Phytocapping for Municipal Solid Waste Landfills: A Sustainable Approach
Abstract
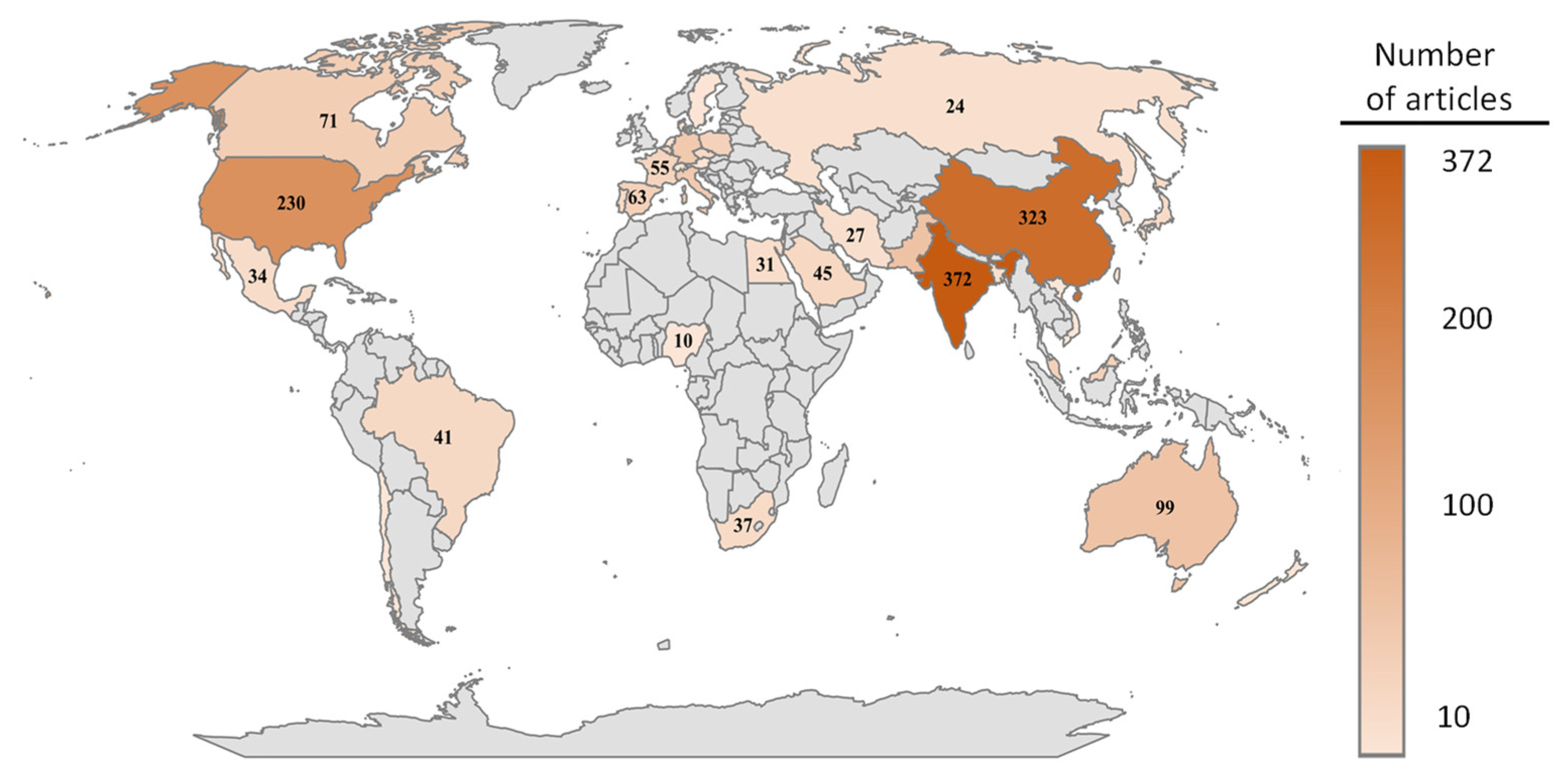
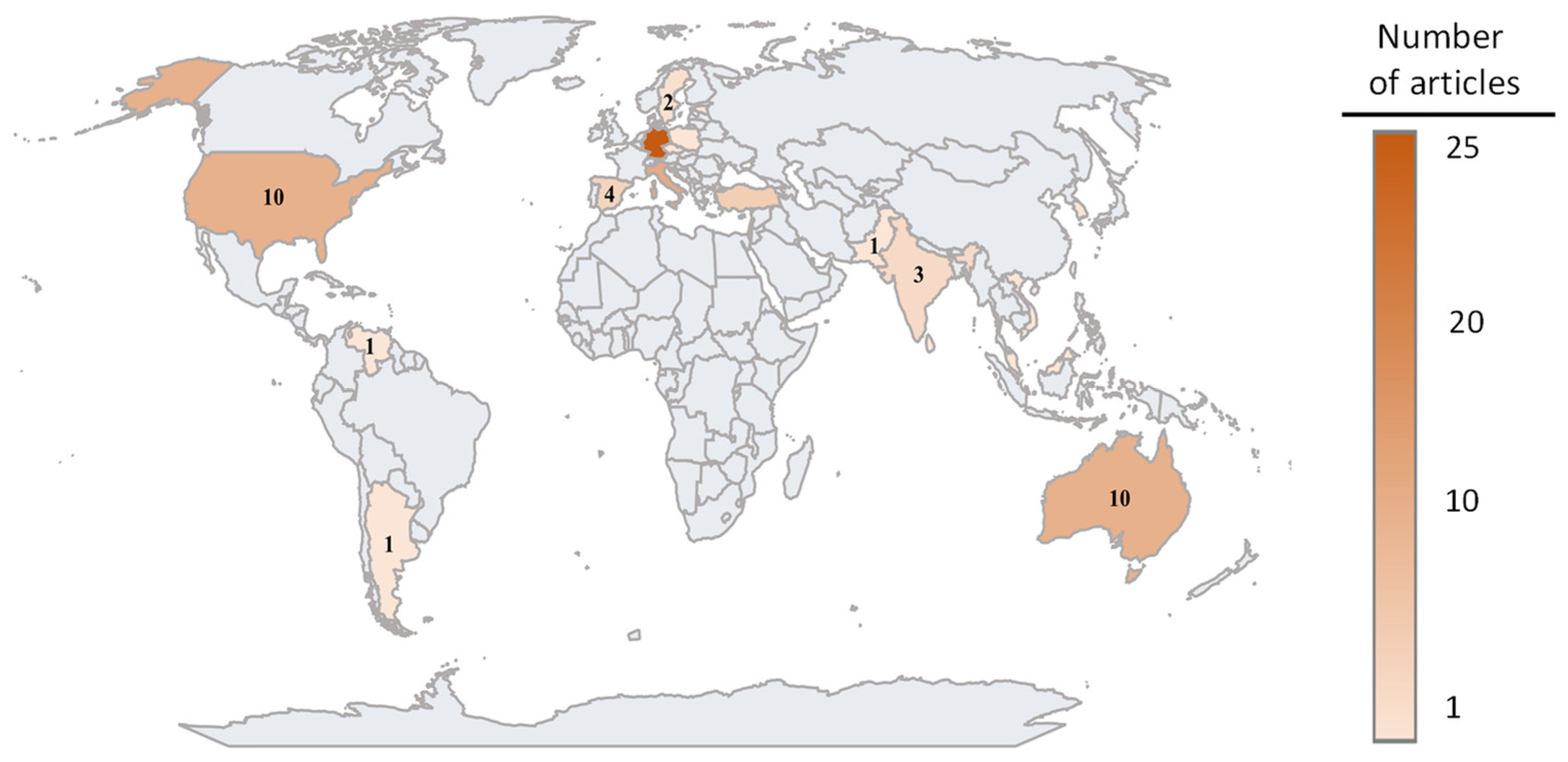
:1. Introduction
2. Development of Landfill Waste Management Technology to Overcome Environmental Challenges
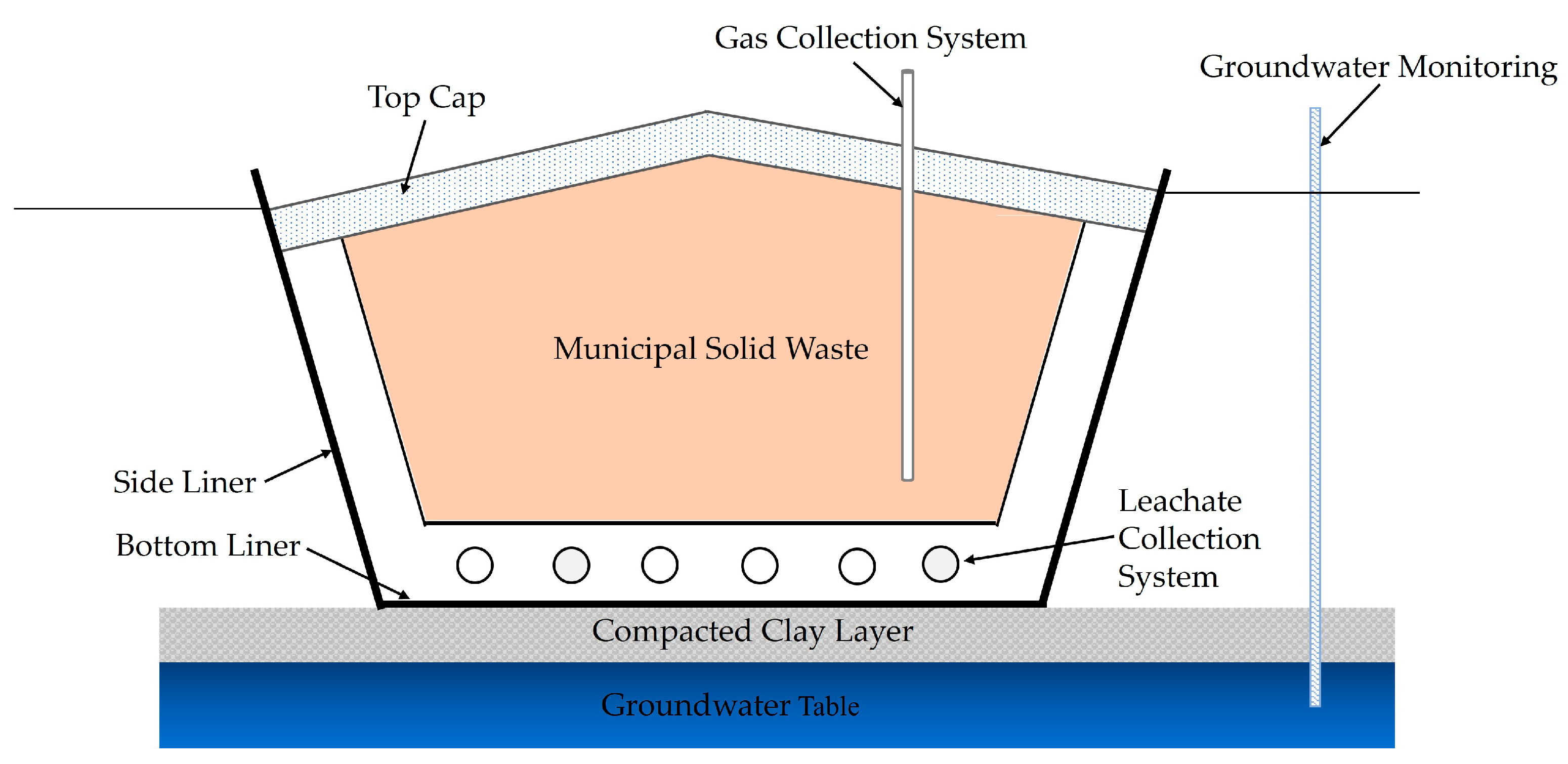
2.1. Conventional Landfill Cell and Capping
2.2. Role of Water Infiltration in Greenhouse Gas Production during Anaerobic Decomposition of MSW and Its Impact on the Environment
2.3. Conventional MSW Landfill Capping and Challenges
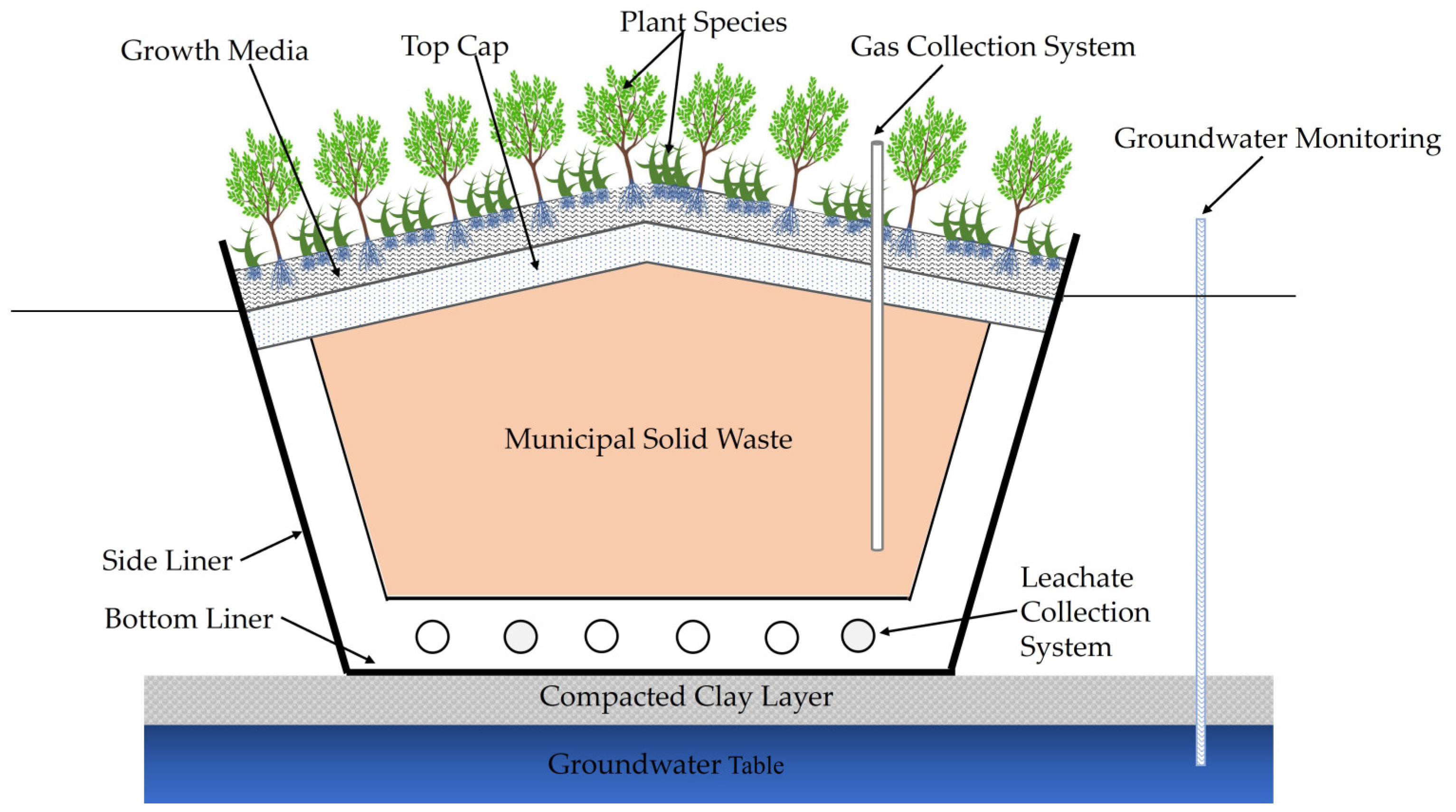
3. Development of Phytocapping Technology
3.1. Mechanisms Involved in MSW Landfill Phytocapping
3.2. Growth Media
3.3. Plant Selection for Phytocapping
3.3.1. Advantages of Native Plants over Non-Native Plants
3.3.2. Studied Plant Species in Phytocapping
3.4. Water Balance Performance Study of Landfill Phytocapping
3.5. Phytocapping: Challenges and Opportunities
4. Economic Benefits and Sustainability of Phytocapping
5. Conclusions
- Engineered landfill technology was developed after the 1970s, and compacted clay caps, GCLs, PVC and HDPE are commonly used in conventional landfill covers to reduce GHG production by minimising water infiltration. However, these approaches are expensive, and their performance is questionable in the long run.
- Phytocapping is a new landfill capping method that consists of a growth medium layer for growing vegetation over a landfill cap. Selecting appropriate plants and designing suitable growth media is challenging, as these depend on multiple climatic and geological variables. A few research studies have been conducted on landfill phytocapping to evaluate the effectiveness of phytocapping and found that phytocapping with CLO growth media is more economical and sustainable than conventional landfill methods.
- However, all this research has been conducted over short time periods and the findings are mainly based on either laboratory or field results. This is not adequate as it does not provide any acceptable design guidelines for growth media; appropriate mixing ratios for growth media amendments to enhance plant growth; initial irrigation requirements; or the mortality and survivability of phytocapping plants in different landfill environments. Therefore, fruitful research must be conducted in field and laboratory conditions to compare the performances of plants and growth media for phytocapping.
