1. Introduction
Anthropogenic carbon dioxide emissions in the last century have significantly outpaced the natural carbon cycle, which has caused ever-growing concern about climate change. Carbon dioxide is an abundant, renewable, inexpensive, and, in the foreseeable future, environmentally harmful compound that, as such, serves as a promising feedstock in various industries. A plethora of research and review articles have been published to further motivate CO
2 mitigation and provide additional application potential [
1,
2]. Among others, C-C bond formation is considered a superior method for carbon dioxide utilization since the newly formed C-C bond has a higher energy density. Although most of the currently available research provides information on CO
2 reduction to methane/methanol and the synthesis of amides, dioxolanes, and dialkyl carbonates, carboxylation reactions with CO
2 as a reactant for incorporation of the entire CO
2 moiety into the new C-C bond formation still require further research [
3]. One such class of compounds are carboxylic acids (R-COOH), which are very versatile and important chemicals in the medicinal, pharmaceutical, chemical, and other industries and can also be used as model compounds in the synthesis of other value-added products [
4,
5,
6].
Almost every household contains at least one product that has salicylic acid in its composition. This aromatic carboxylic acid is mostly used in the agricultural, medicinal, and cosmetic industries, while it holds its highest value in the production of acetylsalicylic acid, an anti-inflammatory drug better known as aspirin [
7,
8]. Preparation of salicylic acid through carboxylation of phenol, a traditional Kolbe–Schmitt reaction, has been a standard procedure for over 100 years, and since then various Kolbe–Schmitt-type reactions have been used for the production of various aromatic hydroxyl acids [
9,
10]. Phenol, a lignin-derived model compound, is a great representative of other more complex aromatics and, as such, poses great potential for sustainable production of aromatic value-added products from renewable and currently poorly valorized biomass lignin. The first and, at the time, most comprehensive review of Kolbe–Schmitt reactions was published in 1957 by Lindsey and Jeskey [
11], summarizing the difference between Kolbe, Schmitt, and Marasse methods. Among all, the simplest version is the Marasse method, where phenol and potassium carbonate are heated under pressurized carbon dioxide, while other methods are more complex and unreliable. In summary, regardless of the method used, the reaction (shown in
Scheme 1) involves two steps: (1) the production, isolation, and purification of the alkali metal phenoxide; and (2) the carboxylation of the alkali metal phenoxide to the corresponding aromatic hydroxyl acid. Step (1) is usually carried out by dissolving phenol and selected alkali metal hydroxide or carbonate into a selected solvent, heating up to dryness, and further in-vacuo drying the precipitate to obtain the desired metal phenoxide. However, complexity, time, and energy consumption for preparation and purification make this process less desirable.
Regardless, Kolbe–Schmitt-type reactions are still highly important industrial processes for the production of hydroxybenzoic acids, which are valuable feedstocks for the production of various pharmaceuticals and fine chemicals, while utilizing CO
2 as a cheap, non-dangerous, but environmentally harmful C1 source. Carboxylation of aromatic compounds is exclusively carried out under homogeneous conditions, where the basic catalyst and aromatic substrate are both present in solid form and heated while the CO
2 gas is applied under pressure [
5,
6,
7]. Articles as old as 70 years have been published on such reactions, whereas recent studies still have not managed to significantly improve or thoroughly explain the prevailing mechanisms. It has been shown that the Marasse method on p-cresol takes place only with K
2CO
3, while Na
2CO
3, NaHCO
3, Li
2CO
3, MgCO
3, and CaCO
3 showed no activity [
12]. Regardless of the CO
2 pressure or reaction time used, the reactions were shown to take place only at temperatures above 373 K; however, the most crucial factor for the activity and selectivity was the presence of water, which is highly detrimental. A similar effect of water was proven by Cason et al. [
13] on the carboxylation of catechol (dihydroxybenzene). Furthermore, Baine et al. [
14] have shown that for phenol carboxylation, the Marasse method with K
2CO
3 and phenol behaves identically like the carboxylation of intermediate potassium phenoxide. This finding laid the groundwork for further studies that mostly used carboxylation of metal phenoxides instead of direct carboxylation of phenolic compounds in the presence of basic catalysts. One of which was by Iijima et al. [
15], who obtained the best conversion of potassium phenoxide under supercritical CO
2 conditions. A year later, they published an additional article using supercritical CO
2, however, for direct carboxylation of phenol [
16]. Yet again, among all tested bases (lithium, sodium, potassium, rubidium, calcium, and barium carbonates), K
2CO
3 was shown to be most active (37%) and selective (99%) towards salicylic acid, while other metallic oxides (alumina, zirconia, and ceria) were shown to be non-active.
