Metal Analysis of Leachate from the Organic Fraction of Urban Solid Waste (MSW) from the Municipality of Belém/PA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of the Study Area
2.2. Calculation of the Mass Required for the Gravimetric Composition
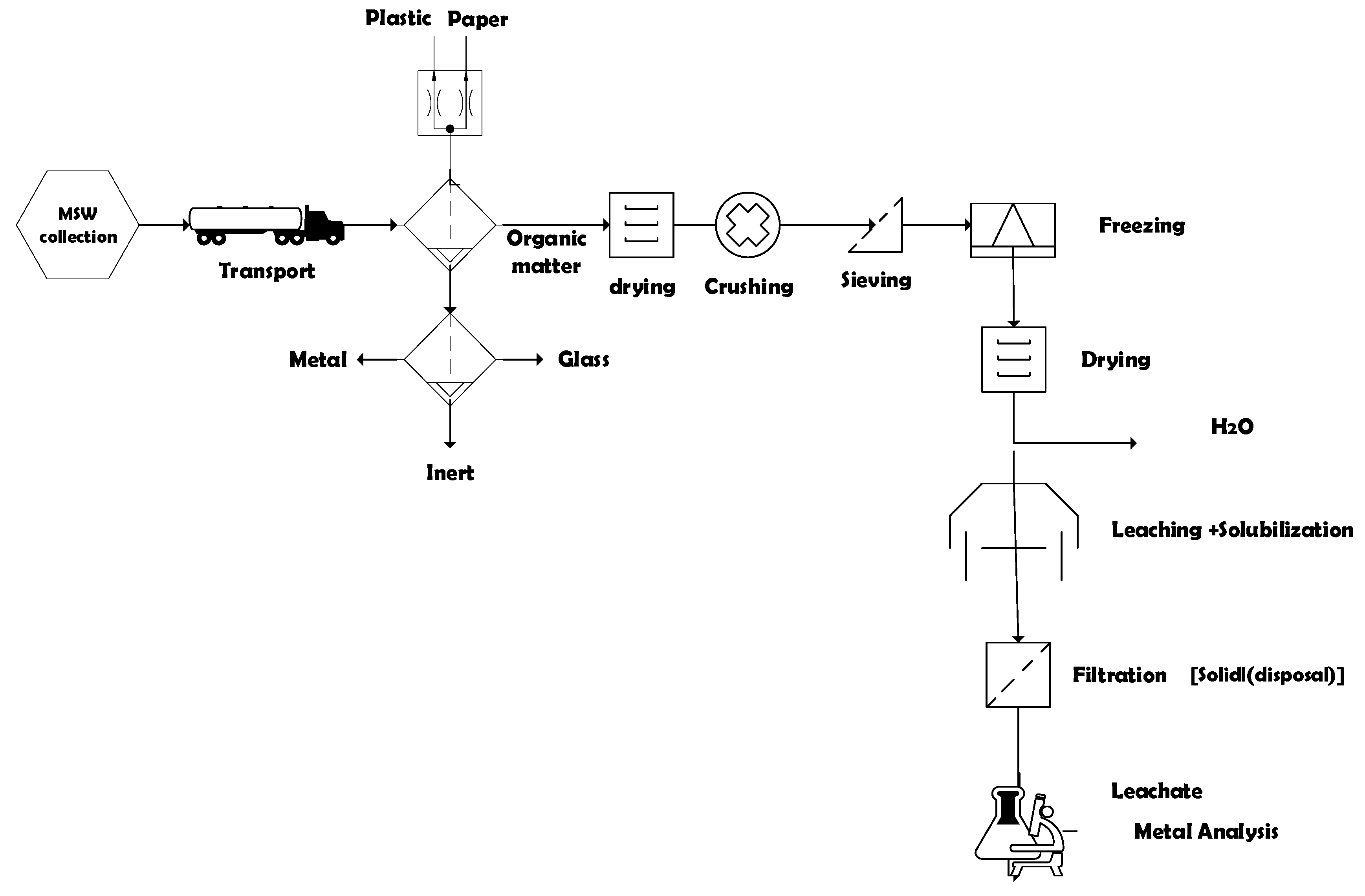
2.3. Gravimetric Collection and Composition
2.4. Pre-Treatment of Samples (Drying, Crushing and Sieving Content)
2.4.1. Laboratory Determinations
Leaching and Solubilization Tests
Determination of Heavy Metals (Sample Preparation and Digestion)
2.4.2. Method and Statistical Analysis
3. Results
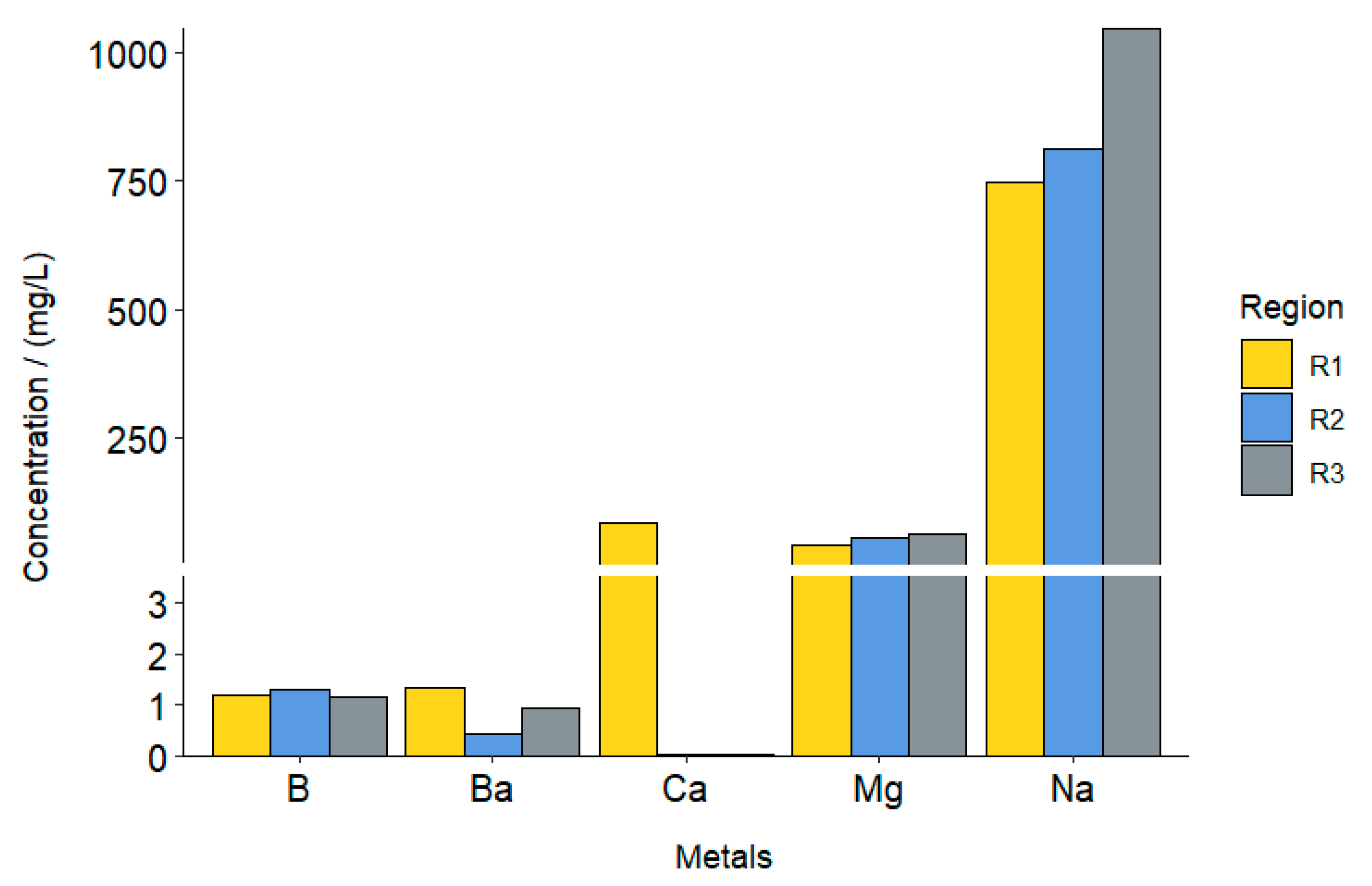
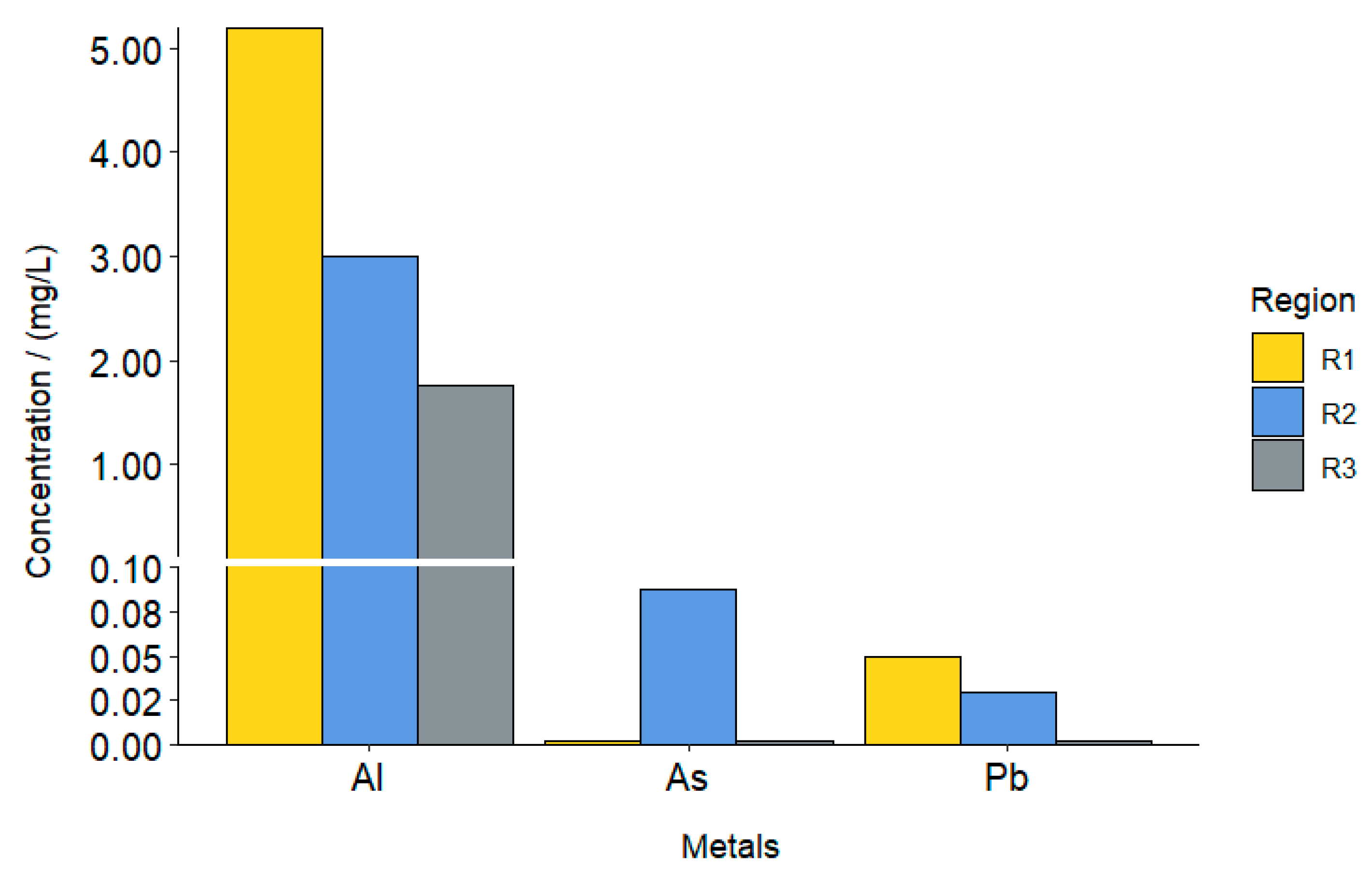
3.1. Analysis of the Leachate Extract of the Organic Fraction
3.2. Verification of Compliance with Legal Sanitary and Environmental Standards
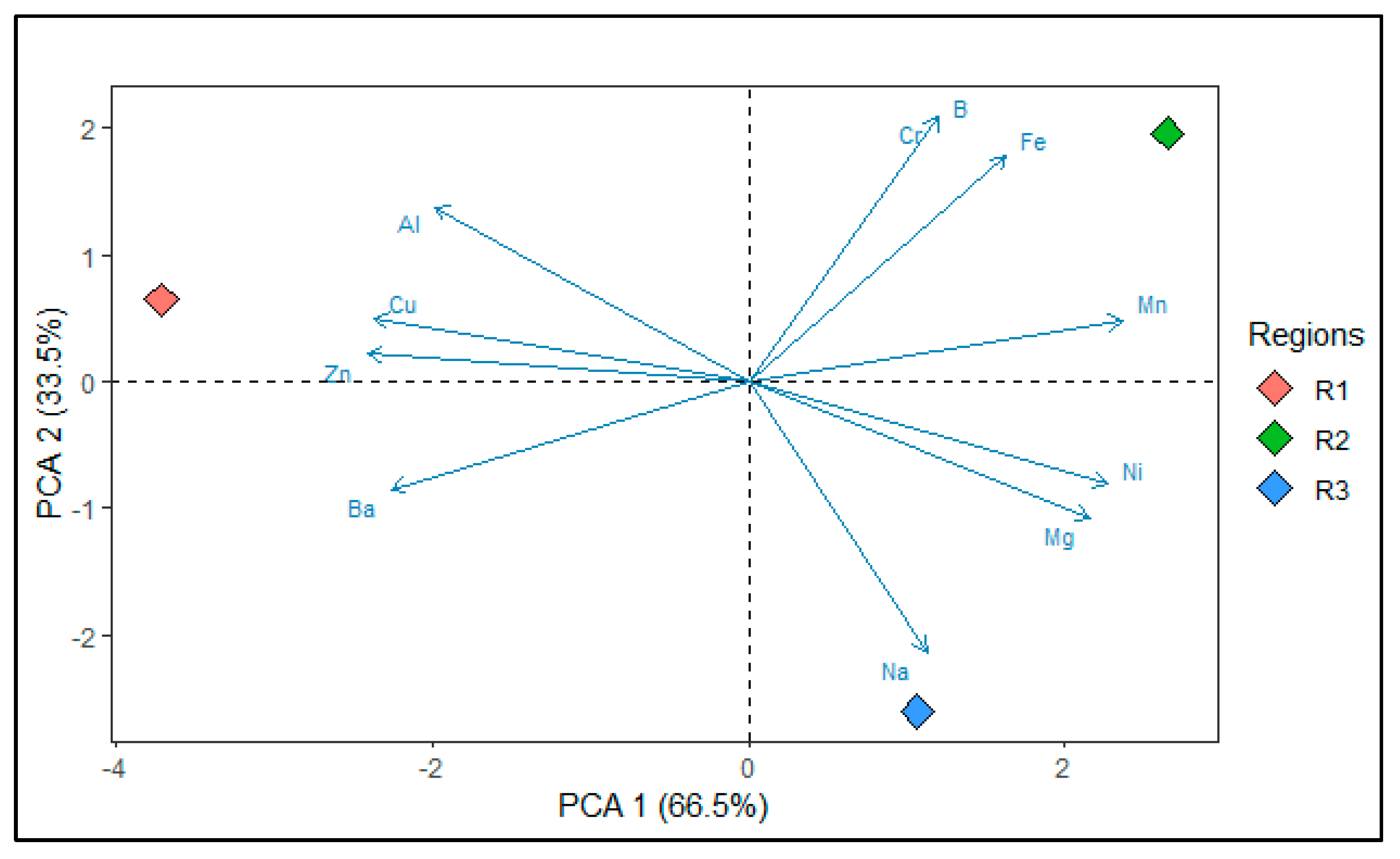
3.3. Pearson Correlation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsai, F.M.; Bui, T.-D.; Tseng, M.-L.; Wu, K.-J. A causal municipal solid waste management model for sustainable cities in Vietnam under uncertainty: A comparison. Resour. Conserv. Recycl. 2020, 154, 104599. [Google Scholar] [CrossRef]
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management; World Bank: New York, NY, USA, 2012. [Google Scholar]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Associação Brasileira de Empresas de Limpeza Pública e Resíduos Especiais–ABRELPE. Panorama 2021; ABRELPE: São Paulo, Brazil, 2021. [Google Scholar]
- Da Silva, D.R.B.; Costa Filho, I.S.; de Souza, W.C.S.; dos Santos, F.V.; de Machado, F.P.C.; de Brandão, S.I.W.; Pereira, F.C.; da Assunção, C.M.A.; Silva, R.H.Y.; Russo, M.A.T.; et al. Aspectos Quantitativos e Qualitativos de Resíduos Sólidos Urbanos nos Municípios de Ananindeua, Belém e Marituba. In Cooperação Intersetorial e Inovação: Ferramentas para a Gestão Sustentável de Resíduos Sólidos; Pereira, C., Fricke, K., Eds.; Technische Universität Braunschweig: Braunschweig, Germany, 2022. [Google Scholar]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Tratamento de chorume de aterro: Revisão e oportunidade. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef]
