Temperature Management Strategy for Urban Air Mobility Batteries to Improve Energy Efficiency in Low-Temperature Conditions
Abstract
:1. Introduction
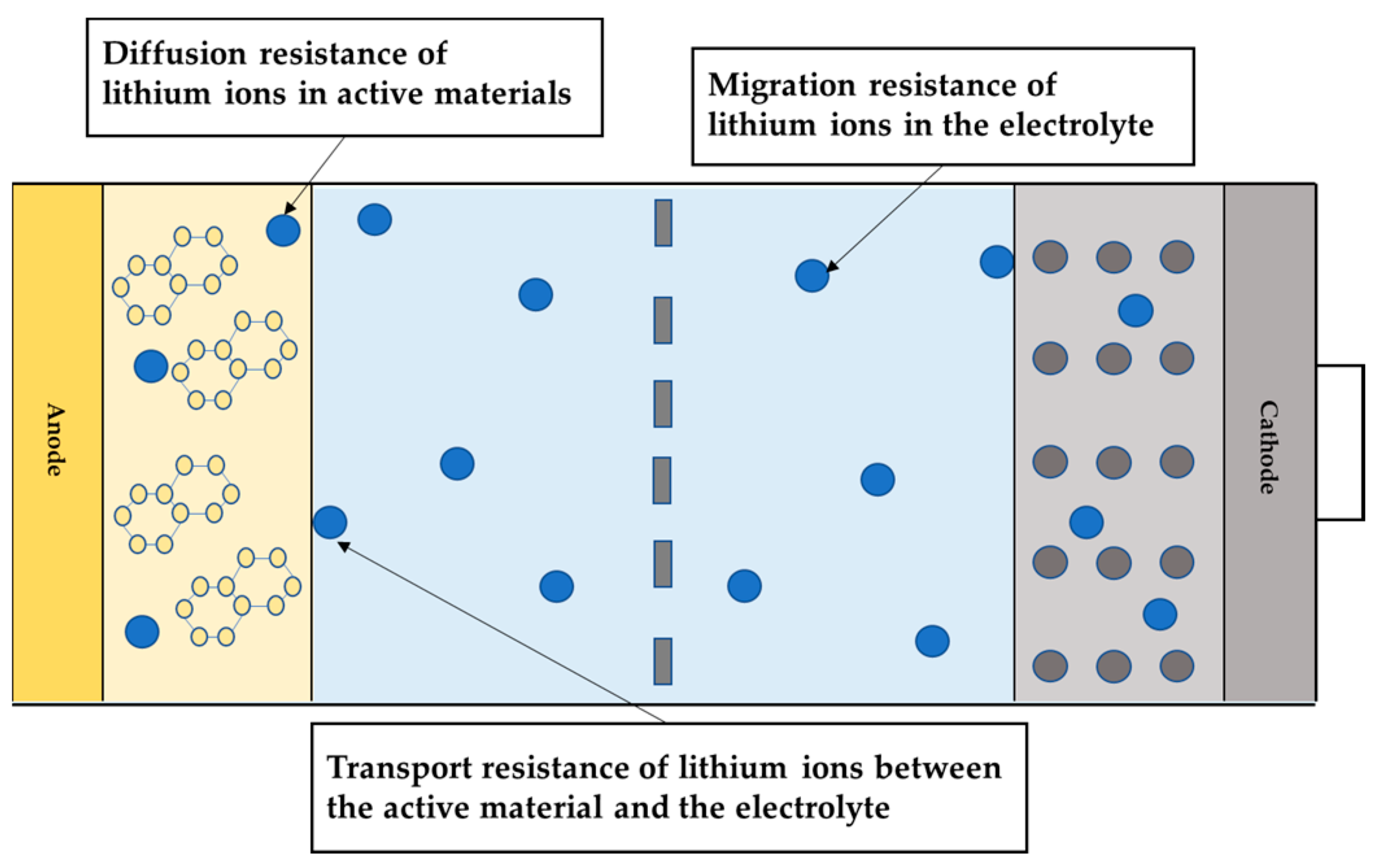
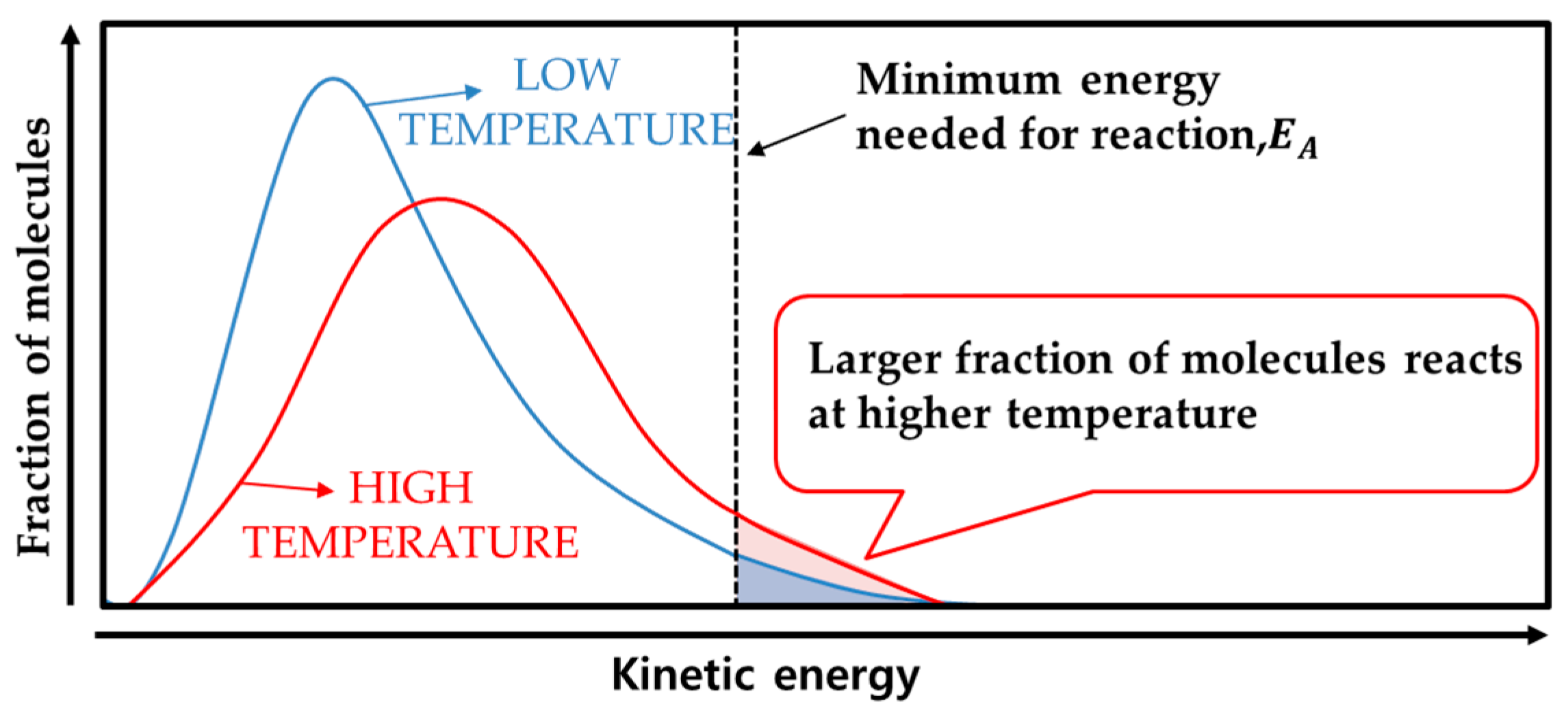
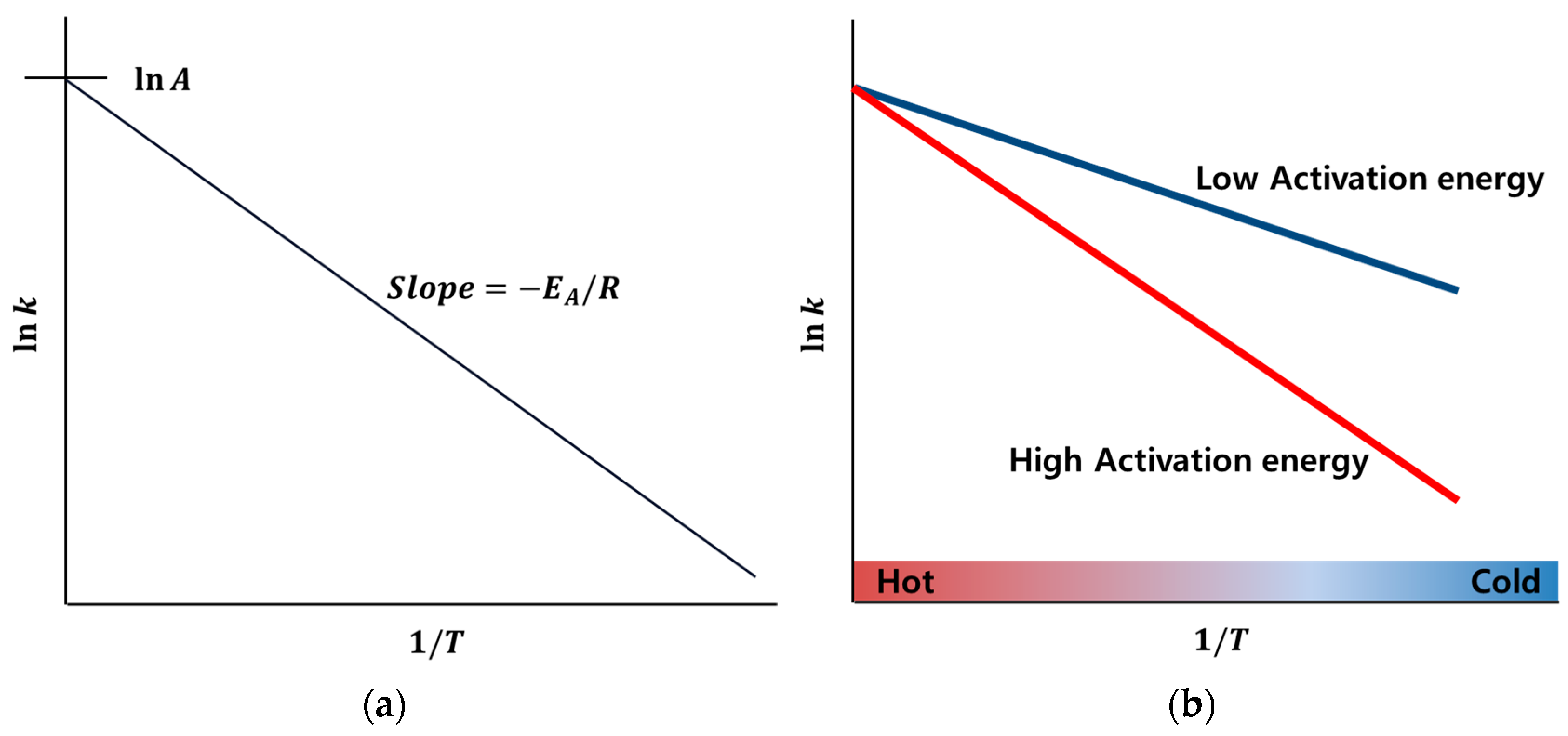
2. Low-Temperature Characteristics of Lithium-Ion Batteries
3. Lithium-Ion Battery Preheating Technology
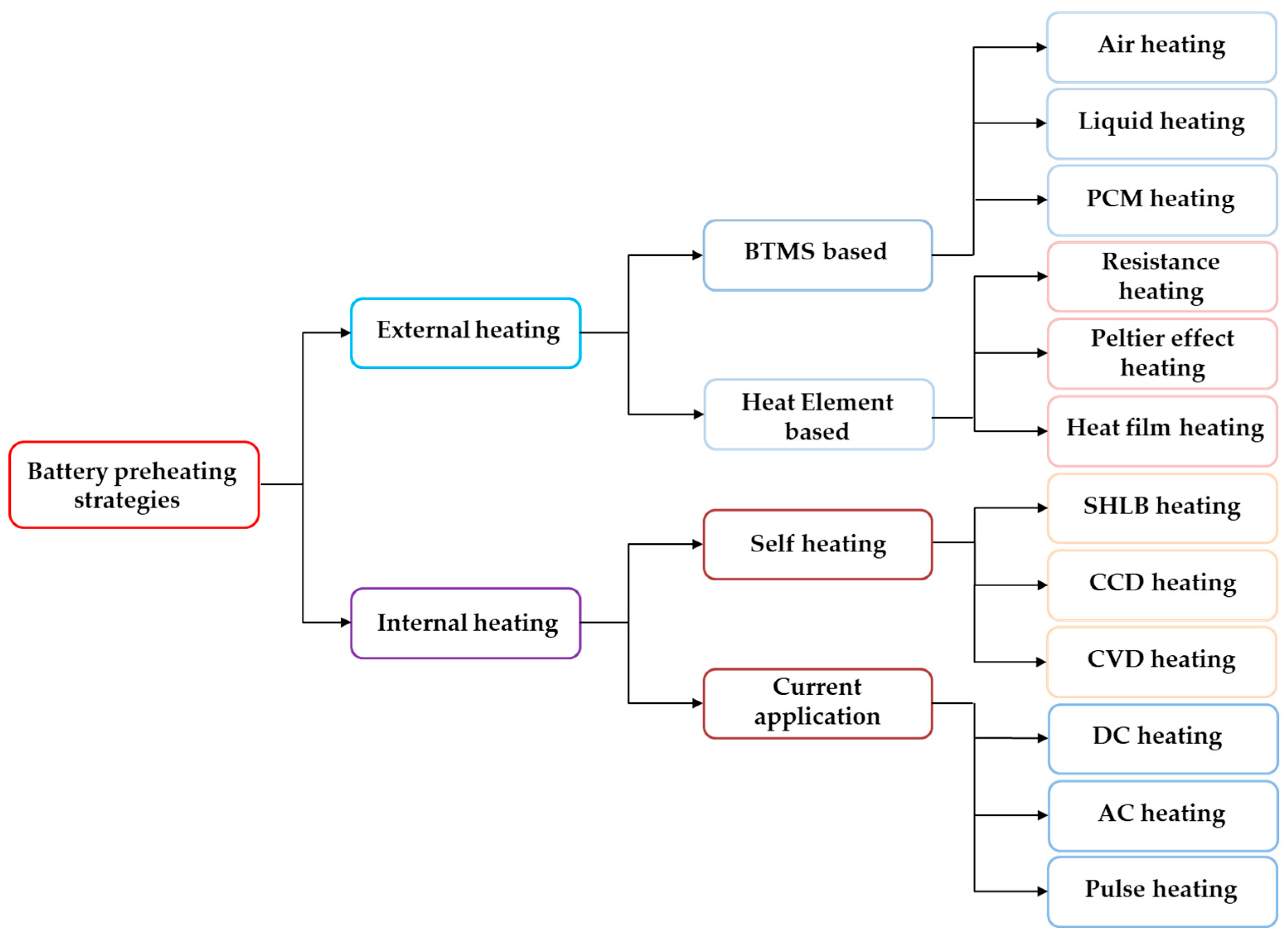
3.1. Classification of Lithium-Ion Battery Preheating Methods
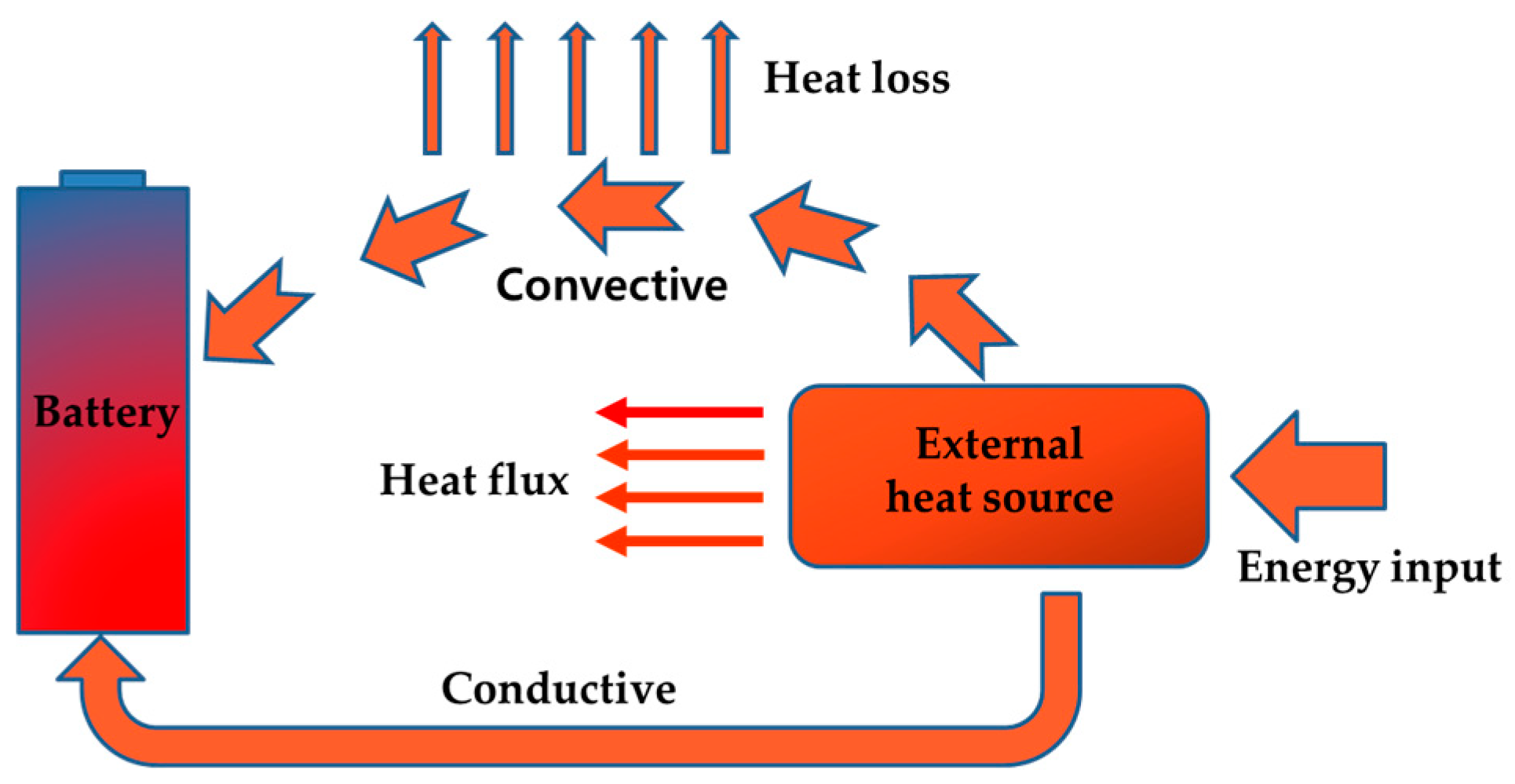
3.1.1. External Heating Strategy
3.1.2. Internal Heating Strategy
3.2. Lithium-Ion Battery Heating Model
Heating-Film-Based Preheating Model
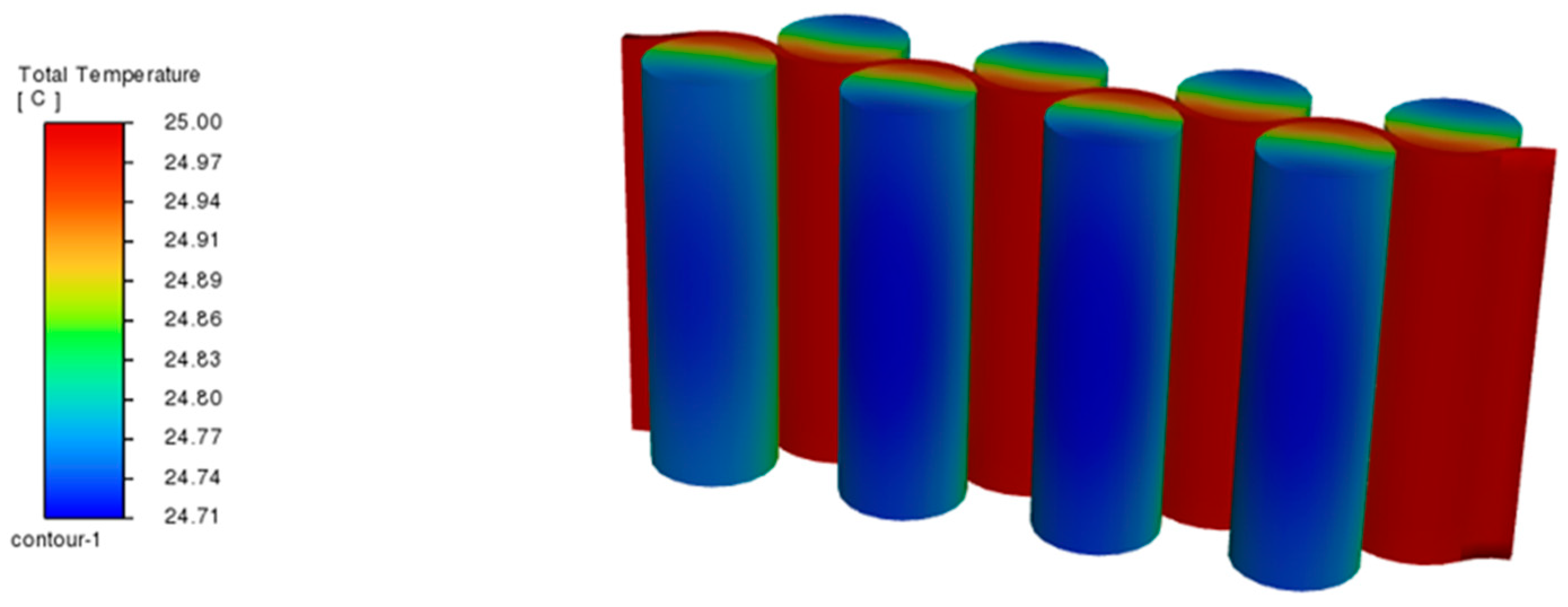
4. Experimental Method