This work represents a comprehensive study of a complete, two-step Kolbe–Schmitt reaction with a systematic approach and thorough explanation of the reaction mechanism and possible routes, the used analytics, and, most importantly, the effect of various preparation procedures. While performing only the second step of the reaction (carboxylation of the metal phenoxide), recent studies mostly use Kolbe–Schmitt-type reactions for carboxylation of various aromatic hydroxides, while this work consolidates both steps of the original Kolbe–Schmitt carboxylation of phenol. In addition to providing up-to-date and more detailed possibilities of phenol carboxylation than Cameron et al. [
12], Lindsey et al. [
11], Baine et al. [
14], or others used over 70 years ago, this work also provides detailed and more precise analytical methods. In addition to this, the effect of various preparation procedures has been thoroughly described, as has the effect various bases (NaOH, KOH, Na
2CO
3, K
2CO
3) have on the yields of obtained mono- and dicarboxylated phenols. Lastly, this work combines two very promising sustainability domains: the valorization of lignin-derived model compounds as a renewable source of aromatics and the utilization of carbon dioxide as an abundant and, in the foreseeable future, environmentally harmful compound, which results in sustainable production of value-added products of high importance in various industries.
2. Materials and Methods
All the precursors, reactants, and gases have been purchased from commercial suppliers and used as is, without any further purification. Isopropanol (≥99.5%, Merck KGaA, Darmstadt, Germany, reference number 107022511), tetrahydrofuran (>95 wt.%, EMD Millipore, Burlington, MA, USA), and toluene (≥99.9%, Merck Emsure KGaA, Darmstadt, Germany, LOT#: K52240225013) were used as solvents, while carbon dioxide (2.2, Messer, Bad Soden am Taunus, Germany) was used as reaction gas. For the preparation of metal phenoxides, four different bases were used: sodium hydroxide (≥98%, pellets (anhydrous), Honeywell Fluka, Charlotte, NC, USA, LOT#: J3260), potassium hydroxide (≥85%, pellets, Honeywell Fluka, Charlotte, NC, USA, LOT#: K0410), sodium carbonate (≥99.5%, anhydrous, Honeywell Fluka, Charlotte, NC, USA, LOT#: J1110), and potassium carbonate (99%, anhydrous, Alfa Aesar, Ward Hill, MA, USA), which were reacted with phenol (99.5%, Acros Organics, Fair Lawn, NJ, USA, LOT#: A0411827), while commercial sodium phenoxide (98%, Alfa Aesar, Ward Hill, MA, USA, LOT#: N06G041) was used as is. For calibration curves used in the high performance liquid chromatography (HPLC), various potential phenolic carbonates were obtained: salicylic acid (SA) (≥99.0%, Sigma-Aldrich, St. Louis, MO, USA, LOT#: MKCK6947), 3-hydroxybenzoic acid (3HBA) (99%, Sigma Aldrich, St. Louis, MO, USA, LOT#: BCCB1724), 4-hydroxybenzoic acid (4HBA) (≥99%, Sigma Aldrich, St. Louis, MO, USA, LOT#: BCCB8991), 2-hydroxyterephthalic acid (2HtPh) (97%, Sigma Aldrich, St. Louis, MO, USA), 4-hydroxyisophthalic acid (4HiPh) (>98.0%, TCI Tokyo Chemical Industry, Tokyo, Japan, LOT#: 7RFGK-MQ), 5-hydroxyisophthalic acid (5HiPh) (99%, Acros Organics, Fair Lawn, NJ, USA), 4-hydroxyphthalic acid (4HPh) (>98.0%, TCI Tokyo Chemical Industry, Tokyo, Japan, LOT#: NBX5C-OA). In-house distilled water was used together with formic acid (98%, Sigma Aldrich, St. Louis, MO, USA) as an internal standard for dissolving the obtained solid products and diluting the liquid samples for the HPLC analysis.
