Sustainable Exploitation of Wine Lees as Yeast Extract Supplement for Application in Food Industry and Its Effect on the Growth and Fermentative Ability of Lactiplantibacillus plantarum and Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Wine Lees Yeast Extract Powder
2.3. Inoculum Preparation
2.4. Test Culture Media, Growth Monitoring and Cell Enumeration
2.5. Data Analysis
2.6. Evaluation of Fermentative Ability
2.7. Determination of pH, Reducing Sugars and Ethanol Content in Fermentation Media
2.8. Statistical Analysis
3. Results and Discussion
3.1. Growth Kinetics of Lactiplantibacillus plantarum 2035
3.2. Growth Kinetics of Saccharomyces cerevisiae NCYC187
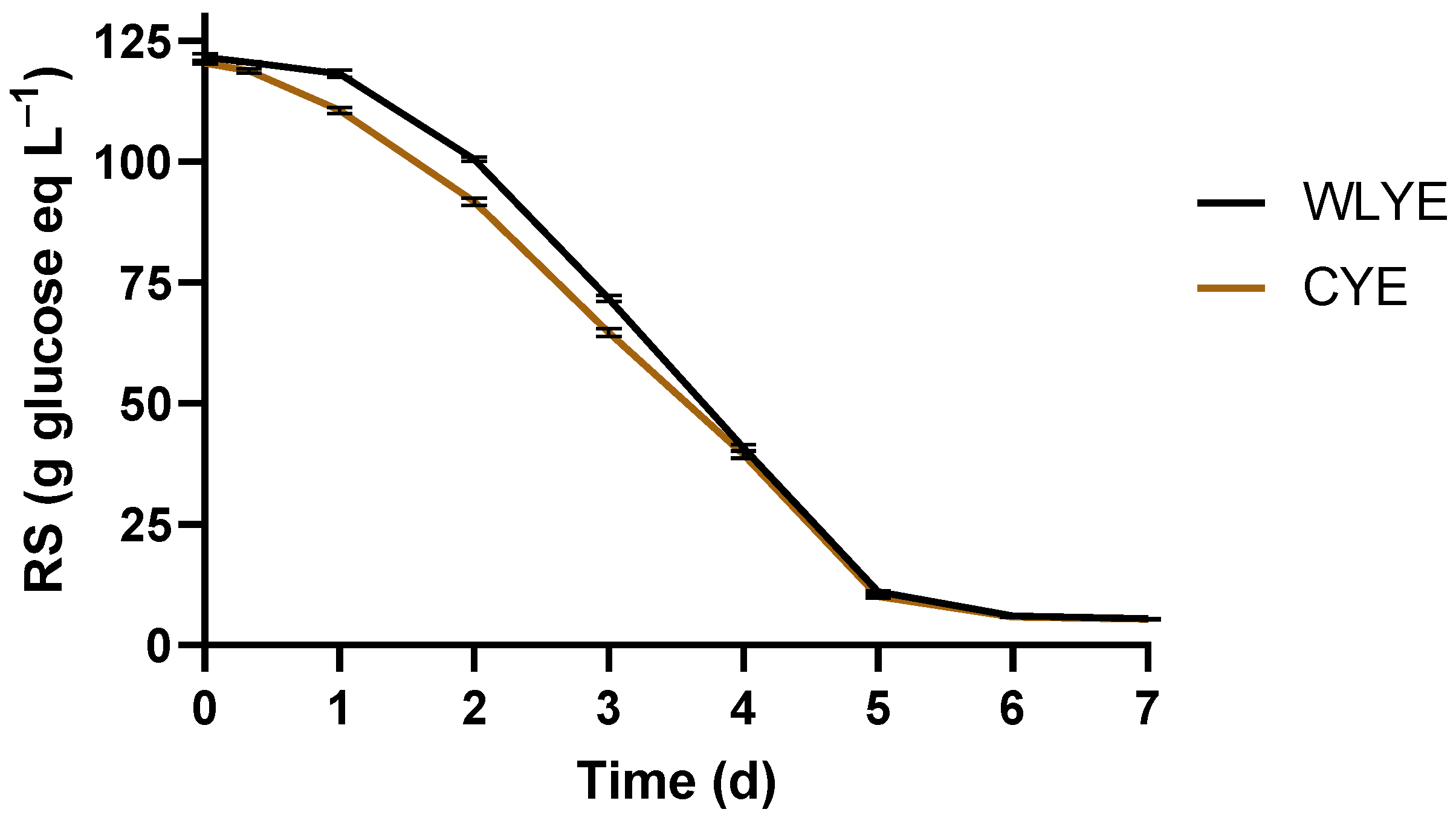
3.3. Fermentative Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OIV World Statistics. Available online: https://www.oiv.int/what-we-do/global-report?oiv (accessed on 16 January 2024).