- Bengtsson, L.; Bendz, D.; Hogland, W.; Rosqvist, H.; Åkesson, M. Water balance for landfills of different age. J. Hydrol. 1994, 158, 203–217. [Google Scholar] [CrossRef]
- Mull, E.J. Approaches Toward Sustainable Urban Solid Waste Management: Sahakaranagar Layout. Master’s Thesis, Lund University, Lund, Sweden, 2005. [Google Scholar]
- Mor, S.; Ravindra, K.; Dahiya, R.P.; Chandra, A. Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environ. Monit. Assess. 2006, 118, 435–456. [Google Scholar] [CrossRef]
- Guerrero, L.A.; Maas, G.; Hogland, W. Solid waste management challenges for cities in developing countries. Waste Manag. 2013, 33, 220–232. [Google Scholar] [CrossRef]
- Joardar, J.C.; Halder, M. Chemical Characterization of Khulna City Waste and Its Effects on Surrounding Soils through Lateral Movement. In Proceedings of the Waste Safe 2013—3rd International Conference on Solid Waste Management in the Developing Countries, Khulna, Bangladesh, 10–12 February 2013. [Google Scholar]
- Kanmani, S.; Gandhimathi, R. Assessment of heavy metal contamination in soil due to leachate migration from an open dumping site. Appl. Water Sci. 2013, 3, 193–205. [Google Scholar] [CrossRef]
- Rodrigues, S.; Henriques, B.; da Silva, E.F.; Pereira, M.; Duarte, A.; Römkens, P. Evaluation of an approach for the characterization of reactive and available pools of twenty potentially toxic elements in soils: Part I–The role of key soil properties in the variation of contaminants’ reactivity. Chemosphere 2010, 81, 1549–1559. [Google Scholar] [CrossRef]
- Wong, C.S.; Li, X.; Thornton, I. Urban environmental geochemistry of trace metals. Environ. Pollut. 2006, 142, 1–16. [Google Scholar] [CrossRef]
- Dong, J.; Yu, M.; Bian, Z.; Wang, Y.; Di, C. Geostatistical analyses of heavy metal distribution in reclaimed mine land in Xuzhou, China. Environ. Earth Sci. 2011, 62, 127–137. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Chromium (IV) Compounds. In IARC Monograph 100C; International Agency for Research on Cancer (IARC): Lyon, France, 2018. [Google Scholar]