Author Contributions
Funding
Conflicts of Interest
References
- Gunarathne, V.; Ashiq, A.; Ramanayaka, S.; Wijekoon, P.; Vithanage, M. Biochar from municipal solid waste for resource recovery and pollution remediation. Environ. Chem. Lett. 2019, 17, 1225–1235. [Google Scholar] [CrossRef]
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management. Urban Development & Local Government Unit 15; World Bank: Washington, DC, USA, 2012; pp. 1–98. [Google Scholar]
- Lamb, D.T.; Venkatraman, K.; Bolan, N.; Ashwath, N.; Choppala, G.; Naidu, R. Phytocapping: An Alternative Technology for the Sustainable Management of Landfill Sites. Crit. Rev. Env. Sci. Tec. 2014, 44, 561–637. [Google Scholar] [CrossRef]
- Venkatraman, K.; Ashwath, N.; Su, N. Phytocapping of Municipal Landfills. In Landfills and Recycling Centers; Nova Science Publishers: New York, NY, USA, 2015; pp. 111–146. [Google Scholar]
- Tchobanoglous, G.; Theisen, H.; Vigil, S. Integrated Solid Waste Management,‘Engineering Principles and Management Issues’; McGraw–Hill Inc.: New York, NY, USA, 1993; p. 949. [Google Scholar]
- Khapre, A.; Kumar, S.; Rajasekaran, C. Phytocapping: An alternate cover option for municipal solid waste landfills. Env. Technol. 2019, 40, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Kumar, H.; Singh, L.; Sawarkar, A.D.; Kumar, M.; Kumar, S. Phytocapping Technology for Sustainable Management of Contaminated Sites: Case Studies, Challenges, and Prospects; Elsevier: London, UK, 2022; pp. 601–616. [Google Scholar]
- El-Fadel, M.; Findikakis, A.N.; Leckie, J.O. Environmental impacts of solid waste landfilling. J. Env. Manag. 1997, 50, 1–25. [Google Scholar] [CrossRef]
- Gallagher, L.; Ferreira, S.; Convery, F. Host community attitudes towards solid waste landfill infrastructure: Comprehension before compensation. J. Environ. Plan. Manag. 2008, 51, 233–257. [Google Scholar] [CrossRef]
- Bateman, S. National landfill survey. Landfill Des. 2005, 1, 7–9. [Google Scholar]
- Benson, C.H. Liners and covers for waste containment. In Proceedings of the Creation of New Geo-Environment, Fourth Kansai International Geotechnical Forum, Osaka, Japan, 2 November 2000; pp. 1–40. [Google Scholar]
- EPA. Environmental Management Of Landfill Facilities. Available online: https://www.epa.sa.gov.au/files/4771343_guide_landfill.pdf (accessed on 5 January 2023).