- Santos, V.; Ramos, P.; Sousa, B.; Valeri, M. Towards a framework for the global wine tourism system. J. Organ. Chang. Manag. 2022, 35, 348–360. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J. Wine lees as a source of antioxidant compounds. Antioxidants 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bibbins, B.; Torrado-Agrasar, A.; Salgado, J.M.; de Souza Oliveira, R.P.; Domínguez, J.M. Potential of lees from wine, beer and cider manufacturing as a source of economic nutrients: An overview. Waste Manag. 2015, 40, 72–81. [Google Scholar] [CrossRef]
- Dimou, C.; Vlysidis, A.; Kopsahelis, N.; Papanikolaou, S.; Koutinas, A.A.; Kookos, I.K. Techno-economic evaluation of wine lees refining for the production of value-added products. Biochem. Eng. J. 2016, 116, 157–165. [Google Scholar] [CrossRef]
- Oliveira, M.; Duarte, E. Integrated approach to winery waste: Waste generation and data consolidation. Front. Environ. Sci. Eng. 2016, 10, 168–176. [Google Scholar] [CrossRef]
- Kokkinomagoulos, E.; Kandylis, P. Grape pomace, an undervalued by-product: Industrial reutilization within a circular economy vision. Rev. Environ. Sci. Bio/Technol. 2023, 22, 739–773. [Google Scholar] [CrossRef]
- Rodríguez-Salgado, I.; Paradelo-Pérez, M.; Pérez-Rodríguez, P.; Cutillas-Barreiro, L.; Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M. Cyprodinil retention on mixtures of soil and solid wastes from wineries. Effects of waste dose and ageing. Environ. Sci. Pollut. Res. 2014, 21, 9785–9795. [Google Scholar] [CrossRef]
- Rugani, B.; Vázquez-Rowe, I.; Benedetto, G.; Benetto, E. A comprehensive review of carbon footprint analysis as an extended environmental indicator in the wine sector. J. Clean. Prod. 2013, 54, 61–77. [Google Scholar] [CrossRef]
- Blanco-Huerta, C.; Rodríguez-Nogales, J.M.; Vila-Crespo, J.; Ruipérez, V.; Fernández-Fernández, E. Impact of high hydrostatic pressure and ultrasounds technologies in the autolytic process of Saccharomyces cerevisiae in a model wine system. Food Biosci. 2024, 57, 103614. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef]
- Bustos, G.; Moldes, A.B.; Cruz, J.M.; Domínguez, J.M. Formulation of low-cost fermentative media for lactic acid production with Lactobacillus rhamnosus using vinification lees as nutrients. J. Agric Food Chem. 2004, 52, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Giamouri, E.; Mavrommatis, A.; Simitzis, P.E.; Mitsiopoulou, C.; Haroutounian, S.A.; Koutinas, A.; Pappas, A.C.; Tsiplakou, E. Redefining the Use of Vinification Waste By-Products in Broiler Diets. Sustainability 2022, 14, 15714. [Google Scholar] [CrossRef]
- Kokkinomagoulos, E.; Kandylis, P. Sustainable exploitation of by-products of vitivinicultural origin in winemaking. Proceedings 2020, 67, 5. [Google Scholar] [CrossRef]
- Scarponi, P.; Caminiti, V.; Bravi, M.; Izzo, F.C.; Cavinato, C. Coupling anaerobic co-digestion of winery waste and waste activated sludge with a microalgae process: Optimization of a semi-continuous system. Waste Manag. 2024, 174, 300–309. [Google Scholar] [CrossRef] [PubMed]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and future strategies for wine yeast lees valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef] [PubMed]
- Schlabitz, C.; Lehn, D.N.; de Souza, C.F.V. A review of Saccharomyces cerevisiae and the applications of its byproducts in dairy cattle feed: Trends in the use of residual brewer’s yeast. J. Clean. Prod. 2022, 332, 130059. [Google Scholar] [CrossRef]
- Pérez-Bibbins, B.; Torrado-Agrasar, A.; Pérez-Rodríguez, N.; Aguilar-Uscanga, M.G.; Domínguez, J.M. Evaluation of the liquid, solid and total fractions of beer, cider and wine lees as economic nutrient for xylitol production. J. Chem. Technol. Biotechnol. 2015, 90, 1027–1039. [Google Scholar] [CrossRef]
- IndustryARC, Yeast Extracts Market–Forecast (2025–2032). Available online: https://www.industryarc.com/Report/18940/yeast-extracts-market (accessed on 17 January 2024).