- Dash, S.; Borah, S.S.; Kalamdhad, A. A modified indexing approach for assessment of heavy metal contamination in Deepor Beel, India. Ecol. Indic. 2019, 106, 105444. [Google Scholar] [CrossRef]
- Singh, P.; Purakayastha, T.J.; Mitra, S.; Bhowmik, A.; Tsang, D.C. River water irrigation with heavy metal load influences soil biological activities and risk factors. J. Environ. Manag. 2020, 270, 110517. [Google Scholar] [CrossRef]
- Zhang, C.; Nie, S.; Liang, J.; Zeng, G.; Wu, H.; Hua, S.; Liu, J.; Yuan, Y.; Xiao, H.; Deng, L.; et al. Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Sci. Total Environ. 2016, 557–558, 785–790. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, H.; Cao, A.; Xu, W. Modeling the carbon cycle of the municipal solid waste management system for urban metabolism. Ecol. Model. 2015, 318, 150–156. [Google Scholar] [CrossRef]
- Stamps, B.W.; Lyles, C.N.; Suflita, J.M.; Masoner, J.R.; Cozzarelli, I.M.; Kolpin, D.W.; Stevenson, B.S. Municipal Solid Waste Landfills Harbor Distinct Microbiomes. Front. Microbiol. 2016, 7, 534. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, C.; Pan, J.; Yang, G.; Sun, C.; Liu, Y.; Chen, X.; Zhao, Z. Leachate from municipal solid waste landfills in a global perspective: Characteristics, influential factors and environmental risks. J. Clean. Prod. 2022, 333, 130234. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, Y.; Hu, Y.; Shi, H. The variation in microbial community structure under different heavy metal contamination levels in paddy soils. Ecotoxicol. Environ. Saf. 2019, 180, 557–564. [Google Scholar] [CrossRef]
- Desai, C.; Parikh, R.Y.; Vaishnav, T.; Shouche, Y.S.; Madamwar, D. Tracking the influence of long-term chromium pollution on soil bacterial community structures by comparative analyses of 16S rRNA gene phylotypes. Res. Microbiol. 2008, 160, 1–9. [Google Scholar] [CrossRef]