- Melchior, S. In-situ studies on the performance of landfill caps (compacted clay liners, geomembranes, geosynthetic clay liners, capillary barriers). In Proceedings of the International Containment Technology Conference and Exhibition, Petersburg, FL, USA, 9–12 February 1997. [Google Scholar]
- Levin, S.; Hammod, M. Application of PVC in a ‘Top Cap’application. In Geosynthetic Testing for Waste Containment Applications; American Society for Testing and Materials: Philadelphia, PA, USA, 1990. [Google Scholar]
- Scott, J.; Beydoun, D.; Amal, R.; Low, G.; Cattle, J. Landfill management, leachate generation, and leach testing of solid wastes in Australia and overseas. Crit. Rev. Env. Sci. Tec. 2005, 35, 239–332. [Google Scholar] [CrossRef]
- Benson, C.; Abichou, T.; Albright, W.; Gee, G.; Roesler, A. Field Evaluation of Alternative Earthen Final Covers. Int. J. PhytoreMediat. 2001, 3, 105–127. [Google Scholar] [CrossRef]
- Xiaoli, C.; Xin, Z.; Ziyang, L.; Shimaoka, T.; Nakayama, H.; Xianyan, C.; Youcai, Z. Characteristics of vegetation and its relationship with landfill gas in closed landfill. Biomass Bioenergy 2011, 35, 1295–1301. [Google Scholar] [CrossRef]
- Nagendran, R.; Selvam, A.; Joseph, K.; Chiemchaisri, C. Phytoremediation and rehabilitation of municipal solid waste landfills and dumpsites: A brief review. Waste Manag. 2006, 26, 1357–1369. [Google Scholar] [CrossRef]
- Abichou, T.; Kormi, T.; Wang, C.; Melaouhia, H.; Johnson, T.; Dwyer, S. Use of evapotranspiration (ET) landfill covers to reduce methane emissions from municipal solid waste landfills. J. Water Resour. Prot. 2015, 7, 1087. [Google Scholar] [CrossRef]
- Yuen, S.; Salt, M.; Sun, J.; Benaud, P.; Zhu, G.; Jaksa, A.; Ghadiri, H.; Greenway, M.; Ashwath, N.; Fourie, A. Phytocapping as a sustainable cover for waste containment systems: Experience of the A-ACAP study. In Proceedings of the Thirteenth International Waste Management and Landfill Symposium, Santa Margherita di Pula, Sardinia, Italy, 3–7 October 2011. [Google Scholar]
- Fletcher, T. Neighborhood change at Love Canal: Contamination, evacuation and resettlement. Land Use Policy 2002, 19, 311–323. [Google Scholar] [CrossRef]
- Anderson, D. Does Landfill Leachate Make Clay Liners More Permeable. Civ. Eng. 1982, 52, 66–69. [Google Scholar]
- Gnanapragasam, N.; Lewis, B.-A.G.; Finno, R.J. Microstructural changes in sand-bentonite soils when exposed to aniline. J. Geotech. Eng. 1995, 121, 119–125. [Google Scholar] [CrossRef]
- Shackelford, C.D.; Benson, C.H.; Katsumi, T.; Edil, T.B.; Lin, L. Evaluating the hydraulic conductivity of GCLs permeated with non-standard liquids. Geotext. Geomembr. 2000, 18, 133–161. [Google Scholar] [CrossRef]
- Halse, Y.H.; Lord, A.; Koerner, R.M. Ductile-to-Brittle Transition Time in Polyethylene Geomembrane Sheet. ASTM Special Technical Publication; ASTM International: West Conshohocken, PA, USA, 1990; pp. 95–109. [Google Scholar]
- Yuen, S.T. Bioreactor Landfills Promoted by Leachate Recirculation: A Full-Scale Study. PhD Thesis, University of Melbourne, Melbourne, Australia, 1999. [Google Scholar]
- EPA. Environmental Management of Landfill Facilities—Solid Waste Disposal. Available online: https://www.epa.sa.gov.au/files/13482_guideline_landfill_solid_consultation.pdf (accessed on 20 October 2022).
- Othman, M.A.; Benson, C.H.; Chamberlain, E.J.; Zimmie, T.F. Laboratory Testing to Evaluate Changes in Hydraulic Conductivity of Compacted Clays Caused by Freeze-Thaw: State-of-the-Art. ASTM Special Technical Publication; ASTM International: West Conshohocken, PA, USA, 1994; Volume 1142, pp. 227–254. [Google Scholar]
- Ashwath, N.; Venkatraman, K. Phytocapping of landfills: A Rockhampton experience. In Proceedings of the WasteQ Conference, Mackay, QLD, Australia, 17–19 October 2007. [Google Scholar]
- Simon, F.-G.; Müller, W.W. Standard and alternative landfill capping design in Germany. Environ. Sci. Policy 2004, 7, 277–290. [Google Scholar] [CrossRef]
- Themelis, N.J.; Ulloa, P.A. Methane generation in landfills. Renew. Energy 2007, 32, 1243–1257. [Google Scholar] [CrossRef]
- Hartz, K.; Ham, R. Moisture level and movement effects on methane production rates in landfill samples. Waste Manag. Res. 1983, 1, 139–145. [Google Scholar] [CrossRef]
- Rees, J.F. Optimisation of methane production and refuse decomposition in landfills by temperature control. J. Chem. Technol. Biotechnol. 1980, 30, 458–465. [Google Scholar] [CrossRef]
- Boeckx, P.; Van Cleemput, O. Methane Oxidation in a Neutral Landfill Cover Soil: Influence of Moisture Content, Temperature, and Nitrogen-Turnover; 0047-2425; Wiley: Hoboken, NJ, USA, 1996. [Google Scholar]
- Visvanathan, C.; Pokhrel, D.; Cheimchaisri, W.; Hettiaratchi, J.; Wu, J.S. Methanotrophic activities in tropical landfill cover soils: Effects of temperature, moisture content and methane concentration. Waste Manag. Res. 1999, 17, 313–323. [Google Scholar] [CrossRef]
- Moore, S. Abatement of Methane Emissions. IEA Greenhouse Gas Research and Development Program: Cheltenham, UK, 1998. [Google Scholar]
- Börjesson, G.; Svensson, B.H. Seasonal and diurnal methane emissions from a landfill and their regulation by methane oxidation. Waste Manag. Res. 1997, 15, 33–54. [Google Scholar]
- USEPA. Inventory of US Greenhouse gas Emissions and Sinks; US Environmental Protection Agency, Office of Policy, Planning and Evaluation: Washington, DC, USA, 1994.
- Hansen, J.E.; Sato, M.; Lacis, A.; Ruedy, R.; Tegen, I.; Matthews, E. Climate forcings in the industrial era. Proc. Natl. Acad. Sci. USA 1998, 95, 12753–12758. [Google Scholar] [CrossRef] [PubMed]
- Abichou, T.; Powelson, D.; Chanton, J.; Escoriaza, S.; Stern, J. Characterization of methane flux and oxidation at a solid waste landfill. J. Environ. Eng. 2006, 132, 220–228. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Averyt, K.; Marquis, M. Climate Change 2007–The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007; Volume 4. [Google Scholar]
- McOmber, R.; Moore, C. Field evaluation of landfill methane movement and methane control systems. In Proceedings of the Land Disposal: Municipal Solid Waste, Proceedings of the 7th Annual Research Symposium, Philadelphia, PA, USA, 16–18 March 1981; pp. 104–115. [Google Scholar]
- Parker, A. Landfill gas problems—Case histories. In Proceedings of the Landfill Gas Symposium; Wiley: Harwell, UK, 1981; pp. 161–175. [Google Scholar]
- Raybould, J.; Anderson, D. Migration of landfill gas and its control by grouting—A case history. Q. J. Eng. Geol. Hydrogeol. 1987, 20, 75–83. [Google Scholar] [CrossRef]
- Shafer, R.; Renta-Babb, A.; Bandy, J.; Smith, E.; Malone, P. Landfill Gas Control at Military Installations; Construction Engineering Research Lab (Army): Champaign, IL, USA, 1984. [Google Scholar]
- Stearns, R.; Petoyan, G. Utilization of landfills as building sites. Waste Manag. Res. 1984, 2, 75–83. [Google Scholar] [CrossRef]
- Feng, S.; Leung, A.K.; Ng, C.W.W.; Liu, H. Theoretical analysis of coupled effects of microbe and root architecture on methane oxidation in vegetated landfill covers. Sci. Total Environ. 2017, 599, 1954–1964. [Google Scholar] [CrossRef]
- Bian, R.; Shi, W.; Chai, X.; Sun, Y. Effects of plant radial oxygen loss on methane oxidation in landfill cover soil: A simulative study. Waste Manag. 2020, 102, 56–64. [Google Scholar] [CrossRef]
- Ko, J.H.; Xu, Q.; Jang, Y.-C. Emissions and control of hydrogen sulfide at landfills: A review. Crit. Rev. Env. Sci. Tec. 2015, 45, 2043–2083. [Google Scholar] [CrossRef]
- Haslina, H.; NorRuwaida, J.; Dewika, M.; Rashid, M.; Khairunnisa, M.; Azmi, M.A.D. Landfill leachate treatment methods and its potential for ammonia removal and recovery-A review. Proc. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012064. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, Q.-Y.; Zheng, J.; Li, Y.-H.; Xiang, S.; Sun, X.-J.; He, X.-S. Volatile and semi-volatile organic compounds in landfill gas: Composition characteristics and health risks. Environ. Int. 2023, 174, 107886. [Google Scholar] [CrossRef]
- Al-Yaqout, A.; Hamoda, M. Evaluation of landfill leachate in arid climate—A case study. Environ. Int. 2003, 29, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Danthurebandara, M.; Van Passel, S.; Nelen, D.; Tielemans, Y.; Van Acker, K. Environmental and socio-economic impacts of landfills. In Proceedings of the Linnaeus ECO-TECH Kalmer, Kalmar, Sweden, 26–28 November; 2012; pp. 40–52. [Google Scholar]
- Mann, H.; Schmadeke, C. Investigation leads to solution for landfill leachate seepage. Public Work. 1986, 117, 54. [Google Scholar]
- Lehane, M. Environment in Focus: A Discussion Document on Key Environmental Indicators for Ireland; Environmental Protection Agency: Wexford, Ireland, 1999.
- Badv, K. An overview on the effect of open dump solid waste landfill in Urmia City, Iran, on the surrounding soil and groundwater resources. In Proceedings of the International Conference on Environmental Science and Technology, Kalamata, Greece, 16–18 July 2002. [Google Scholar]
- Neufeld, D. An ecosystem approach to planning for groundwater: The case of Waterloo Region, Ontario, Canada. Hydrogeol. J. 2000, 8, 239–250. [Google Scholar] [CrossRef]
- Bocanegra, E.; Massone, H.; Martinez, D.; Civit, E.; Farenga, M. Groundwater contamination: Risk management and assessment for landfills in Mar del Plata, Argentina. Environ. Geol. 2001, 40, 732–741. [Google Scholar] [CrossRef]
- Rao, K.J.; Shantaram, M.V. Soil and Water Pollution Due to Open Landfills; Environmental Protection Agency: Washington, DC, USA, 2021.