- Global Market Insights, Yeast Extract Market Size By Technology (Autolyzed Yeast, Hydrolyzed Yeast), By Source (Bakers Yeast, Brewers Yeast, Torula Yeast), By Application (Food & Beverages, Animal Feed, Pharmaceuticals), By Form (Powder, Paste) & Forecast, 2023–2032. Available online: https://www.gminsights.com/industry-analysis/yeast-extract-market (accessed on 16 January 2024).
- Salgado, J.M.; Rodriguez, N.; Cortes, S.; Domínguez, J.M. Development of cost-effective media to increase the economic potential for larger-scale bioproduction of natural food additives by Lactobacillus rhamnosus, Debaryomyces hansenii, and Aspergillus niger. J. Agric. Food Chem. 2009, 57, 10414–10428. [Google Scholar] [CrossRef]
- Dimou, C.; Kopsahelis, N.; Papadaki, A.; Papanikolaou, S.; Kookos, I.K.; Mandala, I.; Koutinas, A.A. Wine lees valorization: Biorefinery development including production of a generic fermentation feedstock employed for poly (3-hydroxybutyrate) synthesis. Food Res. Int. 2015, 73, 81–87. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Moldes, A.B.; Domínguez, J.M. Tartaric acid recovery from distilled lees and use of the residual solid as an economic nutrient for Lactobacillus. J. Agric. Food Chem. 2006, 54, 7904–7911. [Google Scholar] [CrossRef]