5. Results and Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Human Settlements Programme. World Cities Report 2022: Envisaging the Future of Cities; United Nations Research Institute for Social Development: New York, NY, USA, 2022. [Google Scholar]
- Afrin, T.; Yodo, N. A survey of road traffic congestion measures towards a sustainable and resilient transportation system. Sustainability 2020, 12, 4660. [Google Scholar] [CrossRef]
- Garrow, L.A.; German, B.J.; Leonard, C.E. Urban air mobility: A comprehensive review and comparative analysis with autonomous and electric ground transportation for informing future research. Transp. Res. Part C Emerg. Technol. 2021, 132, 103377. [Google Scholar] [CrossRef]
- Yedavalli, P.; Mooberry, J. An Assessment of Public Perception of Urban Air Mobility (UAM). Airbus UTM: Defining Future Skies. 2019. Available online: https://www.airbus.com/sites/g/files/jlcbta136/files/2022-07/Airbus-UTM-public-perception-study%20-urban-air-mobility.pdf (accessed on 18 August 2024).
- Hong, A.; Park, A.-S.; Kim, M.-S. Policies/industry trends and issues related to urban air mobility (UAM). Electron. Telecommun. Res. Inst. 2023, 38, 36–46. [Google Scholar]
- Revision of Airworthiness Standards for Normal, Utility, Acrobatic, and Commuter Category Airplanes. Federal Register. Available online: https://www.federalregister.gov/documents/2016/12/30/2016-30246/revision-of-airworthiness-standards-for-normal-utility-acrobatic-and-commuter-category-airplanes/ (accessed on 30 December 2016).