- Dunnet, S.C. Current issues at the South Fremantle landfill site, Western Australia. Rural. Remote Environ. Health 2004, 3, 40–51. [Google Scholar]
- Chu, L.M. Landfill Aftercare and Maintenance. In Sustainable Solid Waste Management; Elsevier: Amsterdam, The Netherlands, 2016; pp. 633–652. [Google Scholar]
- Daniel, D.E. Clay liners. In Geotechnical Practice for Waste Disposal; Chapman & Hall: London, UK, 1993; pp. 137–163. [Google Scholar]
- Albright, W.H.; Benson, C.H.; Gee, G.W.; Abichou, T.; McDonald, E.V.; Tyler, S.W.; Rock, S.A. Field performance of a compacted clay landfill final cover at a humid site. J. Geotech. Geoenviron. Eng. 2006, 132, 1393–1403. [Google Scholar] [CrossRef]
- Benson, C.H.; Thorstad, P.A.; Jo, H.-Y.; Rock, S.A. Hydraulic performance of geosynthetic clay liners in a landfill final cover. J. Geotech. Geoenviron. Eng. 2007, 133, 814–827. [Google Scholar] [CrossRef]
- Widomski, M.K.; Stępniewski, W.; Musz-Pomorska, A. Clays of different plasticity as materials for landfill liners in rural systems of sustainable waste management. Sustainability 2018, 10, 2489. [Google Scholar] [CrossRef]
- Albright, W.H.; Benson, C.H.; Gee, G.W.; Abichou, T. Examining the alternatives. Civ. Eng. 2003, 73, 70. [Google Scholar]
- Venkatraman, K. Phytocapping of Municipal Landfills: Evaluating the Performance of 21 Tree Species and Two Soil Depths. Ph.D. Thesis, Queensland University, Brisbane, QLD, Australia, 2013. [Google Scholar]
- Bouazza, A. Geosynthetic clay liners. Geotext. Geomembr. 2002, 20, 3–17. [Google Scholar] [CrossRef]
- Lin, L.-C.; Benson, C.H. Effect of wet-dry cycling on swelling and hydraulic conductivity of GCLs. J. Geotech. Geoenviron. Eng. 2000, 126, 40–49. [Google Scholar] [CrossRef]
- Board, M.; Laine, D. Coralling Liner Nightmares. MSW Manag. 1995, 9, 48–50. [Google Scholar]
- Crozier, F.; Walker, T. CQA+ GLLS = TEC: How much does your liner leak? Wastes Manag. 1995, 1, 24–26. [Google Scholar]
- Stark, T.D.; Newman, E.J.; Aust, R. Back-analysis of a PVC geomembrane-lined pond failure. Geosynth. Int. 2008, 15, 258–268. [Google Scholar] [CrossRef]
- Ankeny, M.; Coons, L.; Majumdar, N.; Kelsey, J.; Miller, M. Performance and cost considerations for landfill caps in semiarid climates. In Landfill Capping in the Semi-Arid West: Problems, Perspectives, and Solutions; Reynolds, T.D., Morris, R.C., Eds.; Environmental Science and Research Foundation: Idaho Falls, ID, USA, 1997; Volume 2, pp. 243–262. [Google Scholar]
- Fonia, A.; Singh, P.; Singh, V.; Kumar, D.; Tripathi, B.N. Molecular mechanisms of heavy metal hyperaccumulation in plants. In Phytoremediation of Environmental Pollutants; CRC Press: Boca Raton, FL, USA, 2017; pp. 99–116. [Google Scholar]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Kalve, S.; Sarangi, B.K.; Pandey, R.A.; Chakrabarti, T. Arsenic and chromium hyperaccumulation by an ecotype of Pteris vittata–prospective for phytoextraction from contaminated water and soil. Curr. Sci. 2011, 100, 888–894. [Google Scholar]
- García-Salgado, S.; García-Casillas, D.; Quijano-Nieto, M.A.; Bonilla-Simón, M.M. Arsenic and heavy metal uptake and accumulation in native plant species from soils polluted by mining activities. Water Air Soil. Pollut. 2012, 223, 559–572. [Google Scholar] [CrossRef]
- Srivastava, M.; Ma, L.Q.; Santos, J.A.G. Three new arsenic hyperaccumulating ferns. Sci. Total Environ. 2006, 364, 24–31. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Yang, Y.; Li, B.; Wu, Y.; Sun, H.; Yang, Y. Cadmium accumulation characteristics in turnip landraces from China and assessment of their phytoremediation potential for contaminated soils. Front. Plant Sci. 2016, 7, 1862. [Google Scholar] [CrossRef]
- Peng, K.; Luo, C.; You, W.; Lian, C.; Li, X.; Shen, Z. Manganese uptake and interactions with cadmium in the hyperaccumulator—Phytolacca Americana L. J. Hazard. Mater. 2008, 154, 674–681. [Google Scholar] [CrossRef]
- Marques, A.P.; Rangel, A.O.; Castro, P.M. Remediation of heavy metal contaminated soils: Phytoremediation as a potentially promising clean-up technology. Crit. Rev. Env. Sci. Tec. 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Sakakibara, M.; Ohmori, Y.; Ha, N.T.H.; Sano, S.; Sera, K. Phytoremediation of heavy metal-contaminated water and sediment by Eleocharis acicularis. CLEAN–Soil. Air Water 2011, 39, 735–741. [Google Scholar] [CrossRef]
- Wang, J.; Feng, X.; Anderson, C.W.; Xing, Y.; Shang, L. Remediation of mercury contaminated sites–a review. J. Hazard. Mater. 2012, 221, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sanz, A.; Millán, R.; Sierra, M.J.; Alarcón, R.; García, P.; Gil-Díaz, M.; Vazquez, S.; Lobo, M.C. Mercury uptake by Silene vulgaris grown on contaminated spiked soils. J. Env. Manag. 2012, 95, S233–S237. [Google Scholar] [CrossRef] [PubMed]
- Chaney, R.L.; Broadhurst, C.L.; Centofanti, T. Phytoremediation of soil trace elements. Trace Elem. Soils 2010, 2, 311–352. [Google Scholar]
- Bani, A.; Pavlova, D.; Echevarria, G.; Mullaj, A.; Reeves, R.D.; Morel, J.-L.J.-L.; Sulçe, S. Nickel hyperaccumulation by the species of Alyssum and Thlaspi (Brassicaceae) from the ultramafic soils of the Balkans. Bot. Serbica 2010, 34, 3–14. [Google Scholar]
- Koptsik, G. Problems and prospects concerning the phytoremediation of heavy metal polluted soils: A review. Eurasian Soil. Sci. 2014, 47, 923–939. [Google Scholar] [CrossRef]
- WoS. Web of Science Database. Available online: https://clarivate.com/ (accessed on 17 January 2023).
- Kumar, V.; Singh, K.; Shah, M.P.; Kumar, M. Chapter 22—Phytocapping: An eco-sustainable green technology for environmental pollution control. In Bioremediation for Environmental Sustainability; Kumar, V., Saxena, G., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 481–491. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Dickinson, N.M.; Baker, A.J.; Doronila, A.; Laidlaw, S.; Reeves, R.D. Phytoremediation of inorganics: Realism and synergies. Int. J. Phytoremediat. 2009, 11, 97–114. [Google Scholar] [CrossRef]
- Prabha, J.; Kumar, M.; Tripathi, R. Opportunities and challenges of utilizing energy crops in phytoremediation of environmental pollutants: A review. In Bioremediation for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2021; pp. 383–396. [Google Scholar]
- Dwyer, S.F. Finding a better cover. Civ. Eng. 2001, 71, 58–63. [Google Scholar]
- Hauser, V.L.; Weand, B.L.; Gill, M.D. Natural covers for landfills and buried waste. J. Environ. Eng. 2001, 127, 768–775. [Google Scholar] [CrossRef]
- Bundt, M.; Albrecht, A.; Froidevaux, P.; Blaser, P.; Flühler, H. Impact of preferential flow on radionuclide distribution in soil. Environ. Sci. Technol. 2000, 34, 3895–3899. [Google Scholar] [CrossRef]
- Glinski, D.; Lipic, J. Soil Physical Conditions and Plant Roots; CRC Press Inc.: Boca Raton, FL, USA, 1990. [Google Scholar]
- Johnson, A.; Roy, I.; Matthews, G.; Patel, D. An improved simulation of void structure, water retention and hydraulic conductivity in soil with the Pore-Cor three-dimensional network. Eur. J. Soil. Sci. 2003, 54, 477–490. [Google Scholar] [CrossRef]
- Rajkai, K.; Kabos, S.; Van Genuchten, M.T.; Jansson, P.-E. Estimation of water-retention characteristics from the bulk density and particle-size distribution of Swedish soils. Soil. Sci. 1996, 161, 832–845. [Google Scholar] [CrossRef]
- Nixon, D.; Stephens, W.; Tyrrel, S.; Brierley, E. The potential for short rotation energy forestry on restored landfill caps. Bioresour. Technol. 2001, 77, 237–245. [Google Scholar] [CrossRef]
- Islam, K.; Mulchi, C.; Ali, A. Interactions of tropospheric CO2 and O3 enrichments and moisture variations on microbial biomass and respiration in soil. Glob. Change Biol. 2000, 6, 255–265. [Google Scholar] [CrossRef]
- Lamb, D.T.; Heading, S.; Bolan, N.; Naidu, R. Use of Biosolids for Phytocapping of Landfill Soil. Water Air Soil. Poll. 2012, 223, 2695–2705. [Google Scholar] [CrossRef]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.-W. Role of organic amendments on enhanced bioremediation of heavy metal (loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar] [CrossRef]
- Huber-Humer, M.; Röder, S.; Lechner, P. Approaches to assess biocover performance on landfills. Waste Manag. 2009, 29, 2092–2104. [Google Scholar] [CrossRef]
- Börjesson, G.; Sundh, I.; Svensson, B. Microbial oxidation of CH4 at different temperatures in landfill cover soils. FEMS Microbiol. Ecol. 2004, 48, 305–312. [Google Scholar] [CrossRef]