- Balmaseda, A.; Miot-Sertier, C.; Lytra, G.; Poulain, B.; Reguant, C.; Lucas, P.; Nioi, C. Application of white wine lees for promoting lactic acid bacteria growth and malolactic fermentation in wine. Int. J. Food Microbiol. 2024, 413, 110583. [Google Scholar] [CrossRef] [PubMed]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Kallis, M.; Sideris, K.; Kopsahelis, N.; Bosnea, L.; Kourkoutas, Y.; Terpou, A.; Kanellaki, M. Pistacia terebinthus resin as yeast immobilization support for alcoholic fermentation. Foods 2019, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Li, Y.K.; Song, H.C.; Tao, Y.S.; Russo, N. Phenolic matrix effect on aroma formation of terpenes during simulated wine fermentation–Part I: Phenolic acids. Food Chem. 2021, 341, 128288. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Champagne, C.P.; Gaudreau, H.; Conway, J. Effect of the production or use of mixtures of bakers’ or brewers’ yeast extracts on their ability to promote growth of lactobacilli and pediococci. Electron. J. Biotechnol. 2003, 6, 185–197. [Google Scholar] [CrossRef]
- Horn, S.J.; Aspmo, S.I.; Eijsink, V.G.H. Growth of Lactobacillus plantarum in media containing hydrolysates of fish viscera. J. Appl. Microbiol. 2005, 99, 1082–1089. [Google Scholar] [CrossRef]
