infrared spectroscopy for authentication of Picea abies seed provenance. New For. 2017, 48, 629–642. [Google Scholar] [CrossRef]
- Tigabu, M.; Farhadi, M.; Stener, L.G.; Odén, P.C. Visible + Near Infrared spectroscopy as taxonomic tool for identifying birch species. Silva Fenn. 2018, 52, 9996. [Google Scholar] [CrossRef]
- Teixeira, A.M.; Sousa, C. A review on the application of vibrational spectroscopy to the chemistry of nuts. Food Chem. 2019, 277, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Camargos, J.A.A.; Gonçalez, J.C.A. A colorimetria aplicada como instrumento na elaboração de uma tabela de cores de madeira. Bras. Florest. 2001, 71, 30–41. [Google Scholar]
- Kraus, J.E.; Arduin, M. Manual Básico de Métodos em Morfologia Vegetal; EDUR: Seropédica, Brazil, 1997; 198p. [Google Scholar]
- Obermüller, F.A.; Daly, D.C.; Oliveira, E.C.; Souza, H.F.T.P.; Oliveira, H.M.; Souza, L.S.; Silveira, M. Guia Ilustrado e Manual de Arquitetura Foliar Para Espécies Madeireiras da Amazônia Ocidental; GK Noronha: Rio Branco, Brazil, 1999; 101p. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR R: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package version 1.0.5. 2017. Available online: https://cran.r-project.org/package=factoextra (accessed on 15 February 2021).
- Kuhn, M.; Wing, J.; Weston, S.; Williams, A.; Keefer, C.; Engelhardt, A.; Benesty, M. Package ‘caret’. 2020. Available online: https://cran.r-project.org/package=caret (accessed on 15 February 2021).
- Dartora, N.; De Souza, L.M.; Santana-Filho, A.P.; Iacomini, M.; Valduga, A.T.; Gorin, P.A.; Sassaki, G.L. UPLC-PDA–MS evaluation of bioactive compounds from leaves of Ilex paraguariensis with different growth conditions, treatments and ageing. Food Chem. 2011, 4, 1453–1461. [Google Scholar] [CrossRef]
- Davis, P.A.; Caylor, S.; Whippo, C.W.; Hangarter, R.P. Changes in leaf optical properties associated with light-dependent chloroplast movements. Plant Cell Environ. 2011, 34, 2047–2059. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.D.; Noble, S.D. Spectrographic measurement of plant pigments from 300 to 800 nm. Remote Sens. Environ. 2014, 148, 119–123. [Google Scholar] [CrossRef]
- Kira, O.; Linker, R.; Gitelson, A. Non-destructive estimation of foliar chlorophyll and carotenoid contents: Focus on informative spectral bands. Int. J. Appl. Earth Obs. Geoinf. 2015, 38, 251–260. [Google Scholar] [CrossRef]
- Maire, V.; Wright, I.J.; Prentice, I.C.; Batjes, N.H.; Bhaskar, R.; Van Bodegom, P.M.; Reich, P.B. Global effects of soil and climate on leaf photosynthetic traits and rates. Glob. Ecol. Biogeogr. 2015, 6, 706–717. [Google Scholar] [CrossRef]
- Bravo, S.; Amorós, J.A.; Pérez-de-los-Reyes, C.; García, F.J.; Moreno, M.M.; Sánchez-Ormeño, M.; Higueras, P. Influence of the soil pH in the uptake and bioaccumulation of heavy metals (Fe, Zn, Cu, Pb and Mn) and other elements (Ca, K, Al, Sr and Ba) in vine leaves, Castilla-La Mancha (Spain). J. Geochem. Explor. 2017, 174, 79–83. [Google Scholar] [CrossRef]
- Freitas, F.A.D.; Kopp, M.M.; Sousa, R.O.D.; Zimmer, P.D.; Carvalho, F.I.F.D.; Oliveira, A.C.D. Absorção de P, Mg, Ca e K e tolerância de genótipos de arroz submetidos a estresse por alumínio em sistemas hidropônicos. Ciência Rural. 2006, 1, 72–79. [Google Scholar] [CrossRef]
- Oliveira, R.H.; Rosolem, C.A.; Trigueiro, R.M. Importância do fluxo de massa e difusão no suprimento de potássio ao algodoeiro como variável de água e potássio no solo. Rev. Bras. De Ciências Do Solo 2004, 3, 439–445. [Google Scholar] [CrossRef]
- Ohyama, T.; Sueyoshi, K. Nitrogen Assimilation in Plants; Signpost, Trivandrum: Kerala, India, 2010; 378p. [Google Scholar]
- Yarce, C.J.; Rojas, G. Near infrared spectroscopy for the analysis of macro and micro nutrients in sugarcane leaves. Sugar Ind. 2012, 137, 707–710. [Google Scholar] [CrossRef]
- Xue, J.; Fuentes, S.; Poblete-Echeverria, C.; Viejo, C.G.; Tongson, E.; Du, H.; Su, B. Automated chinese medicinal plants classification based on machine learning using leaf morpho-colorimetry, fractal dimension and visible/near infrared spectroscopy. Int. J. Agric. Biol. Eng. 2019, 2, 123–131. [Google Scholar] [CrossRef]