- Stern, J.C.; Chanton, J.; Abichou, T.; Powelson, D.; Yuan, L.; Escoriza, S.; Bogner, J. Use of a biologically active cover to reduce landfill methane emissions and enhance methane oxidation. Waste Manag. 2007, 27, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Cabral, A.; Moreira, J.; Jugnia, L.-B. Biocover performance of landfill methane oxidation: Experimental results. J. Environ. Eng. 2010, 136, 785–793. [Google Scholar] [CrossRef]
- Jugnia, L.-B.; Aït-Benichou, S.; Fortin, N.; Cabral, A.R.; Greer, C.W. Diversity and dynamics of methanotrophs within an experimental landfill cover soil. Soil. Sci. Soc. Am. J. 2009, 73, 1479–1487. [Google Scholar] [CrossRef]
- Heijden, E.v.d.; Kuyper, T.W. Ecological strategies of ectomycorrhizal fungi of Salix repens: Root manipulation versus root replacement. Oikos 2003, 103, 668–680. [Google Scholar] [CrossRef]
- Ebbs, S.; Bushey, J.; Poston, S.; Kosma, D.; Samiotakis, M.; Dzombak, D. Transport and metabolism of free cyanide and iron cyanide complexes by willow. Plant Cell Environ. 2003, 26, 1467–1478. [Google Scholar] [CrossRef]
- Ettala, M.O.; Yrjönen, K.M.; Rossi, E.J. Vegetation coverage at sanitary landfills in Finland. Waste Manag. Res. 1988, 6, 281–289. [Google Scholar] [CrossRef]
- Phillips, I.; Greenway, M.; Robertson, S. Use of phytocaps in remediation of closed landfills- correct selection of soil materials. Land. Contam. Reclam. 2004, 12, 339–348. [Google Scholar] [CrossRef]
- Albright, W.H.; Benson, C.H.; Gee, G.W.; Roesler, A.C.; Abichou, T.; Apiwantragoon, P.; Lyles, B.F.; Rock, S.A. Field water balance of landfill final covers. J. Environ. Qual. 2004, 33, 2317–2332. [Google Scholar] [CrossRef]
- Weand, B.L.; Horin, J.D.; Hauser, V.L.; Gimon, D.M.; Gill, M.D.; Mehta, M.; Casagrande, D. Landfill Covers for Use at Air Force Installations; Air Force Center for Environmental Excellence, Brooks Air Force Base: Port San Antonio, TX, USA, 1999. [Google Scholar]
- Venkatraman, K.; Ashwath, N. Phytocapping: Importance of tree selection and soil thickness. Water Air Soil. Pollut. Focus. 2009, 9, 421–430. [Google Scholar] [CrossRef]
- Dimitriou, I.; Aronsson, P.; Weih, M. Stress tolerance of five willow clones after irrigation with different amounts of landfill leachate. Bioresour. Technol. 2006, 97, 150–157. [Google Scholar] [CrossRef]
- Justin, M.Z.; Pajk, N.; Zupanc, V.; Zupančič, M. Phytoremediation of landfill leachate and compost wastewater by irrigation of Populus and Salix: Biomass and growth response. Waste Manag. 2010, 30, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Klang-Westin, E.; Eriksson, J. Potential of Salix as phytoextractor for Cd on moderately contaminated soils. Plant Soil. 2003, 249, 127–137. [Google Scholar] [CrossRef]
- Perttu, K.L.; Features Submission, H.C. Biomass production and nutrient removal from municipal wastes using willow vegetation filters. J. Sustain. For. 1994, 1, 57–70. [Google Scholar] [CrossRef]
- Kiniry, J.R.; Williams, J.R.; Major, D.; Izaurralde, R.; Gassman, P.W.; Morrison, M.; Bergentine, R.; Zentner, R. EPIC model parameters for cereal, oilseed, and forage crops in the northern Great Plains region. Can. J. Plant Sci. 1995, 75, 679–688. [Google Scholar] [CrossRef]
- Robinson, B.; Green, S.; Mills, T.; Clothier, B.; van der Velde, M.; Laplane, R.; Fung, L.; Deurer, M.; Hurst, S.; Thayalakumaran, T. Phytoremediation: Using plants as biopumps to improve degraded environments. Soil. Res. 2003, 41, 599–611. [Google Scholar] [CrossRef]
- Gay, L.; Fritschen, L. An energy budget analysis of water use by saltcedar. Water Resour. Res. 1979, 15, 1589–1592. [Google Scholar] [CrossRef]
- Cleverly, J.R.; Smith, S.D.; Sala, A.; Devitt, D.A. Invasive capacity of Tamarix ramosissima in a Mojave Desert floodplain: The role of drought. Oecologia 1997, 111, 12–18. [Google Scholar] [CrossRef]
- Cittadino, A.; Sayevitz, M.; Bonini, M.P. Survival, Growth and Biomass Production. Environ. Eng. Manag. J. 2021, 20, 1157–1161. [Google Scholar] [CrossRef]
- Waisel, Y.; Eshel, A.; Kafkafi, U. Plant Roots—The Hidden Half; Marcel Dekkar Inc.: New York, NY, USA; Basel, Switzerland; Hong Kong, China, 1991. [Google Scholar]
- Moffat, A.; Houston, T. Tree establishment and growth at Pitsea landfill site, Essex, UK. Waste Manag. Res. 1991, 9, 35–46. [Google Scholar]
- Dwyer, S.F.; Stormont, J.C.; Anderson, C.E. Mixed Waste Landfill Design Report; Sandia National Lab. (SNL-NM): Albuquerque, NM, USA, 1999.
- Mok, H.-F.; Majumder, R.; Laidlaw, W.S.; Gregory, D.; Baker, A.J.; Arndt, S.K. Native Australian species are effective in extracting multiple heavy metals from biosolids. Int. J. Phytoremediat. 2013, 15, 615–632. [Google Scholar] [CrossRef]
- Licht, L.; Aitchison, E.; Schnabel, W.; English, M.; Kaempf, M. Landfill capping with woodland ecosystems. Pract. Period. Hazard. Toxic. Radioact. Waste Manag. 2001, 5, 175–184. [Google Scholar] [CrossRef]
- Lal, R. Soil erosion impact on agronomic productivity and environment quality. Crit. Rev. Plant Sci. 1998, 17, 319–464. [Google Scholar] [CrossRef]
- Bielders, C.; Alvey, S.; Cronyn, N. Wind erosion: The perspective of grass-roots communities in the Sahel. Land. Degrad. Dev. 2001, 12, 57–70. [Google Scholar] [CrossRef]
- Licht, L.A.; Isebrands, J. Linking phytoremediated pollutant removal to biomass economic opportunities. Biomass Bioenergy 2005, 28, 203–218. [Google Scholar] [CrossRef]
- Atlas of Living Australia. Available online: https://www.ala.org.au/ (accessed on 20 October 2022).
- AVH. The Australian Virtual Herbarium. Available online: https://avh.chah.org.au/ (accessed on 22 October 2022).
- State Flora. Available online: https://www.stateflora.sa.gov.au/ (accessed on 23 October 2022).
- Future, P.F.A. Available online: https://pfaf.org/user/Plant.aspx? (accessed on 1 August 2023).
- Plant Selector. Botanic Garden of South Australia. Available online: http://plantselector.botanicgardens.sa.gov.au/Plants/Details/2748 (accessed on 1 February 2023).
- Michael, R.N. Landfill Phytocap Development and Performance Evaluation Using Australian Native Plants; University of Melbourne, Department of Civil and Environmental Engineering: Melbourne, Australia, 2010. [Google Scholar]
- Kwit, C.; Collins, B. Native grasses as a management alternative on vegetated closure caps. Environ. Manag. 2008, 41, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Molz, F.J.; Browning, V.D. Effect of vegetation on landfill stabilization. Groundwater 1977, 15, 409–415. [Google Scholar] [CrossRef]
- Hui, L.; Chu, L. Identifying suitable tree species for evapotranspiration covers of landfills in humid regions using seedlings. Urban. For. Urban. Green. 2019, 38, 157–164. [Google Scholar] [CrossRef]
- Albright, W.H.; Benson, C.H. Alternative Cover Assessment Program 2002 Annual Report. US Environmental Protection Agency: Washington, DC, USA, 2002. [Google Scholar]
- Salt, M.; Jaksa, M.; Cox, J.; Lightbody, P. Final cover performance in the Australian Environment-the A-ACAP field trials. In Proceedings of the 7th International Congress on Environmental Geotechnics, Melbourne, Australia, 10–15 November 2014; pp. 1388–1396. [Google Scholar]
- Warren, R.W.; Hakonson, T.E.; Bostick, K.V. Choosing the most effective hazardous waste landfill cover. Remediat. J. 1996, 6, 23–41. [Google Scholar] [CrossRef]
- Jones, C.A. Effect of soil texture on critical bulk densities for root growth. Soil. Sci. Soc. Am. J. 1983, 47, 1208–1211. [Google Scholar] [CrossRef]
- BDA. The Full Cost of Landfill Disposal in Australia. Available online: https://www.dcceew.gov.au/sites/default/files/documents/landfill-cost.pdf (accessed on 21 August 2023).
- Palanivel, T.M.; Sulaiman, H. Generation and composition of municipal solid waste (MSW) in Muscat, Sultanate of Oman. APCBEE Procedia 2014, 10, 96–102. [Google Scholar] [CrossRef]
- Guo, H.; Xu, H.; Liu, J.; Nie, X.; Li, X.; Shu, T.; Bai, B.; Ma, X.; Yao, Y. Greenhouse gas emissions in the process of landfill disposal in China. Energies 2022, 15, 6711. [Google Scholar] [CrossRef]
- EPA, US. Basic Information about Landfill Gas. Available online: https://www.epa.gov/lmop/basic-information-about-landfill-gas (accessed on 23 June 2024).
- Rahman, M.M.; Beecham, S.; Iqbal, A.; Karim, M.R.; Rabbi, A.T.Z. Sustainability assessment of using recycled aggregates in concrete block pavements. Sustainability 2020, 12, 4313. [Google Scholar] [CrossRef]
- Kanwar, V.S.; Shukla, S.K.; John, S.; Kandra, H.S. Sustainable Civil Engineering: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Department of Primary Industries and Regional Development. Composting to Avoid Methane Production—Western Australia; Department of Primary Industries and Regional Development: South Perth, WA, Australia, 2022.