- Federal Urban Air Mobility. Concept of Operations, v1. 0; Federal Aviation Administration: Washington, DC, USA, 2020.
- Federal Urban Air Mobility. Concept of Operations, v2. 0; Federal Aviation Administration: Washington, DC, USA, 2023.
- UAM (Urban Air Mobility), Leading the 3D Future Transportation System. SAMSUNG SDS. Available online: https://www.samsungsds.com/kr/insights/uam.240313.html (accessed on 13 March 2024).
- Cohen, A.P.; Shaheen, S.A.; Farrar, E.M. Urban air mobility: History, ecosystem, market potential, and challenges. IEEE Trans. Intell. Transp. Syst. 2021, 22, 6074–6087. [Google Scholar] [CrossRef]
- Qiao, X.; Chen, G.; Lin, W.; Zhou, J. The Impact of Battery Performance on Urban Air Mobility Operations. Aerospace 2023, 10, 631. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Dubé, Y. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl. Energy 2016, 164, 99–114. [Google Scholar] [CrossRef]
- Vidal, C.; Gross, O.; Gu, R.; Kollmeyer, P.; Emadi, A. xEV Li-ion battery low-temperature effects. IEEE Trans. Veh. Technol. 2019, 68, 4560–4572. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Li, X.; Chen, S.; Xing, S.; Guo, Y. Lithium-Ion Battery State of Health Estimation Based on Internal Resistance Compensation. Available online: https://ssrn.com/abstract=4791836 (accessed on 18 August 2024).