- Kawahigashi, F.; Mendes, M.B.; Gomes da Assunção Júnior, V.; Gomes, V.H.; Fernandes, F.; Hirooka, E.Y.; Kuroda, E.K. Pós-tratamento de lixiviado de aterro sanitário com carvão ativado. Rev. Bras. Eng. Sanitária Ambient. 2014, 19, 235–244. [Google Scholar] [CrossRef]
- Lange, L.C.; Alves, J.F.; Amaral, M.C.S.; de Melo Júnior, W.R. Tratamento de lixiviado de aterro sanitário por processo oxidativo avançado empregando reagente de Fenton. Rev. Bras. Eng. Sanitária Ambient. 2006, 11, 175–183. [Google Scholar] [CrossRef]
- Assunção, F.P.d.C.; Pereira, D.O.; da Silva, J.C.C.; Ferreira, J.F.H.; Bezerra, K.C.A.; Bernar, L.P.; Ferreira, C.C.; Costa, A.F.d.F.; Pereira, L.M.; da Paz, S.P.A.; et al. A Systematic Approach to Thermochemical Treatment of Municipal Household Solid Waste into Valuable Products: Analysis of Routes, Gravimetric Analysis, Pre-Treatment of Solid Mixtures, Thermochemical Processes, and Characterization of Bio-Oils and Bio-Adsorbents. Energies 2022, 15, 7971. [Google Scholar] [CrossRef]
- Pereira, D.O.; da Costa Assunção, F.P.; da Silva, J.C.C.; Ferreira, J.F.H.; Ferreira, R.B.P.; Lola, Á.L.; do Nascimento, Í.C.P.; Chaves, J.P.; do Nascimento, M.S.C.; da Silva Gouvêa, T.; et al. Prediction of Leachate Characteristics via an Analysis of the Solubilized Extract of the Organic Fraction of Domestic Solid Waste from the Municipality of Belém, PA. Sustainability 2023, 15, 15456. [Google Scholar] [CrossRef]
- BELÉM. Lei Municipal nº 9.656, de 30 de dezembro de 2020. Institui a Política Municipal de Saneamento Básico do Município de Belém, o Plano Municipal de Saneamento Básico (PMSB), e o Plano de Gestão Integrada de Resíduos Sólidos (PGIRS), em Atenção ao Disposto no Art. 9º da Lei Federal nº 11.445/2007, com as Atualizações Trazidas pela Lei nº 14.026/2020, o Novo Marco do Saneamento Básico, e dá Outras Providências. Belém, PA, 30 dez. 2020. Available online: https://arbel.belem.pa.gov.br/wp-content/uploads/2022/05/PMGIRS-INTEGRAL.pdf (accessed on 1 August 2022).