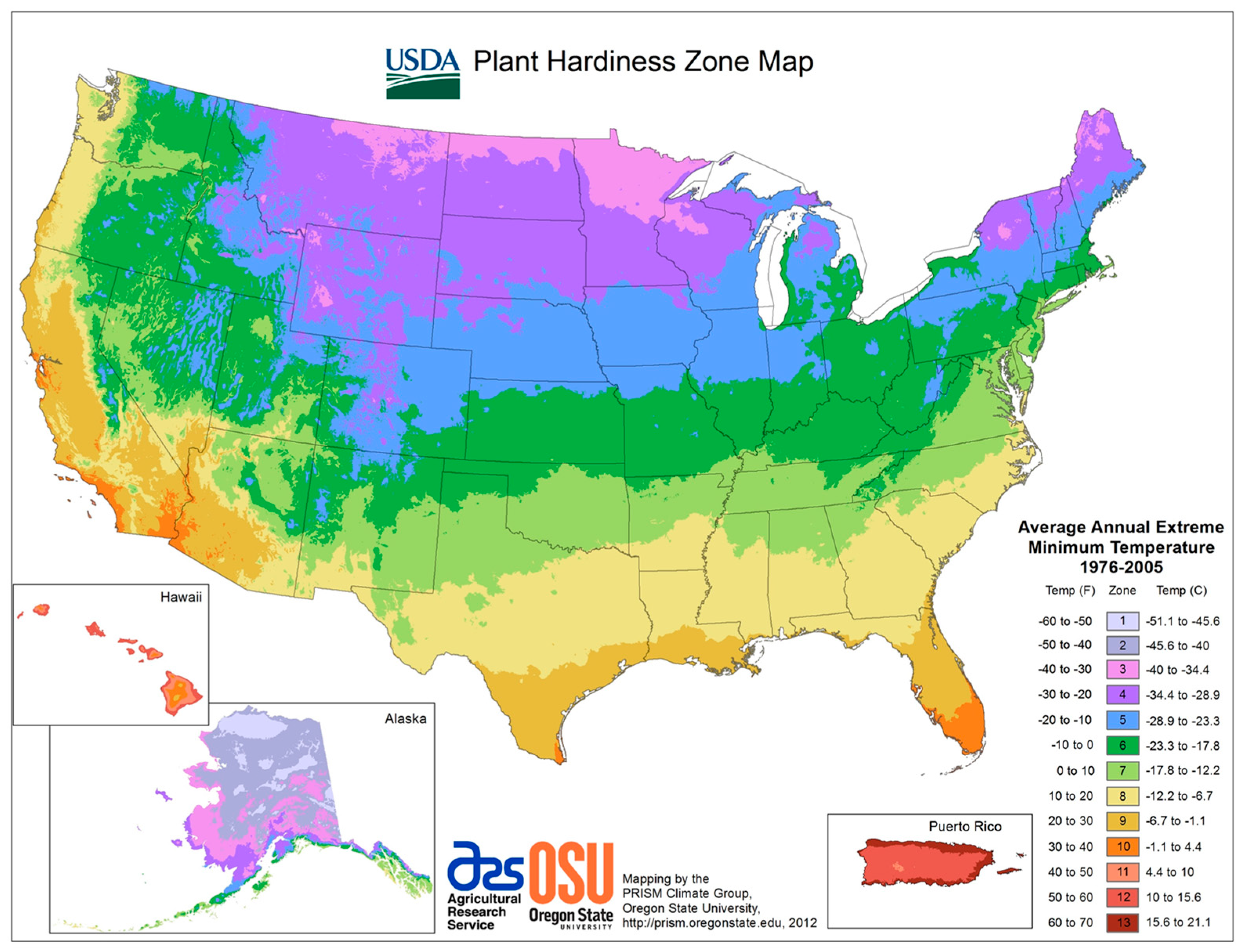
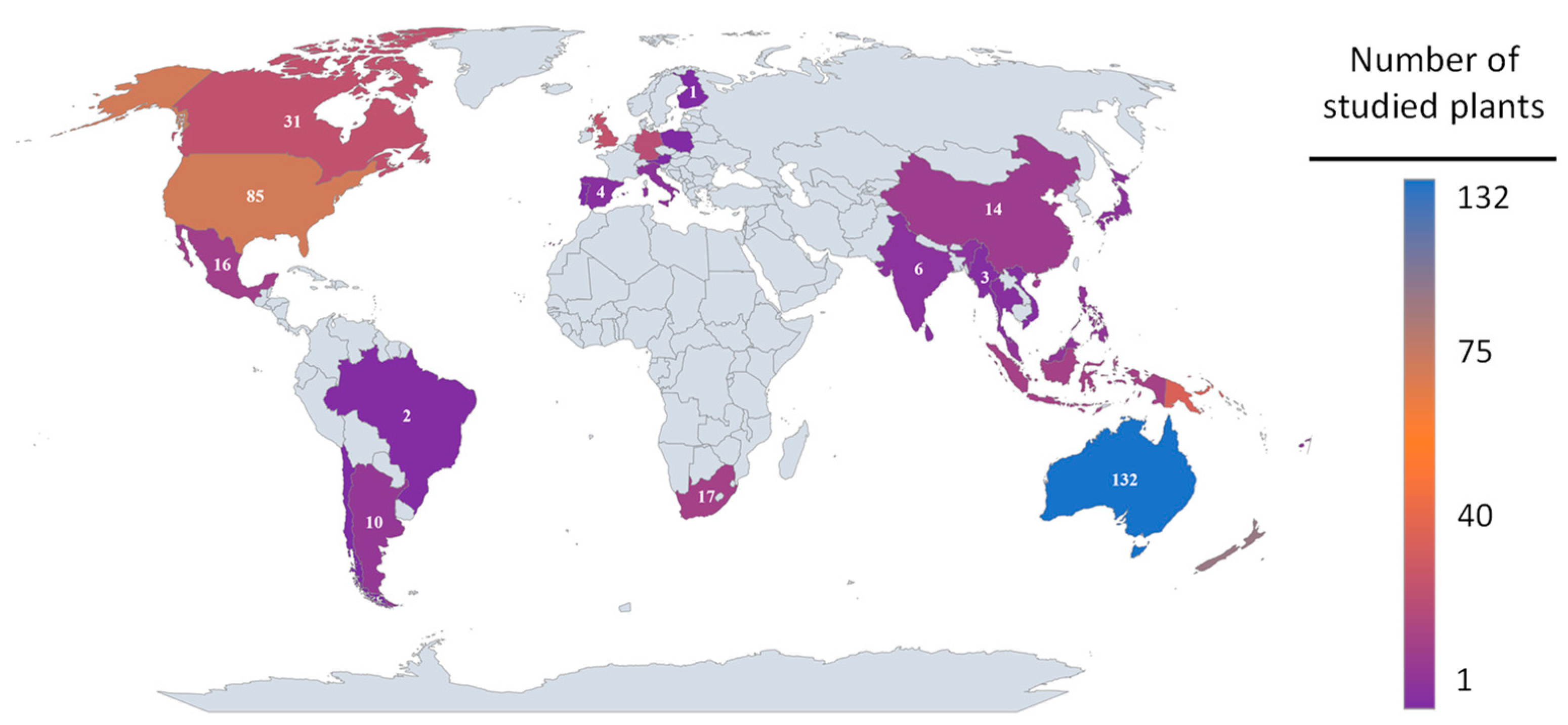


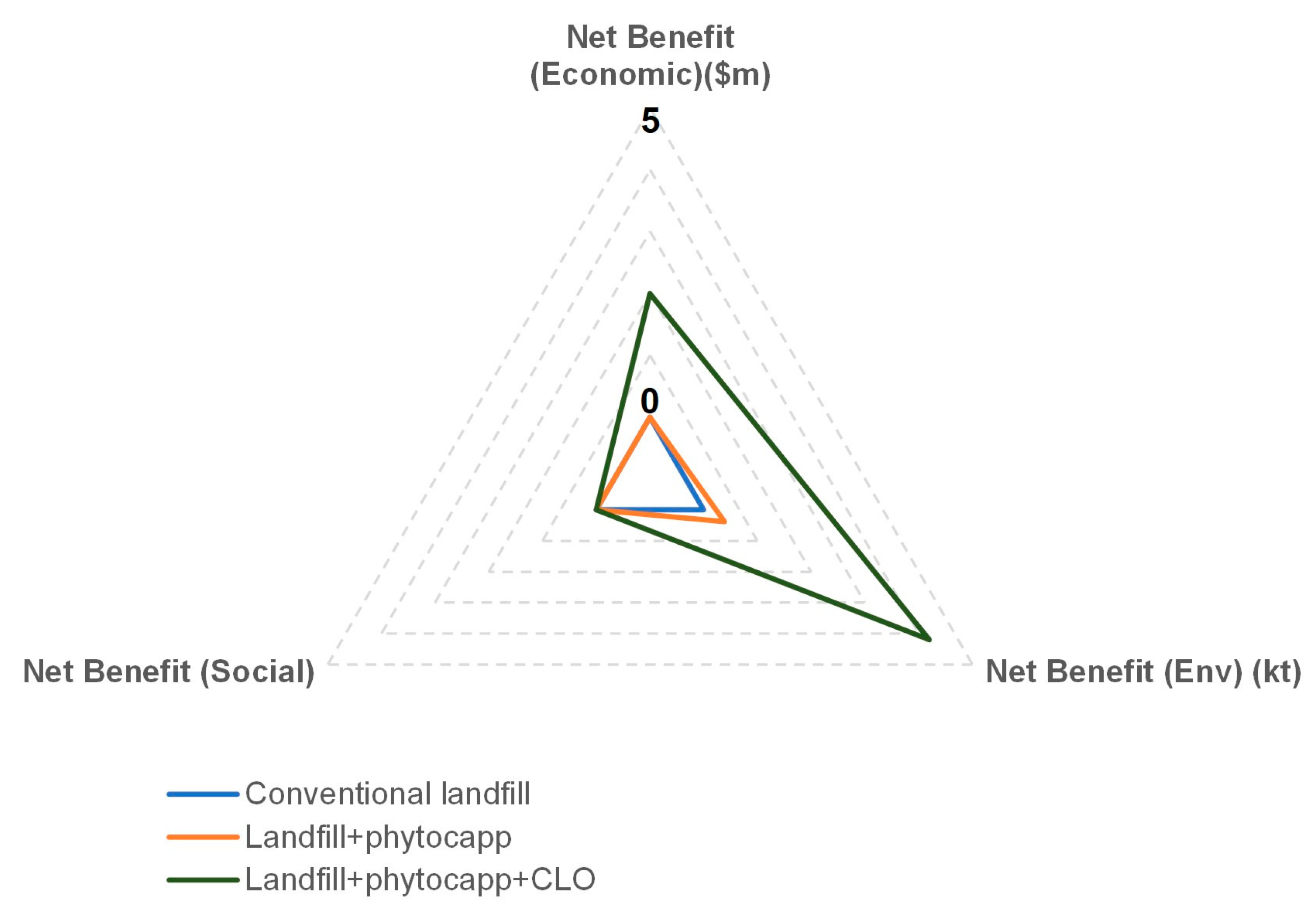
| Plant Species Name and Habitat | Family Name | Availability | Soil Type | pH | Rainfall Tolerance (mm) | Adap-Tability | Max Height (m) | Canopy Dia (m) | USDA Plant Hardi-ness Zone | Resear-Cher(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Acacia harpophylla (T) | Fabaceae | AUS, NSW | LoC | - | 300–700 | D | 25 | - | - | Venkatraman [67] |
| Acacia mangium (T) | Fabaceae | AUS, PNG, ID, MY, | SLC | - | - | D | 30 | - | 10–12 | |
| Pongamia pinnata (T) | Fabaceae | IN, ID, AUS, PNG, PH, CN, FIJI | SL | 6–9 | 500–2500 | D | 15–25 | - | 10–12 | |
| Eucalyptus grandis (T) | Myrtaceae | AUS, NSW, NZ | SLC | - | 990–1780 | - | 50 | - | 9–11 | |
| Eucalyptus raveretiana (T) | Myrtaceae | AUS, NSW | SLC | - | - | - | 21–30 | - | 9–12 | |
| Eucalyptus tereticornis (T) | Myrtaceae | AUS, PNG, NZ | SLC | 7–10 | 500–2000 | - | 20–35 | - | 9–12 | |
| Callistemon viminalis (T) | Myrtaceae | AUS, NZ | LoC | - | - | D | 10 | - | - | |
| Lophostemon confertus (T) | Myrtaceae | AUS, NZ | LoC | 4.5–7 | 900–1700 | C | 30 | - | 9–12 | |
| Melaleuca leucadendra (T) | Myrtaceae | AUS, MY, ID, PNG | SLC | 5.5–8.5 | 650–1600 | D | 30 | - | 10–12 | |
| Melaleuca linariifolia (S) | Myrtaceae | AUS, NZ | SLC | 4.5–7 | - | - | 6–10 | - | 8–11 | |
| Ficus microcarpa (T) | Moraceae | AUS, PNG, NZ, PH | SLC | 4.3–8.6 | 300–2700 | C, D | 8.5 | - | 6–10 | |
| Ficus racemose (T) | Moraceae | CN, IN, MM, MY, PH TH, VN, ID, PNG, AUS, NZ, USA | SLC | 4.5–8.0 | - | - | 12 | - | 9–12 | |
| Populus nigraitalica (T) | Salicaceae | AUS, NZ, USA | SLC | 4.5–8.0 | - | - | 20–30 | - | 3–9 | |
| Syzigium AUStralis (S) | Salicaceae | AUS, NZ, PNG, USA, ID | SLC | 4.5–8.0 | - | - | 3 | - | 6–12 | |
| Casuarina cunninghamiana (T) | Casuarinaceae | AUS, NZ | SLC | 4.5–8.5 | 500–4000 | D | 8–20 | - | 8–11 | |
| Casuarina glauca (T) | Casuarinaceae | AUS, NZ | SL | 4.5–8.5 | - | - | 18 | - | 8–11 | |
| Cupaniopsis anacardioides (T) | Sapindaceae | AUS, PNG, NZ | SLC | - | - | - | 10 | - | - | |
| Dendrocalamus latiflorus (T) | Poaceae | AUS, CN, MM, VN | SLC | - | - | C | 10–20 | - | 10–12 | |
| Hibiscus tiliaceus (T) | Malvaceae | AUS, PNG, ID | SL | 4–9 | 900–2500 | - | 6–8 | - | 10–12 | |
| Eucalyptus camaldulensis (T) | Myrtaceae | AUS, NZ | SL | 4.5–7.5 | 400 | D, L, MF | 20–40 | 10–15 | 8–12 | Michael [137] |
| Eucalyptus melliodora (T) | Myrtaceae | AUS, NZ | SL | 4.2–7.5 | 400 | D, L, MF | 20–35 | 20–30 | 9–11 | |
| Eucalyptus cladocalyx (T) | Myrtaceae | AUS | SLC | 4–9 | 350–600 | D, L, MF | 20–25 | 12–15 | - | |
| Eucalyptus polybractea (T) | Myrtaceae | AUS | SLC | 4.5–8.5 | 300 | D, MF | 2–8 | 4–8 | - | |
| Eucalyptus viridis (T) | Myrtaceae | AUS, NZ | SLC | 4.5–8.5 | 400 | D, MF | 4–8 | 3–5 | - | |
| Acacia mearnsii (T) | Fabaceae | AUS, NZ | SLC | 4.5–8.5 | 500 | D, L, MF | 5–15 | 5–20 | 8–11 | |
| Acacia pycnantha (T) | Fabaceae | AUS, NZ | SLC | 4.5–8.5 | 350 | D, L, MF | 4–6 | 2–6 | 7–10 | |
| Allocasuarina verticillate (T) | Casuarinaceae | AUS, NZ, SAF, GR | SLC | 4–9 | 350 | D, HF, S | 5–8 | 4–6 | 8–11 | |
| Callitris gracilis (T) | Cupressaceae | AUS, NZ, SAF | SL | 4–9 | 300 | D, HF, S | 7–20 | 3–8 | - | |
| Melaleuca lanceolata (T) | Myrtaceae | AUS, USA | SLC | 4–8.5 | 250 | D, HF, S | 3–8 | 3–5 | - | |
| Themeda triandra (G) | Poaceae | AUS, NZ, PNG, SAF, SRI | SLC | 4.5–8.5 | 100 | D, L, MF | 0.9–1.0 | 1.0 | - | |
| Microlaena stipoides (G) | Poaceae | AUS, NZ, PNG, USA | SLC | 4–6 | 200 | D, HF, S | 0.1–0.7 | 0.2–1.0 | 8–10 | |
| Bothriochloa macra (G) | Poaceae | AUS, NZ | SLC | 4.5–7.5 | 450–500 | D, L, MF | 0.4–0.8 | 0.2–0.4 | - | |
| Austrodanthonia caespitosa(G) | Poaceae | AUS, NZ | SLC | 4.5–7.5 | 300–450 | D, L, MF | 0.2–0.8 | 0.2–0.2 | - | |
| Poa labillardierei (G) | Poaceae | AUS, NZ | SLC | 4.5–7.5 | 300–500 | D, L, MF | 0.5–0.6 | 0.4–0.5 | - | |
| Austrostipa elegantissima (G) | Poaceae | AUS | SLC | 7–9 | - | D, L, HF | 0.5–1.0 | 1.0–1.0 | - | |
| Eucalyptus cladocalyx (T) | Myrtaceae | AUS, NZ | SLC | 4.5–8 | 350–600 | D, L, MF | 20–25 | 12–15 | - | [127] |
| Eucalyptus polybractea (T) | Myrtaceae | AUS | SLC | 4.5–8 | 300 | D, MF | 2–8 | 4–8 | - | |
| Allocasuarina verticillate (T) | Casuarinaceae | AUS, NZ, SAF, GR | SLC | 4.5–8.5 | 350 | D, HF, S | 5–8 | 4–6 | 8–11 | |
| Atriplex nummularia (S) | Chenopodiaceae | AUS | SLC | 4–8.5 | 230–650 | D, HF, S | 2–4 | 1–3 | 7–10 | |
| Acacia mearnsii (T) | Fabaceae | AUS, NZ, SAF | SL | 5.0–7.2 | 660–2280 | D, L, MF | 10 | - | 8–11 | |
| Grevillea robusta (T) | Proteaceae | AUS, NZ | SLC | 4.5–8 | 450–550 | D, L, MF | 8–20 | 5–14 | 9–11 | |
| Salix reicharDii (T) | Salicaceae | AUS, NZ | SLC | 4.5–8 | 400–500 | D, L, MF | 8–10 | - | - | |
| Cynodon dactylon (G) | Poaceae | AUS, FIJI, NZ, PNG, USA, CI | SLC | 4.5–8.5 | - | - | 0.5 | 0.3 | 6–9 | [112] |