- Zhao, Z.; Wang, A.; Chen, A.; Zhao, Y.; Hu, Z.; Wu, K.; Luo, J. Leveraging Ion Pairing and Transport in Localized High-Concentration Electrolytes for Reversible Lithium Metal Anodes at Low Temperatures. Angew. Chem. 2024, e202412239. [Google Scholar] [CrossRef]
- Ringsby, A.J.; Fong, K.D.; Self, J.; Bergstrom, H.K.; McCloskey, B.D.; Persson, K.A. Transport phenomena in low temperature lithium-ion battery electrolytes. J. Electrochem. Soc. 2021, 168, 080501. [Google Scholar] [CrossRef]
- Stolz, L.; Winter, M.; Kasnatscheew, J. Practical relevance of charge transfer resistance at the Li metal electrode| electrolyte interface in batteries? J. Solid State Electrochem. 2024, 1–6. [Google Scholar] [CrossRef]
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]
- Blinder, S.; Nordman, C. Collision theory of chemical reactions. J. Chem. Educ. 1974, 51, 790. [Google Scholar] [CrossRef]
- Atkins, P.W.; De Paula, J. Physical Chemistry, 9th ed.; W. H. Freeman and Company: New York, NY, USA, 2010; pp. 800–801. [Google Scholar]
- Brown, T.L.; LeMay, J.H.E.; Bursten, B.E.; Murphy, C.J.; Woodward, P. Chemistry: The Central Science, 12th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2009; pp. 577–579. [Google Scholar]
- Di Ventra, M.; Kim, S.-G.; Pantelides, S.; Lang, N. Temperature effects on the transport properties of molecules. Phys. Rev. Lett. 2001, 86, 288. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zeng, X.; Li, Z. Understanding High-Temperature Chemical Reactions on Metal Surfaces: A Case Study on Equilibrium Concentration and Diffusivity of CxHy on a Cu (111) Surface. JACS Au 2022, 2, 443–452. [Google Scholar] [CrossRef]
- Mei, P.; Zhang, Y.; Zhang, W. Low-temperature lithium-ion batteries: Challenges and progress of surface/interface modifications for advanced performance. Nanoscale 2023, 15, 987–997. [Google Scholar] [CrossRef]