- IBGE. Instituto Brasileiro de Geografia e Estatística. Censo Brasileiro de 2010; IBGE: Rio de Janeiro, Brazil, 2012. Available online: https://censo2010.ibge.gov.br/ (accessed on 1 August 2022).
- Fesseha, S.N.; Bin, F. The Assessment of Solid Waste Products Management in Ethiopians Municipal Urban Areas. Int. J. Soc. Sci. Manag. 2015, 2, 165–179. [Google Scholar] [CrossRef]
- Associação Brasileira De Normas Técnicas. ANBT. NBR 10.004/2004–Resíduos Sólidos–Classificação; Associação Brasileira De Normas Técnicas: Rio de Janeiro, Brazil, 2004. [Google Scholar]
- NBR 10005; Lixiviação de Resíduos Sólidos—Procedimento. ABNT: Rio de Janeiro, Brazil, 2004.
- NBR 10006; Solubilização de Resíduos Sólidos—Procedimento. ABNT: Rio de Janeiro, Brazil, 2004.
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- NBR ISO/IEC 17025; General Requirements for the Competence of Testing and Calibration Laboratories. ABNT: Rio de Janeiro, Brazil, 2017.
- Croghan, C.W.; Egeghy, P.P. Methods for Dealing with Values Below the Limit of Detection Using SAS; North Carolina State University, Institute for Advanced Analytics: Raleigh, NC, USA, 2003. [Google Scholar]
- Hewett, P.; Ganser, G.H. A comparison of several methods for analyzing censored data. Ann. Occup. Hyg. 2007, 51, 611–632. [Google Scholar]
- Zhang, F.; Li, C.; Shi, Y.; Meng, L.; Zan, F.; Wu, X.; Wang, L.; Sheng, A.; Crittenden, J.C.; Chen, J. Evaluation on leachability of heavy metals from tailings: Risk factor identification and cumulative influence. Environ. Sci. Pollut. Res. 2023, 30, 64565–64575. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, H.; Sheng, Y.; Ma, Z.; Zhang, J.; Liu, F.; Chen, S.; Meng, Q.; Bai, Y. Distribution, Source Apportionment, and Health Risk Assessment of Heavy Metals in Groundwater in a Multi-mineral Resource Area, North China. Expo. Health 2022, 14, 807–827. [Google Scholar] [CrossRef]
- Kumari, P.; Gupta, N.C.; Kaur, A.; Singh, K. Application of Principal Component Analysis and Correlation for Assessing Groundwater Contamination in and around Municipal Solid Waste Landfill of Ghazipur, Delhi. J. Geol. Soc. India 2019, 94, 595–604. [Google Scholar] [CrossRef]
- Krugel, J. Estudo da Concentração do Percolado de Aterro Industrial por Evaporação Visando à Redução da Carga Poluidora. Masters’s Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2013. [Google Scholar]
- Samadder, S.; Prabhakar, R.; Khan, D.; Kishan, D.; Chauhan, M. Analysis of the contaminants released from municipal solid waste landfill site: A case study. Sci. Total Environ. 2017, 580, 593–601. [Google Scholar] [CrossRef]
- Conselho Nacional do Meio Ambiente. Resolução nº 396, de 03 de Abril de 2008. Dispõe Sobre a Classificação e Diretrizes Ambientais para o Enquadramento das águas Subterrâneas e dá Outras Providências; Diário Oficial da União: Brasilia, Brazil, 2008; p. 89. [Google Scholar]
- Conselho Nacional de Meio Ambiente. Resolução nº 430, de 13 de Maio de 2011. Dispõe Sobre as Condições e Padrões de Lançamento de Efluentes; CONAMA: Brasilia, Brazil, 2005. [Google Scholar]
- FAO. Plant Nutrition for Food Security: A Guide for Integrated Nutrient Management; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; Available online: http://www.fao.org (accessed on 2 June 2023).