- Frizon, C.N.T.; Nisgoski, S. Color Parameters to Predict Moisture and Tannin Content in Yerba Mate Process. Floresta E Ambiente 2020, 2, 2–9. [Google Scholar] [CrossRef]
- Cabral-Malheiros, G.; Hecktheuer, L.H.R.; Canto, M.W.D.; Balsamo, G.M. O tempo e o tipo de embalagem sobre a erva-mate tipo chimarrão durante armazenagem em condições ambientais. Ciência Rural. 2010, 3, 654–660. [Google Scholar] [CrossRef]
- Nisgoski, S.; Carneiro, M.E.; Lengowski, E.C.; Schardosin, F.Z.; De Muñiz, G.I.B. Potential use of visible and near-infrared spectroscopy for pine species discrimination by examination of needles. South. For. 2015, 4, 243–247. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, N.M. Non-destructive assessment of chlorophyll, carotenoid and anthocyanin content in higher plant leaves: Principles and algorithms. In Remote Sensing for Agriculture and the Environment; Peripheral Editions: Athens, Greece, 2004; pp. 78–94. [Google Scholar]
- Penuelas, J.; Filella, I. Visible and near infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 1998, 4, 151–156. [Google Scholar] [CrossRef]
- Schenk, J.S.; Workman, J.J., Jr.; Westerhaus, M.O. Application of NIR spectroscopy to agricultural products. In Handbook of Near-Infrared Analysis, 3rd ed.; Burns, D.A., Ciurczak, E.W., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 347–386. [Google Scholar]
- Schwanninger, M.; Rodrigues, J.C.; Fackler, K. A review of band assignments in near infrared spectra of wood and wood components. J. Near Infrared Spectrosc. 2011, 19, 287–308. [Google Scholar] [CrossRef]
- Mees, C.; Souard, F.; Delporte, C.; Deconinck, E.; Stoffelen, P.; Stévigny, C.; De Braekeleer, K. Identification of coffee leaves using FT-NIR spectroscopy and SIMCA. Talanta 2018, 177, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Lohr, D.; Tillmann, P.; Druege, U.; Zerche, S.; Rath, T.; Meinken, E. Non-destructive determination of carbohydrate reserves in leaves of ornamental cuttings by near-infrared spectroscopy (NIRS) as a key indicator for quality assessments. Biosyst. Eng. 2017, 158, 51–63. [Google Scholar] [CrossRef]
- Tang, R.; Chen, X.; Li, C. Detection of nitrogen content in rubber leaves using near-infrared (NIR) spectroscopy with correlation-based successive projections algorithm (SPA). Appl. Spectrosc. 2018, 72, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Migacz, I.P.; Manfron, J.; Farago, P.V.; Raman, V.; Muñiz, G.I.B.; Nisgoski, S. VIS/NIR spectra and color parameters according to leaf age of some Eucalyptus species: Influence on their classification and discrimination. For. Syst. 2022, 31, e013. [Google Scholar] [CrossRef]
- Richardson, A.D.; Reeves, J.B.; Gregoire, T.G. Multivariate analyses of visible/near infrared (VIS/NIR) absorbance spectra reveal underlying spectral differences among dried, ground conifer needle samples from different growth environments. New Phytol. 2003, 161, 291–301. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Carranza-Jimenez, L.; Sinca, F.; Tupayachi, R.; Anderson, C.B.; Martinez, P. Functional and biological diversity of foliar spectra in tree canopies throughout the Andes to Amazon region. New Phytol. 2014, 204, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Cavender-Bares, J.; Meireles, J.E.; Couture, J.J.; Kaproth, M.A.; Kingdon, C.C.; Singh, A.; Serbin, S.P.; Center, A.; Zuniga, E.; Townsend, P.A.; et al. Associations of leaf spectra with genetic and phylogenetic variation in oaks: Prospects for remote detection of biodiversity. Remote Sens. 2016, 8, 221. [Google Scholar] [CrossRef]
- Borraz-Martínez, S.; Boqué, R.; Simó, J.; Mestre, M.; Gras, A. Development of a methodology to analyze leaves from Prunus dulcis varieties using near infrared spectroscopy. Talanta 2019, 204, 320–328. [Google Scholar] [CrossRef]
- Nisgoski, S.; Schardosin, F.Z.; Batista, F.R.R.; De Muñiz, G.I.B.; Carneiro, M.E. Potential use of NIR spectroscopy to identify Cryptomeria japonica varieties from southern Brazil. Wood Sci. Technol. 2016, 1, 71–80. [Google Scholar] [CrossRef]
- Xu, L.; Yan, S.M.; Cai, C.B.; Zhong, W.; Yu, X.P. Rapid analysis of geographical origins and age of Torreya grandis seeds by NIR spectroscopy and pattern recognition methods. J. Spectrosc. 2013, 2013, 468509. [Google Scholar] [CrossRef]