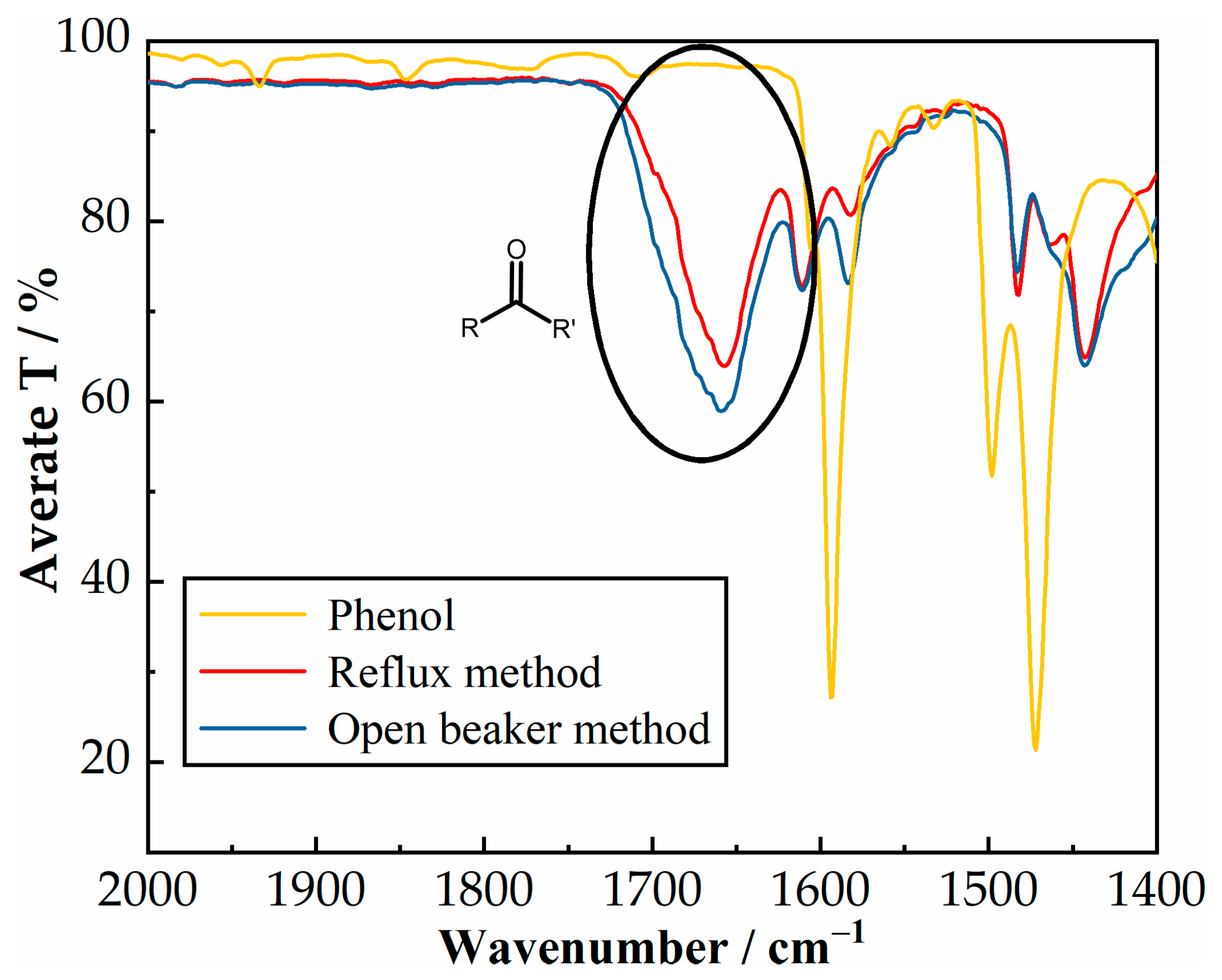
The preparation of alkali metal phenoxides was carried out in two different ways, as described by Cameron et al. in 1949 [
12]. Although this article is moderately outdated, not very detailed, and lacks correlation between the obtained results and preparation methods, in our work certain steps were modified. Since metal hydroxides are provided in pelletized form, they were primarily crushed using a ball mill, dried overnight in the oven, and stored in the desiccator until needed for use. Metal carbonates are provided as small particles; therefore, they were only oven-dried overnight (110 °C, ~18 h) prior to usage. The open beaker method involved mixing the alkali metal, solvent, and phenol in the beaker and heating it to dryness, after which it was additionally dried in the vacuum oven. The reflux method involved dissolving phenol in 10 mL of solvent, while the alkali metals were introduced into the round-bottom flask with 30 mL of solvent. Half of the phenol was introduced immediately, and the second half after 1.5 h. The synthesis was carried out for 3 h overall under reflux at 120 °C, after which it was vacuum filtered to obtain solid metal phenoxides, which were then dried overnight in the vacuum oven at 40 °C. The contents were introduced into glass liners, and the carboxylation step of metal phenoxides was carried out in a Parr 5000 Multi Reactor System (Parr Instrument Company, Moline, IL, USA) with six 75 mL parallel batch reactors and individual temperature and pressure controls. Agitation was performed with magnetic stirrers at 1000 min
−1. After introducing the metal phenoxide into the reactor, the reactor itself was purged three times with 10 bar of CO
2, and then 40 bar of CO
2 was released into the reactor. The temperature was set to 473 K (200 °C) with a heat-up rate of 7 K min
−1, and once the temperature was reached, the start of the reaction was noted. After 5 h of reaction, the reactors were cooled to room temperature, their contents vacuum dried overnight, weighed, dissolved, and prepared for high-performance liquid chromatography (HPLC) analysis, or analyzed as they were using Fourier transform infrared spectroscopy (FTIR). The used HPLC machine was the Agilent 1100 with the Acclaim Organic Acid column (250 mm length, 4 mm diameter, 5 µm particle size) and the pre-column guard cartridge with identical packing (10 mm length, 4 mm diameter, 5 µm particle size). The ATR-FTIR analyses of solid samples were carried out on Spectrum Two Perkin Elmer (Manchester, UK) using a LiTaO
3 MIR detector, over the frequency range of 400 to 4000 cm
−1, with 4 cm
−1 resolution, 32 scans, and a constant sample pressure of 80 units. The obtained concentrations were expressed in terms of the conversion of phenol or as yields of the obtained product, for which Equations (1) and (2) have been used, respectively.