- Zacharias, C.M. Estudo da Toxicidade de Metais Pesados em Solos Agrícolas. Masters’s Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2013. [Google Scholar]
- Edokpayi, J.N.; Durowoju, O.S.; Odiyo, J.O. Assessment of heavy metals in landfill leachate: A case study of Thohoyandou landfill, Limpopo Province, South Africa. In Heavy Metals; Saleh, H.E.-D.M., Aglan, R.F., Eds.; Springer: Cham, Switzerland, 2018; pp. 123–145. [Google Scholar]
- Conselho Nacional de Meio Ambiente. Resolução nº 357, de 17 de Março de 2005. Dispõe Sobre a Classificação dos Corpos de água e Diretrizes Ambientais para o seu Enquadramento; CONAMA: Brasília, Brazil, 2005. [Google Scholar]
- Maiti, S.K.; Hazra, T.; Debsarkar, A.; Dutta, A. Leachate characterization and identifcation of dominant pollutants using leachate pollution index for an uncontrolled landfll site. Glob. J. Environ. Sci. Manag. 2016, 2, 177–186. [Google Scholar] [CrossRef]
- Hussain, S.; Gupta, G.; Mishra, R.; Patel, N.; Gupta, S.; Alzarea, S.I.; Kazmi, I.; Kumbhar, P.; Disouza, J.; Dureja, H.; et al. Unlocking the secrets: Volatile Organic Compounds (VOCs) and their devastating effects on lung cancer. Pathol. Res. Pr. 2024, 255, 155157. [Google Scholar] [CrossRef]
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Riguetti, P.F.; Cardoso, C.A.L.; Cavalheiro, A.A.; Lenzi, E.; Fiorucci, A.R.; da Silva, M.S. Manganês, zinco, cádmio, chumbo, mercúrio e crômio no chorume de aterro sanitário em Dourados, MS, Brasil. Rev. Ambiente Água Taubaté 2015, 10, 101–111. [Google Scholar]
- Oliveira, D.L.; Santana, G.P. Influência do aterro municipal de Manaus sobre as águas superficiais da circunvizinhança: Um enfoque ao estudo de metais pesados. Caminhos de Geogr. Uberlândia 2010, 11, 75–83. [Google Scholar] [CrossRef]
- Lindamulla, L.; Jegatheesan, V.; Jinadasa, K.; Nanayakkara, K.; Othman, M. Integrated mathematical model to simulate the performance of a membrane bioreactor. Chemosphere 2021, 284, 131319. [Google Scholar] [CrossRef]
- Hussein, M.; Yoneda, K.; Mohd-Zaki, Z.; Amir, A.; Othman, N. Heavy metals in leachate, impacted soils and natural soils of different landfills in Malaysia: An alarming threat. Chemosphere 2021, 267, 128874. [Google Scholar] [CrossRef]
- Wong, A.H.H.; Chin, W.S.M. A case study of long-term CCA preservative leaching from treated hardwood poles in a humid tropical condition. Section 5 Sustainability and Environment. In Proceedings of the the 47th Annual Meeting, Lisbon, Portugal, 15–19 May 2016. [Google Scholar]
- Fu, J.; Zhao, C.; Luo, Y.; Liu, C.; Kyzas, G.Z.; Luo, Y.; Zhao, D.; An, S.; Zhu, H. Heavy metals in surface sediments of the Jialu River, China: Their relations to environmental factors. J. Hazard. Mater. 2014, 270, 102–109. [Google Scholar] [CrossRef]