- Tigabu, M.; Oden, P.C.; Lindgren, D. Identification of seed sources and parents of Pinus sylvestris L. using visible–near infrared reflectance spectra and multivariate analysis. Trees 2005, 19, 468–476. [Google Scholar] [CrossRef]
- Loewe, V.; Navarro-Cerrillo, R.M.; García-Olmo, J.; Riccioli, C.; Sánchez-Cuesta, R. Discriminant analysis of Mediterranean pine nuts (Pinus pinea L.) from Chilean plantations by near infrared spectroscopy (NIRS). Food Control. 2017, 73, 634–643. [Google Scholar] [CrossRef]
- Amendola, L.; Firmani, P.; Bucci, R.; Marini, F.; Biancolillo, A. Authentication of Sorrento walnuts by NIR spectroscopy coupled with different chemometric classification strategies. Appl. Sci. 2020, 10, 4003. [Google Scholar] [CrossRef]
- Manuel, M.N.B.; Silva, A.C.; Lopes, G.S.; Ribeiro, L.P.D. One-class classification of special agroforestry Brazilian coffee using NIR spectrometry and chemometric tools. Food Chem. 2022, 366, 130480. [Google Scholar] [CrossRef] [PubMed]
- Castro, W.; De-la-Torre, M.; Avila-George, H.; Torres-Jimenez, J.; Guivin, A.; Acevedo-Juárez, B. Amazonian cacao-clone nibs discrimination using NIR spectroscopy coupled to naïve Bayes classifier and a new waveband selection approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 270, 120815. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.; Contreras, D.; Freer, J.; Juiz, J.; Valenzuela, S. Supervised pattern recognition techniques for classification of Eucalyptus species from leaves NIR spectra. J. Chil. Chem. Soc. 2008, 53, 1709–1713. [Google Scholar] [CrossRef]
- Huang, B.; Liu, J.; Jiao, J.; Lu, J.; Lv, D.; Mao, J.; Zhao, Y.; Zhang, Y. Applications of machine learning in pine nuts classification. Sci. Rep. 2022, 12, 8799. [Google Scholar] [CrossRef]
- Rojas-Rioseco, M.; Castillo, R.P.; Gonzalez-Campos, J.; Ipinza, R.; Sanhueza, M.I.; Hasbún, R. Phylogeographic origin authentication of Araucaria araucana (Mol.) K Koch seedlings through the application of spectroscopy techniques in different infrared ranges and chemometric methods. New For. 2023, 54, 467–489. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, J.; Tang, Y.; Chen, W.; Xu, Y. A review of the application of near-infrared spectroscopy (NIRS) in forestry. Appl. Spectrosc. Rev. 2022, 57, 300–317. [Google Scholar] [CrossRef]



| Community | Deep | P | K | Na | Al | Ca | Ca + Mg | pH |
|---|---|---|---|---|---|---|---|---|
| (cm) | (mg/dm3) | (cmol/dm3) | ||||||
| Tukamã | 0–20 | 8 | 110 | 17 | 0.8 | 0.2 | 0.6 | 4.73 |
| Tukayá | 0–20 | 6 | 41 | 4 | 1.6 | 0.4 | 0.7 | 4.54 |
| Community | L* | a* | b* | C* | h | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Tukamã | 32.17 a | 3.83 | 2.31 a | 0.98 | 8.43 a | 3.00 | 8.85 a | 10.36 | 72.06 a | 10.47 |
| Tukayá | 33.50 a | 4.22 | 1.62 a | 0.92 | 9.76 a | 3.01 | 9.95 a | 11.26 | 79.14 b | 7.20 |
| Sample Model | Accuracy | Precision |
|---|---|---|
| Leaves | 98.45% | 98.53% |
| Nuts with shell | 89.00% | 89.00% |
| Nuts without shell | 90.91% | 91.16% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisgoski, S.; dos Santos, J.X.; Vieira, H.C.; Naide, T.L.; Stange, R.; Silva, W.D.S.d.; Souza, D.V.; Gama, N.C.; Hamada, M.O.d.S. Provenance Identification of Leaves and Nuts of Bertholletia excelsa Bonpl by Near-Infrared Spectroscopy and Color Parameters for Sustainable Extraction. Sustainability 2023, 15, 15606. https://doi.org/10.3390/su152115606
Nisgoski S, dos Santos JX, Vieira HC, Naide TL, Stange R, Silva WDSd, Souza DV, Gama NC, Hamada MOdS. Provenance Identification of Leaves and Nuts of Bertholletia excelsa Bonpl by Near-Infrared Spectroscopy and Color Parameters for Sustainable Extraction. Sustainability. 2023; 15(21):15606. https://doi.org/10.3390/su152115606
Chicago/Turabian StyleNisgoski, Silvana, Joielan Xipaia dos Santos, Helena Cristina Vieira, Tawani Lorena Naide, Rafaela Stange, Washington Duarte Silva da Silva, Deivison Venicio Souza, Natally Celestino Gama, and Márcia Orie de Souza Hamada. 2023. "Provenance Identification of Leaves and Nuts of Bertholletia excelsa Bonpl by Near-Infrared Spectroscopy and Color Parameters for Sustainable Extraction" Sustainability 15, no. 21: 15606. https://doi.org/10.3390/su152115606