| Populus species (T) | Salicaceae | AUS, NZ, USA, SAF, CA | SLC | - | - | - | 15–50 | - | - | |
| Ampelopsis arborea (G) | Vitaceae | AUS, NZ, PNG, PH, USA, MY | L | 4–8 | - | C | 10 | - | 6–9 | [138] |
| Crataegus species (S) | Rosaceae | AUS, NZ, PNG, FR, GR, USA, ID, CA | SL | - | - | 5–15 | - | - | ||
| Pinus taeda (T) | Pinaceae | AUS, NZ, USA | SL | 4.5–7 | - | D | 40 | - | 6–9 | |
| Quercus nigra (T) | Fagaceae | USA, NZ, AUS | LC | 4–9 | - | C | 20–30 | - | 5–9 | |
| Quercus species (T) | AUS, CN, CA, FR, GR, IN, ID, ITA, JP, MY, MX | LC | 4.5–7.5 | - | C | 25 | - | |||
| Rhus copallinum (S) | Anacardiaceae | AUS, NZ, PNG, USA, CN | SLC | 4–9 | - | D | 2 | 2 | 4–10 | |
| Rubus species (S) | Rosaceae | AUS, CN, FR, GR, ID, NZ, PNG, USA | SLC | 4–9 | - | D | 3 | - | 5–9 | |
| Andropogon virginicus (G) | Poaceae | AUS, NZ | S | 4–9 | - | D | 1.2 | 5–9 | ||
| Cenchrus echinatus (G) | Poaceae | AUS, PNG, USA | SLC | 4.5–9.5 | - | - | 0.8–1.2 | - | ||
| Cynodon dactylon (G) | Poaceae | AUS, FIJI, NZ, PNG, USA, CI | SLC | 4.5–8.5 | - | - | 0.5 | 0.3 | 6–9 | |
| Cyperus echinatus (G) | Cyperaceae | AUS, CN, FIJI, IN, ID, MX, MM, NZ, PNG, USA | SLC | 4.5–8.5 | - | - | 0.5 | 0.3 | - | |
| Digitaria ciliaris (G) | Poaceae | AUS, NZ, PNG, FIJI, SRI | SLC | 4–9 | - | - | 0.5 | 1.0 | 7–10 | |
| Eremochloa ophiuroides (G) | Poaceae | CN, USA | SLC | 4–9 | - | - | 0.2 | 0.5 | - | |
| Juncus effuse (S) | Juncaceae | AUS, NZ, PNG, USA, GR, FR, CA | SLC | 4.0–8.0 | - | D | 1.5 | 0.5 | - | |
| Paspalum notatum (G) | Poaceae | AUS, MX, USA | SLC | 4.0–8.0 | - | D | 0.75 | 0.3 | - | |
| Collinsonia canadensis (S) | Lamiaceae | AUS, CA, GR, NZ, PNG, USA | SLC | 4–9 | - | - | 0.8 | 0.4 | 4–8 | |
| Erechtites hieracifolia (S) | Asteraceae | NZ | SLC | 4–8.5 | - | - | - | - | - | |
| Eupatorium capillifolium (S) | Asteraceae | AUS, CA, NZ, PH, USA | SLC | 4–9 | - | - | - | - | 3–10 | |
| Kummerowia striata (S) | Fabaceae | AUS, USA, CN, JP | SLC | 4–9 | - | - | 0.3 | - | - | |
| Lepidium virginicum (S) | Brassicaceae | AUS, NZ, USA | SLC | 4–9.5 | - | - | 0.5 | - | - | |
| Lespedeza cuneata (S) | Fabaceae | AUS, USA, CA, CN, JP, PNG | SL | 4–9.5 | - | - | 1.0 | - | - | |
| Robinia pseudoacacia (T) | Fabaceae | AUS, NZ, USA, GR | SLC | 4–9 | - | - | 25 | - | 4–9 | [139] |
| Robinia hispida (S) | Fabaceae | AUS, NZ, PNG, USA, SAF, ID | SLC | 7–11 | - | D | 3.5 | - | 4–8 | |
| Lolium multiflorum (G) | Poaceae | AUS, NZ, UK, USA | SLC | 4–9 | - | - | 0.3 | - | 4–8 | |
| Eleusine indica (G) | Poaceae | AUS, NZ, FIJI, PNG, USA | SLC | 4–9 | - | D | 0.5 | - | 8–11 | |
| Populus species (T) | Salicaceae | AUS, NZ, USA, SAF, CA | SLC | 4–9 | - | 15–50 | - | 4–9 | [128] | |
| Myrica rubra (T) | Myrtaceae | CN, JP, NZ | SLC | 4.5–7 | - | - | 10–20 | - | 9–11 | [140] |
| Schefflera heptaphylla (T) | Araliaceae | AUS, NZ, PNG, PH, ID, FIJI, MY | SLC | 4.5–7 | - | - | 20 | - | - | |
| Schima superba (T) | Theaceae | AUS, NZ, PNG, TH, VN, MY, CN | SLC | 4.5–7 | - | - | 30 | - | - | |
| Bromus diandrus (G) | Poaceae | AUS, FR, NZ, USA, UK | SLC | 4–9 | - | D | 1.0 | - | 6–9 | [112,141] |
| Panicum virgatum (G) | Poaceae | AUS, NZ, USA, CA | SLC | 4–9 | - | D | 1.8 | 0.3 | 10–12 | |
| Cynodon dactylon (G) | Poaceae | AUS, NZ, PNG, USA, FIJI, CI | SLC | - | 625–1750 | D, L, MF | - | - | 6–9 | |
| Lolium multiflorum (G) | Poaceae | AUS, NZ, UK, USA | SLC | 4–9 | - | - | 0.3 | - | 4–8 | |
| Populus x canadensis (T) | Salicaceae | AUS, NZ, USA, CA | SLC | 4–9 | - | - | 40 | 12 | 4–9 | |
| Bromus hordeaceus (G) | Poaceae | AUS, NZ, UK, USA, GR, FR | SLC | 7–9 | - | - | 1.0 | - | 7–10 | |
| Avena barbata (G) | Poaceae | AUS, NZ, USA, ITA, GR, FR | SLC | 4–9 | - | D | 0.8 | - | 4–8 | |
| Bromus madritensis (G) | Poaceae | AUS, NZ, USA, UK, FR | SLC | 4–9 | - | - | 1.2 | 3–7 | ||
| Erodium cicutarium (G) | Geraniaceae | AUS, NZ, USA, UK, ESP, GR, FR, ITY | SLC | 7–9 | - | - | 0.6 | - | - | |
| Brassica nigra (G) | Brassicaceae | AUS, NZ, USA | SL | 4–9 | - | - | 1.2 | 0.6 | 6–9 | |
| Centaurea solstitialis (G) | Asteraceae | AUS, NZ, USA, UK | SLC | 4–9 | - | D | 0.6 | 5–9 | ||
| Lactuca serriola (G) | Asteraceae | AUS, NZ, USA | SL | 4–9 | 1.5 | 0.3 | 6–9 | |||
| Cirsium vulgare (S) | Asteraceae | AUS, NZ, USA, UK, FR | SLC | 4–9 | - | - | 2 | - | - | |
| Sonchus asper (G) | Asteraceae | AUS, NZ, PNG, USA, ID, PL | SLC | 4–9 | - | - | 0.5 | - | - | |
| Dichelostemma capitatum (G) | Asparagaceae | AUS, NZ, SAF, ITA, GR, CA, EPA | SL | 4–9 | - | - | 0.6 | 0.1 | 4–8 | |
| Eschscholzia californica (G) | Papaveraceae | AUS, NZ, USA, GR, UK | SLC | 4–9 | - | - | 0.3 | 0.2 | 6–11 | |
| Castilleja exserta (G) | Orobanchaceae | AUS, USA | SLC | 4–9 | - | - | 0.45 | - | - | |
| Lupinus bicolor (G) | Fabaceae | AUS, NZ, PNG, USA, TH, UK, SAF, MX, ITY, GR, FR, CI, CA | SLC | 4–9 | - | - | 0.1 | - | - | |
| Larrea tridentata (S) | Zygophyllaceae | USA, MX | SLC | 4–9 | - | - | 4 | - | 7–10 | |