- Silva, A.P.R.D.; Longhi, D.A.; Dalcanton, F.; Aragão, G.M.F.D. Modelling the growth of lactic acid bacteria at different temperatures. Braz. Arch. Biol. Technol. 2018, 61, e18160159. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Upadhyay, V.K.; McSweeney, P.L.; Minervini, F.; Gallo, G.; Gobbetti, M. Effect of proteinases of starter bacteria on the growth and proteolytic activity of Lactobacillus plantarum DPC2741. Int. Dairy J. 2003, 13, 145–157. [Google Scholar] [CrossRef]
- Ardré, M.; Doulcier, G.; Brenner, N.; Rainey, P.B. Interaction among bacterial cells triggers exit from lag phase. bioRxiv 2022. [Google Scholar] [CrossRef]
- García-Ríos, E.; Gutiérrez, A.; Salvadó, Z.; Arroyo-López, F.N.; Guillamon, J.M. The fitness advantage of commercial wine yeasts in relation to the nitrogen concentration, temperature, and ethanol content under microvinification conditions. Appl. Environ. Microbiol. 2014, 80, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-López, F.N.; Orlić, S.; Querol, A.; Barrio, E. Effects of temperature, pH and sugar concentration on the growth parameters of Saccharomyces cerevisiae, S. kudriavzevii and their interspecific hybrid. Int. J. Food Microbiol. 