- Alves, R.I.S.; Tonani, K.A.A.; Nikaido, M.; Cardoso, O.O.; Trevilato, T.M.B.; Seguramuñoz, S.I. Avaliação das concentrações de metais pesados em águas superficiais e sedimentos do Córrego Monte Alegre e afluentes, Ribeirão Preto, SP, Brasil. Ambi-Agua Taubaté 2010, 5, 122–132. [Google Scholar] [CrossRef]
- Antweiler, R.C.; Taylor, H.E.; Alpers, C.N. Distribution and geochemistry of selected trace elements in the Sacramento River near Keswick Reservoir. Chem. Geol. 2012, 298–299, 70–78. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Singh, D.S.; Singh, A.K. Environmental repercussions of cane-sugar industries on the Chhoti Gandak river basin, Ganga Plain, India. Environ. Monit. Assess. 2010, 171, 321–344. [Google Scholar] [CrossRef]
- Kuajara, O.; Sanchez, J.; Ballestrin, R.; Teixeira, E. Environmental monitoring of the North Porto Alegre landfill, Brazil. Water Environ. Res. 1997, 69, 1170–1177. [Google Scholar] [CrossRef]
- Oliveira, F.S.J.; Jucá, F.T.J. Acúmulo de metais pesados e capacidade de impermeabilização do solo imediatamente abaixo de uma célula de um aterro de resíduos sólidos. Eng. Sanit. Ambient. 2004, 9, 211–217. [Google Scholar] [CrossRef]
- Celere, M.S.; Oliveira, A.S.; Trevilato, T.M.B.; Segura-Munhõz, S.I. Metais presentes no chorume coletado no aterro sanitário de Ribeirão Preto, São Paulo, Brasil e sua relevância para saúde pública. Caderno de Saúde Pública 2007, 23, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Cort, E.P.D.; Alberti, V.; Rotta, M.; Becegato, V.; Machado, W.C.P.; Onofre, S.B. Níveis de metais pesados presentes no chorume produzido em aterros sanitários da região sudoeste do Paraná. Geoambiente Jataí 2008, 11, 103–116. [Google Scholar] [CrossRef]
- Cintra, I.S. Estudo da Influência da Recirculação de Chorume Cdu e Chorume Inoculado na Aceleração do Processo de Digestão Anaeróbia de Resíduos Sólidos Urbanos. Masters’s Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2003; 352p. [Google Scholar]
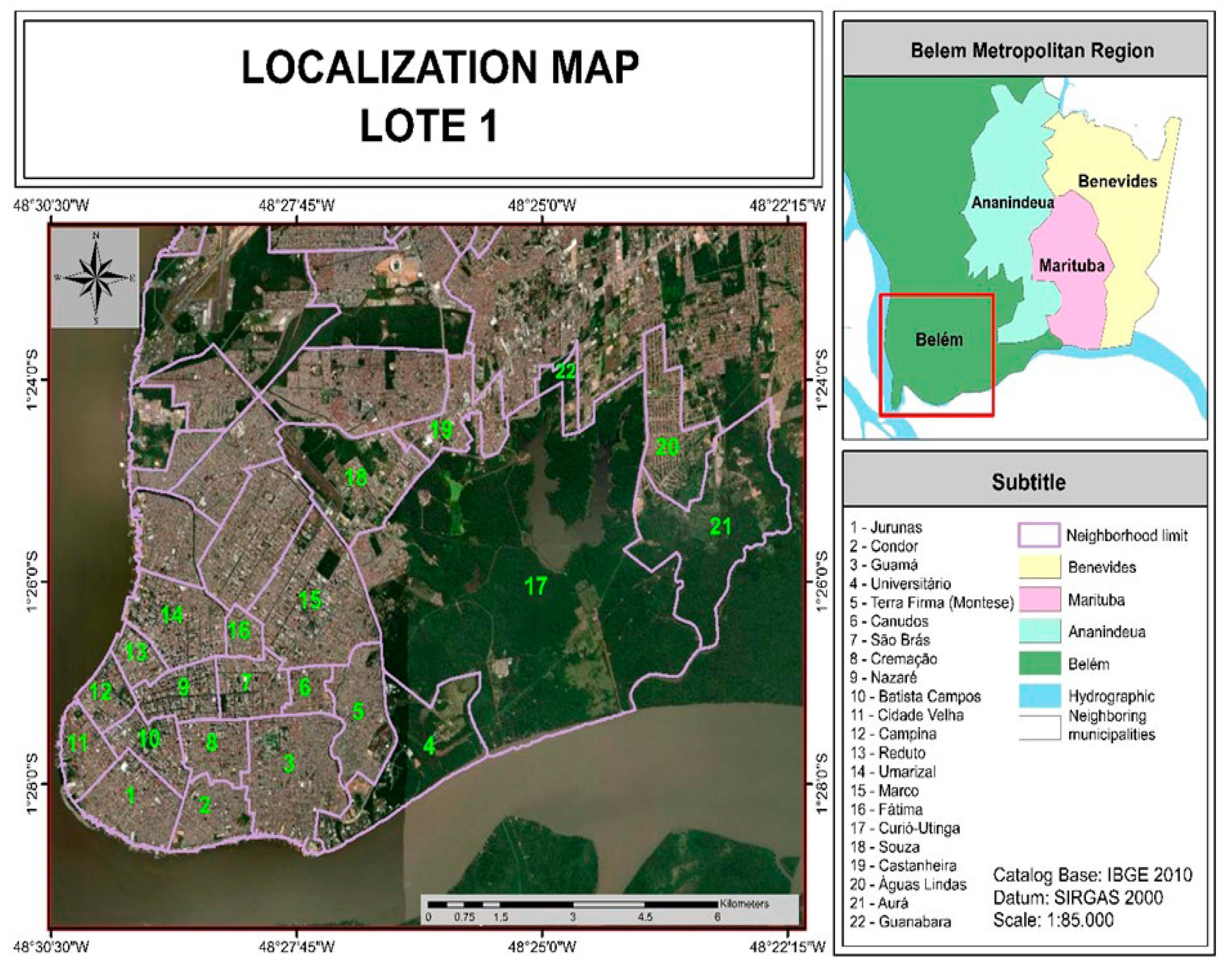




| Region | Class | Sectors | Neighborhoods |
|---|---|---|---|
| 1 | E | 1, 2 and 3 | Aurá, Águas Lindas, Curió-Utinga, Guanabara, Castanheira, Souza e Marco. |
| 2 | D | 4, 5 and 6 | Canudos, Terra Firme, Guamá, Condor, Jurunas e Fátima. |
| 3 | C | 7, 8 and 9 | Umarizal, São Brás, Cremação, Batista Campos, Nazaré, Reduto, Campina e Cidade Velha. |
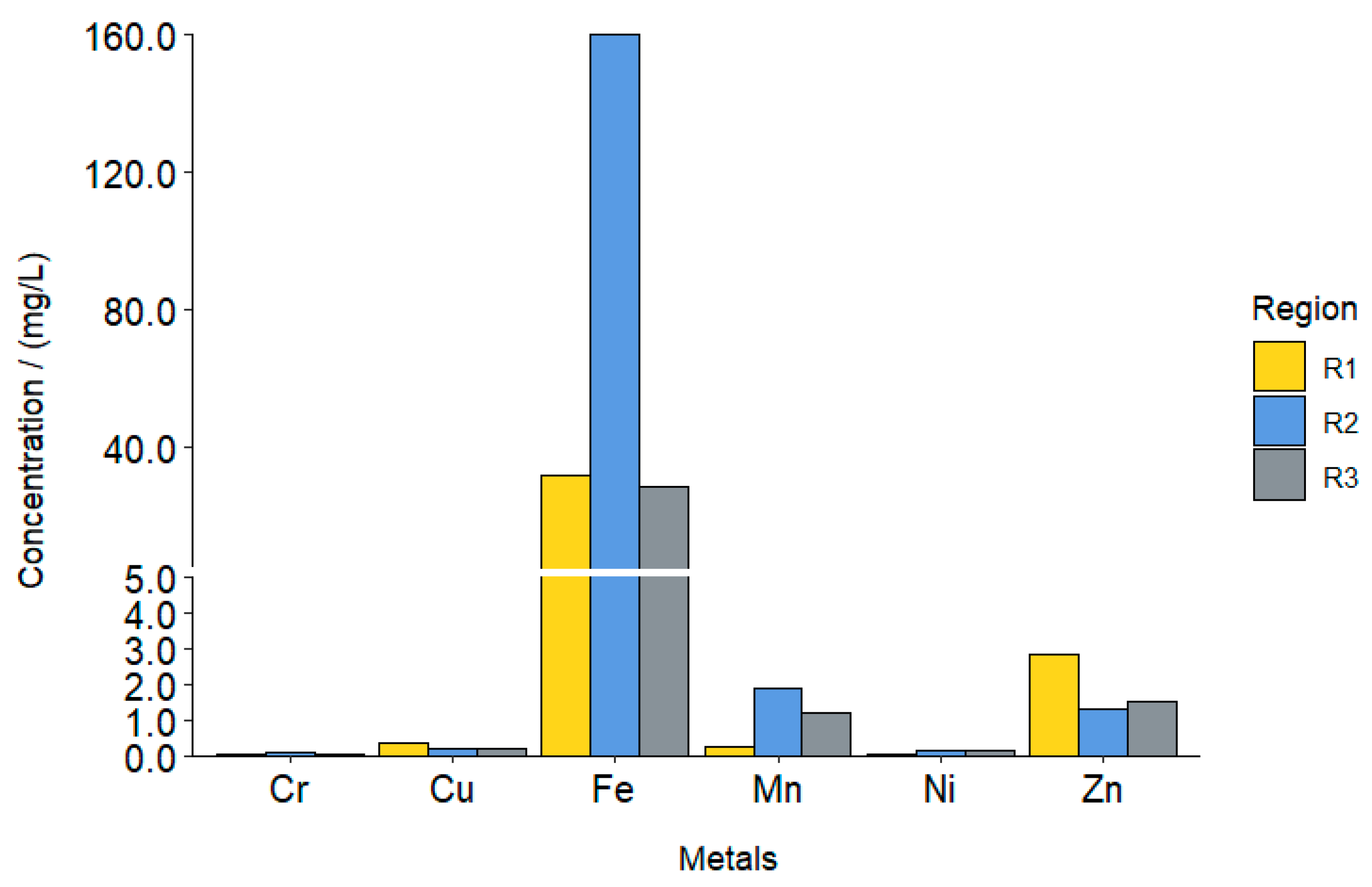
| Heavy Metals (mg/L) | Regions | ||
|---|---|---|---|
| R1 | R2 | R3 | |
| Al | 5.198 | 3.007 | 1.775 |
| As | 0.002 | 0.00875 | <0.002 * |
| Ba | 1.33 | 0.44 | 0.95 |
| B | 1.19 | 1.30 | 1.16 |
| Pb | 0.05 | 0.03 | <0.002 * |
| Cu | 0.35 | 0.22 | 0.23 |
| Cr | 0.060 | 0.075 | 0.055 |
| Fe | 32.2 | 160.4 | 28.5 |
| Mn | 0.265 | 1.910 | 1.205 |
| Ni | 0.06 | 0.14 | 0.15 |
| Na | 748 | 811.3 | 1047.8 |
| Zn | 2.85 | 1.30 | 1.55 |
| Ca | 82.9 | <0.02 * | <0.02 * |