| Salsola tragus (G) | Chenopodiaceae | AUS, NZ, USA, UK, ESP, FI | SL | 4–9 | - | - | 0.5 | - | - | |
| Sorghastrum nutans (S) | Poaceae | USA, AUS, MX, BR | - | - | - | - | 2.0 | - | - | |
| Schizachyrium scoparium | Poaceae | AUS, NZ, USA, PNG, JP, FR, GR, CA | - | - | - | - | - | - | ||
| Andropogon gerardii (S) | Poaceae | AUS, NZ, USA, UK, FIJI, ARG | S | 4–9 | - | - | 2.0 | - | 4–8 | |
| Bouteloua curtipendula (G) | Poaceae | AUS, USA, GR, MX, CA, ARG | - | - | - | - | 1.0 | - | 4–9 | |
| Festuca arundinacea (G) | Poaceae | AUS, NZ, USA, UK, ITY, FR, CA, PT | - | 5.5–7 | - | - | 1.2 | - | - | |
| Pseudoroegneria spicata (G) | Poaceae | AUS, NZ, USA, UK, SAF, PNG, MX, MY, JP | - | - | - | - | - | - | ||
| Elymus trachycaulus (G) | Poaceae | AUS, NZ, USA, UK, GR, CA, ARG | - | - | - | - | 0.3–1.5 | - | - | |
| Medicago sativa (G) | Fabaceae | AUS, NZ, USA, UK, GR, ITY, FR, CA, CN | SLC | 4–9 | - | D | 1.0 | 4–11 | ||
| Melilotus indicus (G) | Fabaceae | AUS, NZ, USA, UK, FR, GR | SLC | 4–9 | - | - | 1.0 | 0.6 | 5–9 | |
| Pascopyrum smithii (G) | Poaceae | AUS, NZ, PNG, SAF, USA, UK, | - | - | - | - | - | - | - | |
| Poa secunda (G) | Poaceae | AUS, NZ, PNG, USA, UK, SRI, SAF, PH, MX, ITY, ID, GR, FR, CA, CH | - | - | - | - | - | - | - | |
| Festuca ovina (G) | Poaceae | AUS, NZ, USA, UK | SL | 4–9 | - | D | 0.3 | - | 4–8 | |
| Bouteloua gracilis (G) | Poaceae | AUS, USA, MX | SLC | 4–9 | - | D | 0.6 | - | 7–10 | |
| Nassella viridula (G) | Poaceae | AUS, NZ, USA, UK, MX, CA | SLC | 4–9 | - | D | 1.2 | - | - | |
| Hesperostipa comata (G) | Poaceae | AUS, PNG, USA, CA, ID, TH | - | - | - | 1.0 | - | - | ||
| Bromus carinatus (G) | Poaceae | AUS, NZ, USA, UK, CA | SLC | 4–9 | - | D | 0.8 | - | 4–8 | |
| Nassella pulchra (G) | Poaceae | AUS, NZ, PNG, USA, UK, SAF, MX, MY, JP, ID, ARG, CA | SLC | 4–9 | - | - | 0.5–1.5 | - | - | |
| Lupinus succulentus (G) | Fabaceae | AUS, NZ, PNG, USA, UK, MX, ITY, GR, FR, CA, ARG | SLC | 4–9 | - | - | 1.0 | - | - | |
| Leymus triticoides (G) | Poaceae | USA, AUS | SLC | 4–9 | - | D | 1.2 | - | - | |
| Elymus lanceolatus (G) | Poaceae | AUS, NZ, USA, UK, PNG, GR, CA, ARG, ID, ITY, SAF, SWE | - | - | - | - | 1.3 | - | - | |
| Bromus marginatus (G) | Poaceae | AUS, NZ, USA | SLC | 4–9 | - | - | 1–1.5 | - | - | |
| Koeleria macrantha (G) | Poaceae | AUS, USA, UK, SAF, CA | SLC | 4–9 | - | - | 0.5 | - | - | |
| Yucca filamentosa (G) | Asparagaceae | USA, NZ | SLC | 4–9 | - | - | 1.2 | 0.6 | 4–10 | |
| Bromus commutatus (G) | Poaceae | NZ, UK, AUS, USA | - | - | - | - | 0.4–1.2 | - | - | |
| Poa compressa (G) | Poaceae | AUS, USA, UK, NZ | - | - | - | - | 0.3–0.4 | - | - | |
| Poa pratensis (G) | Poaceae | AUS, NZ, UK, USA | SLC | 4–9 | - | - | 1.0 | - | 3–9 | |
| Achillea millefolium (G) | Asteraceae | AUS, NZ, USA, UK, SWE, CA | SLC | 4–9 | - | - | 0.6 | 0.6 | 4–8 | |
| Ericameria nauseosa (S) | Asteraceae | USA, CA, NZ | SLC | 4–9 | - | - | 2.0 | 2.0 | 7–9 | |
| Rosa acicularis (S) | Rosaceae | AUS, NZ, USA, PNG, MX, ITY, IN, GR, CI, CN, CA, BRA | SLC | 4–9 | - | - | 1–3 | - | - | |
| Balsamorhiza sagittate (G) | Asteraceae | USA | SLC | 4–9 | - | - | 0.3 | - | 4–8 | |
| Liatris punctata (G) | Asteraceae | AUS, NZ, USA, PNG, PH, SWE, ID, ITA, GR, BRA, ARG | SLC | 4–9 | - | - | 0.6 | - | 3–7 | |
| Linum lewisii (G) | Linaceae | AUS, NZ, USA, UK, ESP, ITA, FR, PH | - | - | - | - | 0.8 | - | - | |
| Lupinus sericeus (G) | Fabaceae | AUS, NZ, USA, PNG, GR, MX, FR, CA, SWE, ITA, ARG | - | - | - | - | 0.5 | - | - | |
| Astragalus cicer (G) | Fabaceae | AUS, USA, UK, SWE, ESP, NZ, MX, ITA, IN, GR, FR, CN, AUT, ARG, CA, CL | - | - | - | - | 0.6–1.0 | - | - | |
| Agropyron cristatum (G) | Poaceae | AUS, NZ, USA | - | - | - | - | 0.3–0.5 | - | 3–9 | |
| Ericameria nauseosa (S) | Asteraceae | NZ, USA, CA | SLC | 4–9 | - | - | 2.0 | 2.0 | 7–9 | |
| Acacia melanoxylon (T) | Fabaceae | AUS, NZ, USA, ARG, FR, GR | SLC | 4–9 | - | - | 30 | - | 9–11 | [20] |
| Melaleuca salignus (T) | Myrtaceae | AUS | - | - | - | - | 15 | - | - | |
| Corymbia intermedia (T) | Myrtaceae | AUS, PNG, SRI | - | - | - | - | 20–30 | - | - | |
| Eucalyptus tereticornis (T) | Myrtaceae | AUS, PNG, NZ, SAF, USA, IN | SLC | 4–9 | - | - | 20–35 | - | 9–12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arifuzzaman; Rahman, M.M.; Karim, M.R.; Hewa, G.A.; Rawlings, R.; Iqbal, A. Phytocapping for Municipal Solid Waste Landfills: A Sustainable Approach. Sustainability 2024, 16, 8230. https://doi.org/10.3390/su16188230
Arifuzzaman, Rahman MM, Karim MR, Hewa GA, Rawlings R, Iqbal A. Phytocapping for Municipal Solid Waste Landfills: A Sustainable Approach. Sustainability. 2024; 16(18):8230. https://doi.org/10.3390/su16188230
Chicago/Turabian StyleArifuzzaman, Md Mizanur Rahman, Md Rajibul Karim, Guna Alankarage Hewa, Robyn Rawlings, and Asif Iqbal. 2024. "Phytocapping for Municipal Solid Waste Landfills: A Sustainable Approach" Sustainability 16, no. 18: 8230. https://doi.org/10.3390/su16188230