2009, 131, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Onetto, C.; McCarthy, J.; Solomon, M.; Borneman, A.R.; Schmidt, S.A. Enhancing fermentation performance through the reutilisation of wine yeast lees. OENO One 2024, 58. [Google Scholar] [CrossRef]
- Balmaseda, A.; Rozès, N.; Leal, M.Á.; Bordons, A.; Reguant, C. Impact of changes in wine composition produced by non-Saccharomyces on malolactic fermentation. Int. J. Food Microbiol. 2021, 337, 108954. [Google Scholar] [CrossRef]
- Onetto, C.A.; Bordeu, E. Pre-alcoholic fermentation acidification of red grape must using Lactobacillus plantarum. Antonie Leeuwenhoek 2015, 108, 1469–1475. [Google Scholar] [CrossRef]
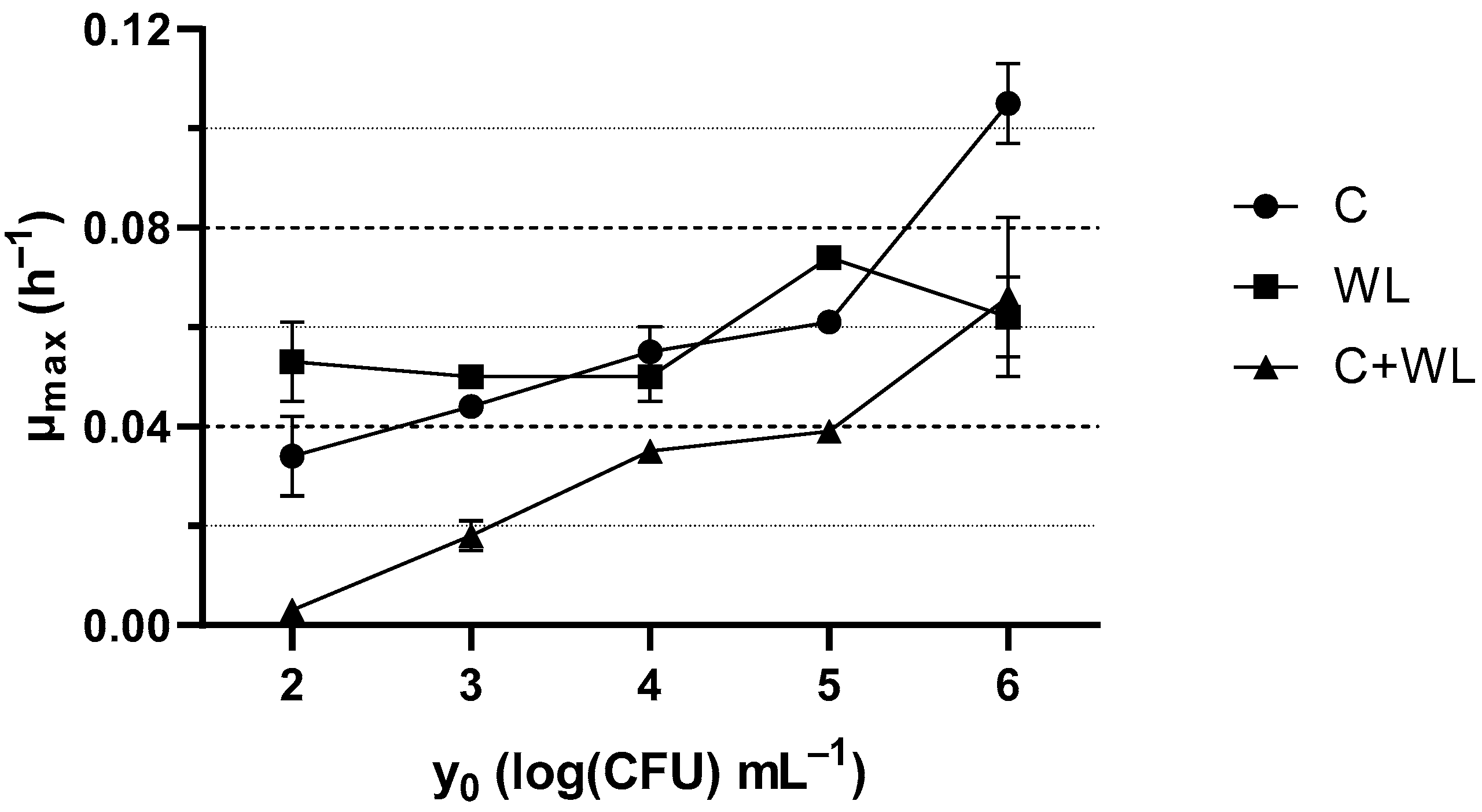
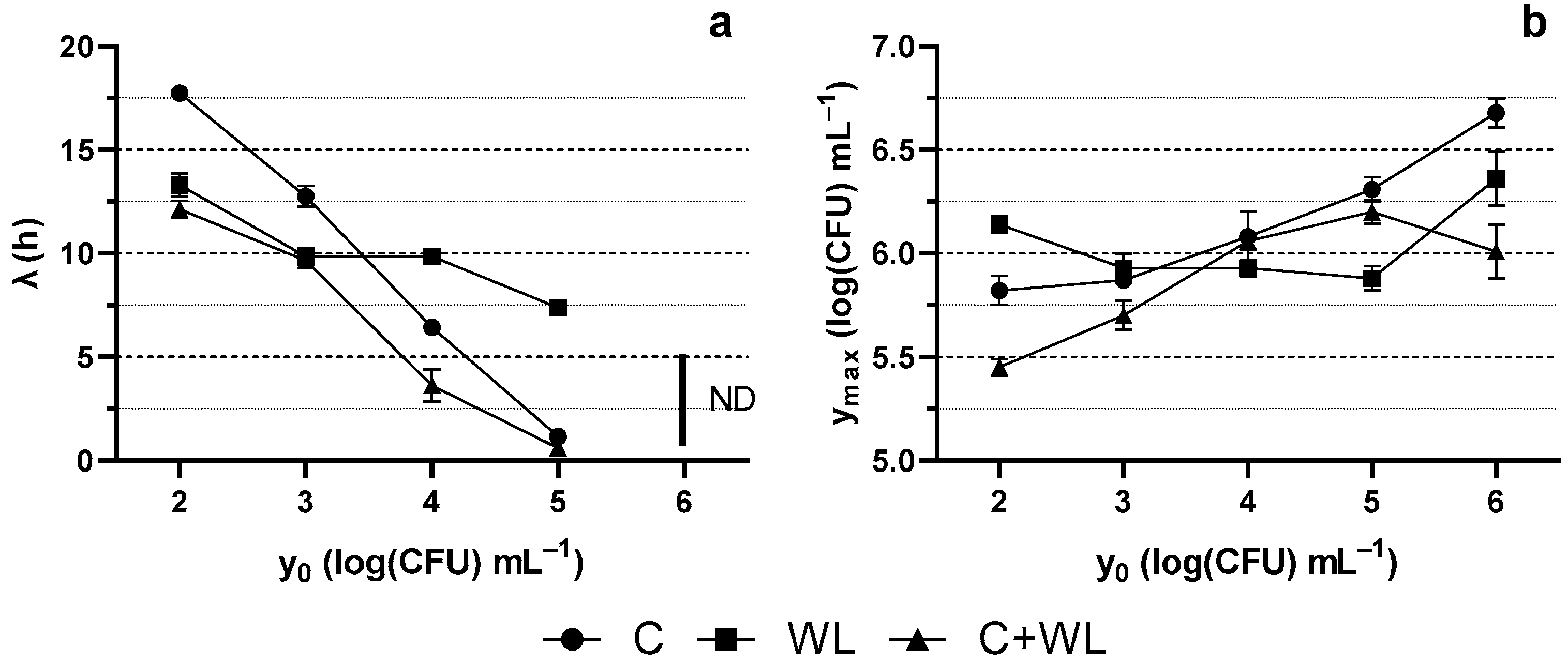
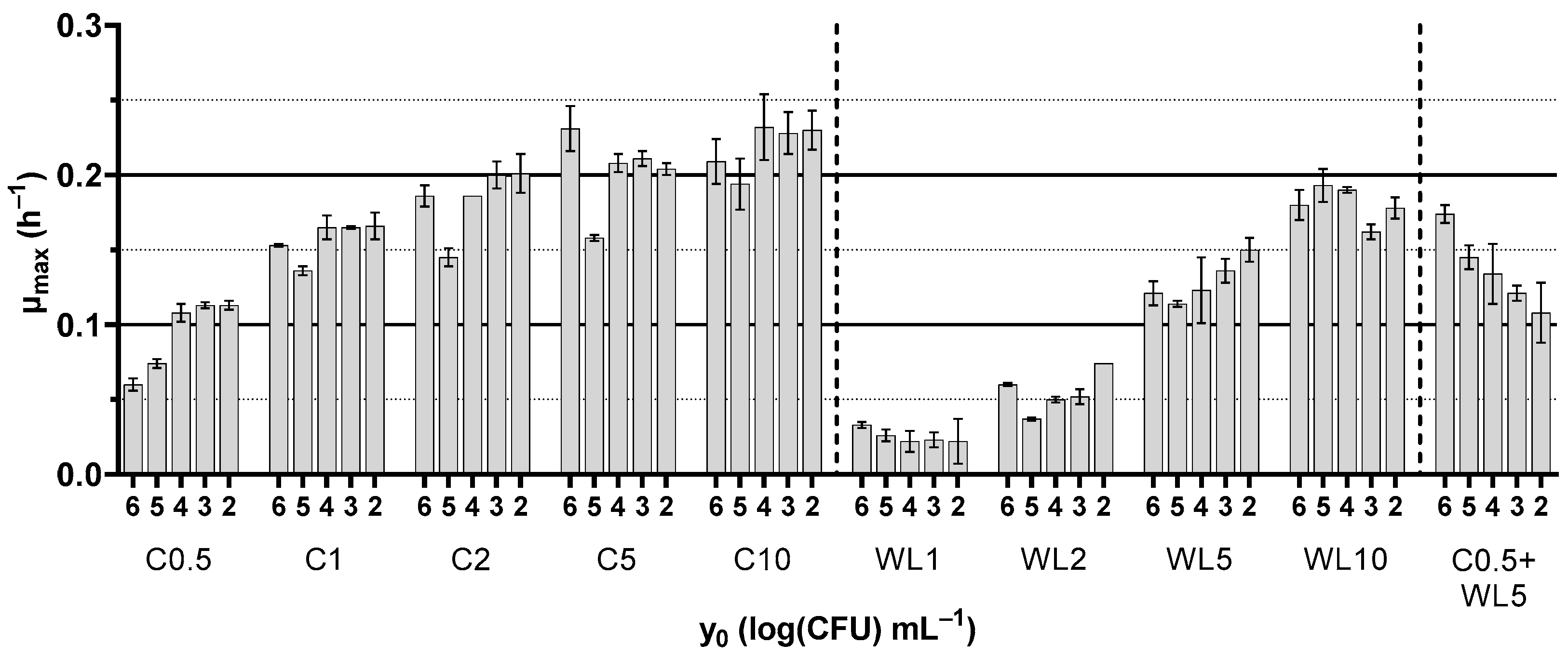


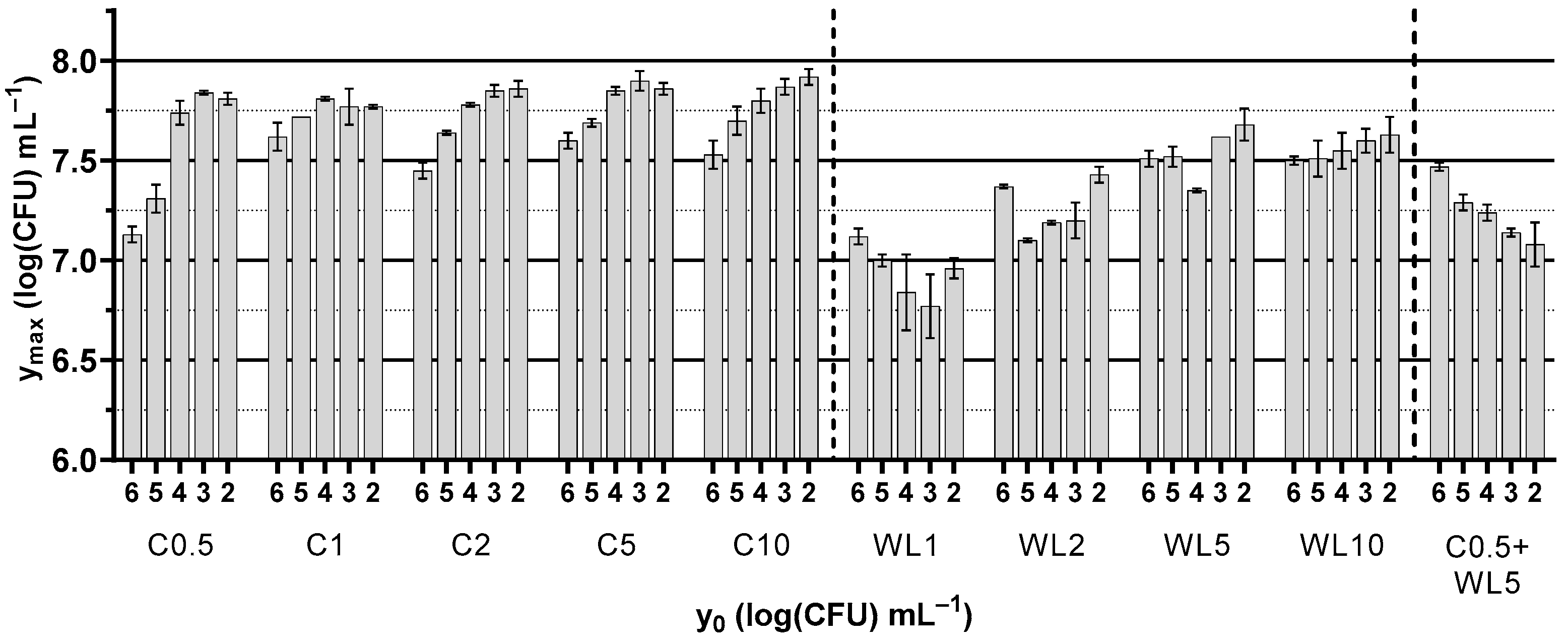

| Culture Media Base | Type of Yeast Extract | Concentration (g L−1) | Coding |
|---|---|---|---|
| Lactiplantibacillus plantarum 2035 | |||
| MRS broth | CYE | 5 | C |
| WLYE | 5 | WL | |
| CYE + WLYE | 2.5 + 2.5 | C + WL | |
| Saccharomyces cerevisiae NCYC187 | |||
| Yeast Carbon Base | CYE | 0.5 | C0.5 |
| CYE | 1 | C1 | |