- Piao, N.; Gao, X.; Yang, H.; Guo, Z.; Hu, G.; Cheng, H.-M.; Li, F. Challenges and development of lithium-ion batteries for low temperature environments. Etransportation 2022, 11, 100145. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Chen, Z. Low temperature preheating techniques for Lithium-ion batteries: Recent advances and future challenges. Appl. Energy 2022, 313, 118832. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, Y.; Howey, D.A.; Perez, H.; Foley, A.; Pecht, M. Battery warm-up methodologies at subzero temperatures for automotive applications: Recent advances and perspectives. Prog. Energy Combust. Sci. 2020, 77, 100806. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Zhang, Y.; Lv, S.; Ni, H.; Deng, Y.; Yuan, Y. A review of the power battery thermal management system with different cooling, heating and coupling system. Energies 2022, 15, 1963. [Google Scholar] [CrossRef]
- Wu, S.; Xiong, R.; Li, H.; Nian, V.; Ma, S. The state of the art on preheating lithium-ion batteries in cold weather. J. Energy Storage 2020, 27, 101059. [Google Scholar] [CrossRef]
- Yu, X.; Lu, Z.; Zhang, L.; Wei, L.; Cui, X.; Jin, L. Experimental study on transient thermal characteristics of stagger-arranged lithium-ion battery pack with air cooling strategy. Int. J. Heat Mass Transf. 2019, 143, 118576. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Meng, H.; Guo, L.; Zhang, Z. Numerical analysis of the thermal performance of a liquid cooling battery module based on the gradient ratio flow velocity and gradient increment tube diameter. Int. J. Heat Mass Transf. 2021, 175, 121338. [Google Scholar] [CrossRef]
- Ianniciello, L.; Biwolé, P.H.; Achard, P. Electric vehicles batteries thermal management systems employing phase change materials. J. Power Sources 2018, 378, 383–403. [Google Scholar] [CrossRef]
- Wang, B.; Yan, M. Research on the Optimization of the Heating Effect of Lithium-Ion Batteries at a Low Temperature Based on an Electromagnetic Induction Heating System. Energies 2024, 17, 3678. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Zhang, G.; Ge, S.; Xu, T.; Ji, Y.; Yang, X.-G.; Leng, Y. Lithium-ion battery structure that self-heats at low temperatures. Nature 2016, 529, 515–518. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, C.Y. Heating strategies for Li-ion batteries operated from subzero temperatures. Electrochim. Acta 2013, 107, 664–674. [Google Scholar] [CrossRef]