where:
is the initial concentration of phenol stoichiometrically calculated.
is the concentration of phenol at time t.
is the concentration of product x obtained at time t.
4. Conclusions
Carboxylation of phenol is a two-step process, widely known as the Kolbe–Schmitt method, involving first the reaction between phenol and a strong alkali metal base to obtain metal phenoxide, which, through carboxylation, can further be mostly converted to salicylic acid. A simpler option is the Marasse method, which involves a one-pot reaction between potassium carbonate and phenol under the CO2 atmosphere. This work provides the first comprehensive study of the entire two-step procedure involving the effect of the preparation method of metal phenoxide and the activity and selectivity of NaOH, KOH, Na2CO3, and K2CO3. It was confirmed that the Kolbe–Schmitt method is only possible with NaOH, KOH, and Na2CO3 bases, while the Marasse method is only possible with K2CO3. Regardless of the method used, salicylic acid is the main product, with 4-hydoxybenzoic acid and 4HiPh being minor products. Additionally, the preparation of metal phenoxides has been reported to be more reliable using reflux than the open-beaker method, which involves evaporating the solvent.
The activity of the mentioned bases has confirmed the reports published 70+ years ago, while this work primarily provides, for the first time, a detailed and easily reiterated preparation procedure with state-of-the-art technology and its effect on the activity and selectivity of the entire two-step reaction. Additionally, for the first time, a new analytical method has been reported for the analysis of obtained products, which significantly saves time and energy from previously reported characterization procedures, including detailed HPLC methodology and sample preparation procedures, as well as highly valuable FTIR qualitative and quantitative methods in metal phenoxide analysis. Overall, it is safe to say that the Kolbe–Schmitt reaction individually does not make a dent in conventional carbon capture and utilization (CCU) and carbon capture and storage (CCS) practices; however, it does play an important role in a greater picture and leaves a lot of room for further investigations and a deeper understanding of this highly complex and yet detrimental field. As such, this article acts as a great groundwork for sustainable conversion of lignin-derived phenols with environmentally concerning carbon dioxide into value-added monocarboxylic and dicarboxylic phenolics and, as such, will be a great reference for various fields of sustainability, biomass valorization, and CO2 utilization.