| Mg | 39.83 | 56.82 | 61.92 |
| Metal Analysis (mg/L) (Region 1) CONAMA | |||
|---|---|---|---|
| Metals | 430/2011 | 357/2005 | 396/2008 |
| Al | - | no | no |
| As | yes | no | yes |
| Ba | yes | no | no |
| B | yes | no | no |
| Pb | yes | no | no |
| Cu | yes | no | yes |
| Cr | yes | no | - |
| Fe | no | no | no |
| Mn | no | no | no |
| Ni | yes | no | no |
| Na | - | - | no |
| Zn | yes | no | yes |
| Mg | no | - | - |
| Metal Analysis (mg/L) (Region 2) CONAMA | |||
|---|---|---|---|
| Metals | 430/2011 | 357/2005 | 396/2008 |
| Al | - | no | no |
| As | yes | yes | yes |
| Ba | yes | yes | yes |
| B | yes | no | no |
| Pb | yes | no | no |
| Cu | yes | no | yes |
| Cr | yes | no | - |
| Fe | no | no | no |
| Mn | no | no | no |
| Ni | yes | no | no |
| Na | - | - | no |
| Zn | yes | no | yes |
| Ca | - | - | - |
| Mg | no | - | - |
| Metal Analysis (mg/L) (Region 3) CONAMA | |||
|---|---|---|---|
| Metals | 430/2011 | 357/2005 | 396/2008 |
| Al | - | no | no |
| As | - | - | - |
| Ba | yes | no | no |
| B | yes | no | no |
| Pb | - | - | - |
| Cu | no | no | yes |
| Cr | yes | no | - |
| Fe | no | no | no |
| Mn | no | no | no |
| Ni | yes | no | no |
| Na | - | - | no |
| Zn | yes | no | yes |
| Mg | no | - | - |
| Variables | PC1 | PC2 |
|---|---|---|
| Al | −0.821 | 0.571 |
| Ba | −0.936 | −0.353 |
| B | 0.498 | 0.867 |
| Cr | 0.498 | 0.867 |
| Cu | −0.979 | 0.205 |
| Fe | 0.674 | 0.739 |
| Mn | 0.980 | 0.199 |
| Ni | 0.942 | −0.336 |
| Na | 0.466 | −0.885 |
| Zn | −0.996 | 0.090 |
| Mg | 0.895 | −0.447 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathews, J.C.; da Costa Assunção, F.P.; Pereira, D.O.; da Silva, J.C.C.; Almeida, F.F.S.; Almeida, A.C.P.; Mendonça, N.M.; de Sousa Brandão, I.W.; Menezes, A.O.; Borges, L.E.P.; et al. Metal Analysis of Leachate from the Organic Fraction of Urban Solid Waste (MSW) from the Municipality of Belém/PA. Sustainability 2024, 16, 8370. https://doi.org/10.3390/su16198370
Mathews JC, da Costa Assunção FP, Pereira DO, da Silva JCC, Almeida FFS, Almeida ACP, Mendonça NM, de Sousa Brandão IW, Menezes AO, Borges LEP, et al. Metal Analysis of Leachate from the Organic Fraction of Urban Solid Waste (MSW) from the Municipality of Belém/PA. Sustainability. 2024; 16(19):8370. https://doi.org/10.3390/su16198370
Chicago/Turabian StyleMathews, Josiane Coutinho, Fernanda Paula da Costa Assunção, Diogo Oliveira Pereira, Jéssica Cristina Conte da Silva, Fernando Felipe Soares Almeida, Aline Christian Pimentel Almeida, Neyson Martins Mendonça, Isaque Wilkson de Sousa Brandão, André Oliveira Menezes, Luiz Eduardo Pizarro Borges, and et al. 2024. "Metal Analysis of Leachate from the Organic Fraction of Urban Solid Waste (MSW) from the Municipality of Belém/PA" Sustainability 16, no. 19: 8370. https://doi.org/10.3390/su16198370