- Liu, X.; Hong, X.; Jiang, X.; Li, Y.; Xu, K. Novel approach for liquid-heating Lithium-ion battery pack to shorten low temperature charge time. J. Energy Storage 2023, 68, 107507. [Google Scholar] [CrossRef]


| Item | Specification |
|---|---|
| Model | INR21700-40T |
| Capacity | 4000 mAh |
| Nominal voltage | 3.6 V |
| Max continuous discharge | 45 A |
| Cell dimension | Height: 70 mm, Diameter: 21 mm |
| Part | Density | Heat Capacity | Thermal Conductivity |
|---|---|---|---|
| Cell | 2255 | 980 | 18.2 |
| Heating film | 1420 | 1090 | 0.3 |
| Aluminum plate | 2700 | 900 | 200 |
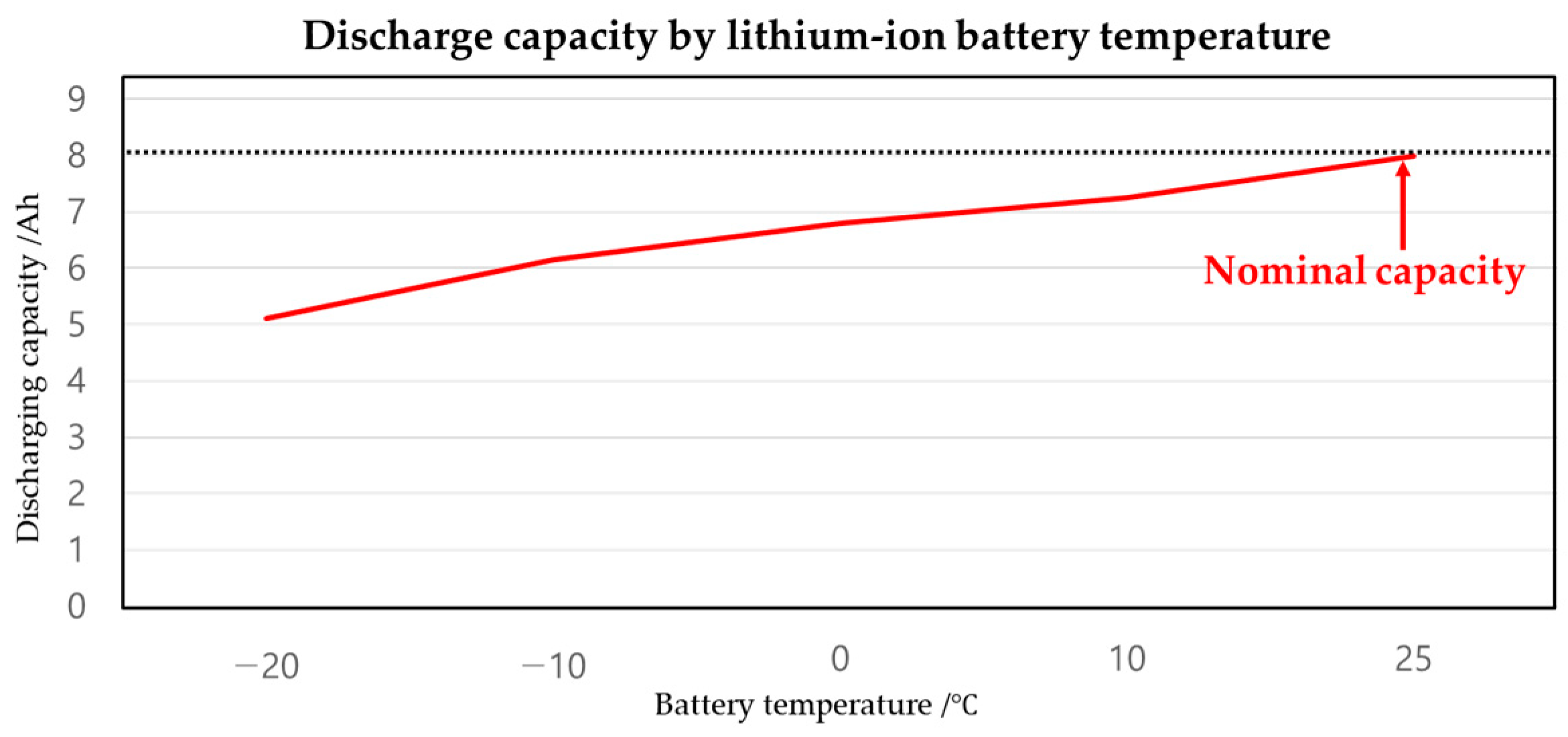
| Temperature | Capacity | Recovery Capacity | Recovery Energy | Consumed Energy | Energy Gain |
|---|---|---|---|---|---|
| −20 | 5.1 Ah | - | - | - | - |
| −10 | 6.16 Ah | 1.06 Ah | 17.808 Wh | 1.66 Wh | 16.148 Wh |
| 0 | 6.8 Ah | 640 mAh | 28.56 Wh | 5.13 Wh | 23.43 Wh |
| 10 | 7.25 Ah | 450 mAh | 36.12 Wh | 11.36 Wh | 24.76 Wh |
| 15 | 7.53 Ah | 280 mAh | 40.824 Wh | 16.66 Wh | 24.164 Wh |
| 20 | 7.78 Ah | 250 mAh | 45.024 Wh | 27.42 Wh | 17.604 Wh |
| 25 | 8 Ah | 230 mAh | 48.72 Wh | 75.11 Wh | −26.39 Wh |
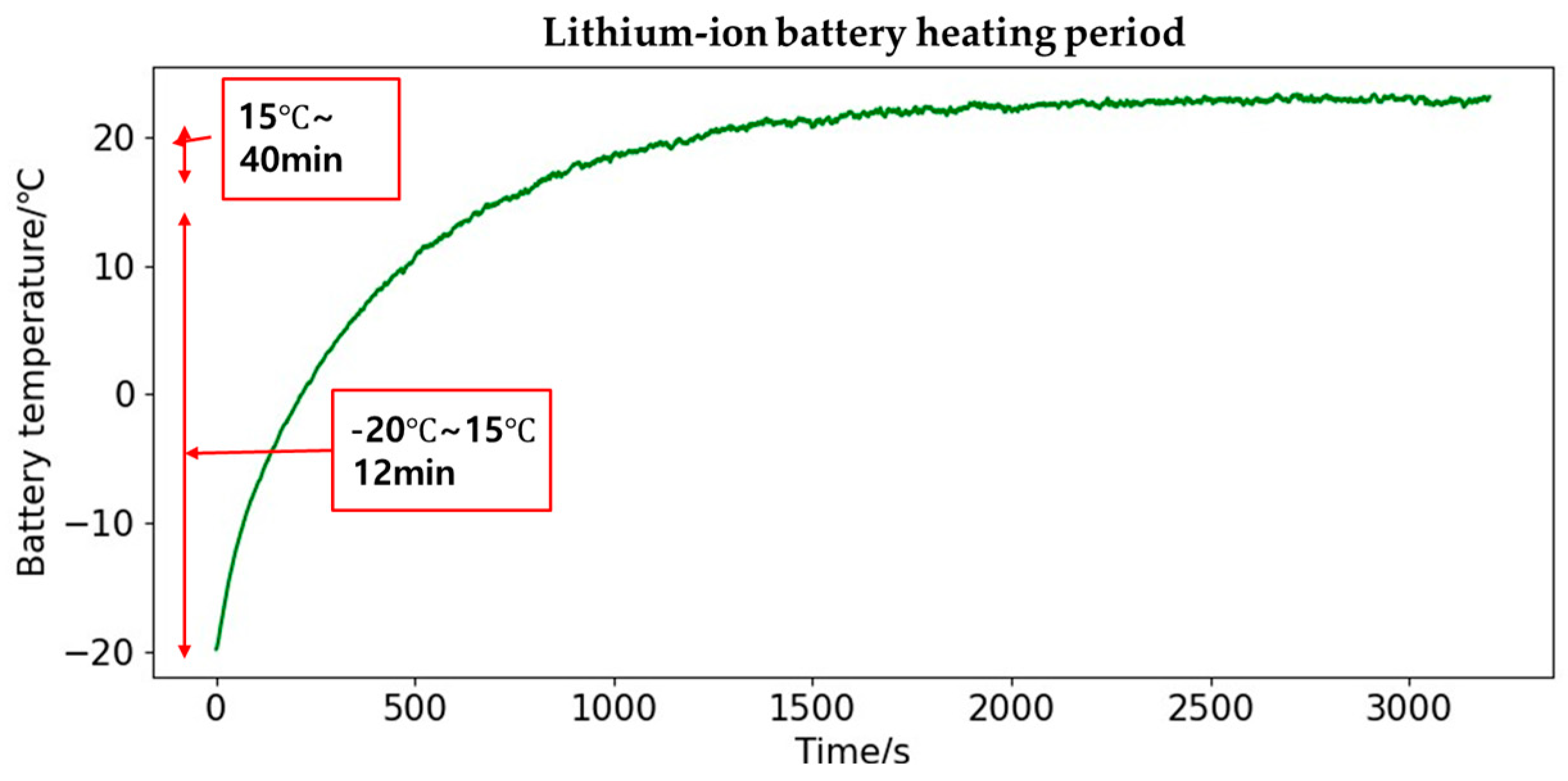
| Temperature | Preheating Period | Consumed Energy | Effectiveness of Preheating |
|---|---|---|---|
| −20 °C~21 °C | 1342 s | 31.38 Wh | High |
| −20 °C~21.5 °C | 1539 s | 36.3375 Wh | High |
| −20 °C~22 °C | 1808 s | 42.78 Wh | High |
| −20 °C~22.5 °C | 2062 s | 47.34 Wh | Medium |
| −20 °C~23 °C | 2661 s | 63.14 Wh | Poor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-W.; Kwon, D.-H.; Cho, I.-H. Temperature Management Strategy for Urban Air Mobility Batteries to Improve Energy Efficiency in Low-Temperature Conditions. Sustainability 2024, 16, 8201. https://doi.org/10.3390/su16188201
Kim S-W, Kwon D-H, Cho I-H. Temperature Management Strategy for Urban Air Mobility Batteries to Improve Energy Efficiency in Low-Temperature Conditions. Sustainability. 2024; 16(18):8201. https://doi.org/10.3390/su16188201
Chicago/Turabian StyleKim, Seon-Woong, Do-Hun Kwon, and In-Ho Cho. 2024. "Temperature Management Strategy for Urban Air Mobility Batteries to Improve Energy Efficiency in Low-Temperature Conditions" Sustainability 16, no. 18: 8201. https://doi.org/10.3390/su16188201