| CYE | 2 | C2 | |
| CYE | 5 | C5 | |
| CYE | 10 | C10 | |
| WLYE | 1 | WL1 | |
| WLYE | 2 | WL2 | |
| WLYE | 5 | WL5 | |
| WLYE | 10 | WL10 | |
| CYE + WLYE | 0.5 + 5 | C0.5 + WL5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkinomagoulos, E.; Kandylis, P. Sustainable Exploitation of Wine Lees as Yeast Extract Supplement for Application in Food Industry and Its Effect on the Growth and Fermentative Ability of Lactiplantibacillus plantarum and Saccharomyces cerevisiae. Sustainability 2024, 16, 8449. https://doi.org/10.3390/su16198449
Kokkinomagoulos E, Kandylis P. Sustainable Exploitation of Wine Lees as Yeast Extract Supplement for Application in Food Industry and Its Effect on the Growth and Fermentative Ability of Lactiplantibacillus plantarum and Saccharomyces cerevisiae. Sustainability. 2024; 16(19):8449. https://doi.org/10.3390/su16198449
Chicago/Turabian StyleKokkinomagoulos, Evangelos, and Panagiotis Kandylis. 2024. "Sustainable Exploitation of Wine Lees as Yeast Extract Supplement for Application in Food Industry and Its Effect on the Growth and Fermentative Ability of Lactiplantibacillus plantarum and Saccharomyces cerevisiae" Sustainability 16, no. 19: 8449. https://doi.org/10.3390/su16198449







