Techno-Economic Assessment of Amine-Based Carbon Capture in Waste-to-Energy Incineration Plant Retrofit
Abstract
:1. Introduction
2. Methodology
2.1. Waste-to-Energy Incineration Plant
2.2. Carbon Capture System Scheme
2.3. Carbon Capture System Simulation
2.4. Cost Estimation
2.4.1. Capital Cost (Capex) Estimation
2.4.2. Operational Cost (Opex) Estimation
2.5. Key Performance Indicators
3. Results and Discussions
3.1. Global Energy Efficiency Results
3.2. Cost Evaluation Results
3.3. Limitations of the Study and Research Needs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| kNOK | 0–20 | 20–100 | 100–500 | 500–1000 | 1000–2000 | 2000–5000 | 5000–15,000 | >15,000 |
|---|---|---|---|---|---|---|---|---|
| Equipment | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Erection/Installation | 0.89 | 0.47 | 0.25 | 0.18 | 0.14 | 0.11 | 0.10 | 0.08 |
| Piping | 3.56 | 1.92 | 1.12 | 0.83 | 0.65 | 0.48 | 0.41 | 0.29 |
| Electric | 1.03 | 0.71 | 0.48 | 0.41 | 0.34 | 0.28 | 0.25 | 0.18 |
| Instrument | 3.56 | 1.92 | 1.12 | 0.83 | 0.65 | 0.48 | 0.41 | 0.29 |
| Civil | 0.55 | 0.36 | 0.25 | 0.20 | 0.17 | 0.14 | 0.13 | 0.09 |
| Steel &and Concrete | 1.79 | 1.17 | 0.79 | 0.64 | 0.55 | 0.43 | 0.39 | 0.28 |
| Insulation | 0.67 | 0.34 | 0.18 | 0.14 | 0.11 | 0.09 | 0.05 | 0.04 |
| Direct Cost | 13.05 | 7.89 | 5.19 | 4.23 | 3.61 | 3.01 | 2.74 | 2.25 |
| Engineering Process | 1.23 | 0.43 | 0.24 | 0.18 | 0.15 | 0.13 | 0.11 | 0.09 |
| Engineering Mechanical | 0.98 | 0.24 | 0.10 | 0.05 | 0.04 | 0.03 | 0.01 | 0.01 |
| Engineering Piping | 1.08 | 0.58 | 0.34 | 0.25 | 0.18 | 0.14 | 0.13 | 0.09 |
| Engineering Electric | 1.04 | 0.30 | 0.15 | 0.11 | 0.10 | 0.09 | 0.05 | 0.04 |
| Engineering Instrument | 1.85 | 0.72 | 0.36 | 0.25 | 0.20 | 0.14 | 0.13 | 0.09 |
| Engineering Civil | 0.39 | 0.11 | 0.04 | 0.03 | 0.03 | 0.01 | 0.01 | 0.01 |
| Engineering Steel & Concrete | 0.58 | 0.24 | 0.13 | 0.10 | 0.09 | 0.05 | 0.05 | 0.04 |
| Engineering Insulation | 0.27 | 0.09 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Engineering Cost | 7.42 | 2.71 | 1.39 | 0.98 | 0.80 | 0.60 | 0.50 | 0.38 |
| Procurement | 1.55 | 0.52 | 0.20 | 0.13 | 0.09 | 0.04 | 0.03 | 0.03 |
| Project Control | 0.37 | 0.14 | 0.05 | 0.04 | 0.04 | 0.03 | 0.03 | 0.03 |
| Site Management | 0.66 | 0.42 | 0.28 | 0.24 | 0.20 | 0.17 | 0.15 | 0.11 |
| Project Management | 0.89 | 0.46 | 0.29 | 0.24 | 0.20 | 0.17 | 0.15 | 0.11 |
| Administration Cost | 3.47 | 1.54 | 0.82 | 0.65 | 0.53 | 0.41 | 0.36 | 0.28 |
| Commissioning | 0.72 | 0.33 | 0.17 | 0.10 | 0.10 | 0.05 | 0.05 | 0.04 |
| Contingency | 4.99 | 2.55 | 1.57 | 1.24 | 1.06 | 0.87 | 0.78 | 0.64 |
| Total Plant Cost, FTotal, CS | 29.65 | 15.02 | 9.14 | 7.20 | 6.10 | 4.94 | 4.43 | 3.59 |
| 3000 t CO2 Captured | 6000 t CO2 Captured | 12,000 t CO2 Captured | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Equipment | Material | Peculiarity | Equipment Cost in Aspen [EUR] | EIC for Italy [kEUR] | Peculiarity | Equipment Cost in Aspen [EUR] | EIC for Italy [kEUR] | Peculiarity | Equipment Cost in Aspen [EUR] | EIC for Italy [kEUR] | |
| F-101 | Fan | SS316 m | Duty: 30 kW | 10 | 95 | Duty: 55 kW | 17 | 99 | Duty: 75 kW | 22 | 130 |
| P-101 | Pump | SS316 m | Duty: 1.5 kW | 8 | 77 | Duty: 3 kW | 10 | 90 | Duty: 7.5 kW | 12 | 115 |
| E-101 | Exchanger | SS316 w | Surface: 14.6 m2 | 20 | 94 | Surface: 29.3 m2 | 22 | 103 | Surface: 60.3 m2 | 38 | 178 |
| C-101 | DCC | SS316 w | Diameter: 0.71 m TT Height: 9.7 m | 88 | 418 | Diameter: 0.94 m TT height: 10.4 m | 169 | 638 | Diameter: 1.4 m TT height: 10.8 m | 272 | 881 |
| C-201 | Absorber | SS316 w | Diameter: 0.71 m TT Height: 23.1 m | 263 | 854 | Diameter: 0.94 m TT Height: 23.6 m | 365 | 975 | Diameter: 1.4 m TT Height: 27.7 m | 609 | 1.626 |
| C-202 | Stripper | SS316 w | Diameter: 0.44 m TT Height: 8.8 m | 64 | 303 | Diameter: 0.6 m TT Height: 9.3 m | 76 | 358 | Diameter: 0.84 m TT Height: 9.75 m | 106 | 402 |
| P-201 | Pump | SS316 m | Duty: 3 kW | 9 | 83 | Duty: 7.5 kW | 11 | 101 | Duty: 11 kW | 13 | 122 |
| P-202 | Pump | SS316 m | Duty: 2.2 kW | 8 | 78 | Duty: 4 kW | 10 | 94 | Duty: 7.5 kW | 13 | 119 |
| P-203 | Pump | SS316 m | Duty: 0.75 kW | 8 | 73 | Duty: 0.75 kW | 8 | 73 | Duty: 0.75 kW | 8 | 73 |
| E-201 | Exchanger | SS316 w | Surface: 9.3 m2 | 19 | 89 | Surface: 18.6 m2 | 22 | 103 | Surface: 37 m2 | 30 | 140 |
| E-202 | Exchanger | SS316 w | Surface: 16.9 m2 | 22 | 103 | Surface: 33.9 m2 | 30 | 140 | Surface: 67.6 m2 | 43 | 202 |
| E-203 | Exchanger | SS316 w | Surface: 3.9 m2 | 15 | 116 | Surface: 7.6 m2 | 16 | 123 | Surface: 15.4 m2 | 20 | 94 |
| E-204 | Exchanger | SS316 w | Surface: 10.2 m2 | 21 | 98 | Surface: 20.9 m2 | 27 | 128 | Surface: 40 m2 | 34 | 160 |
References
- Astrup, T.; Møller, J.; Fruergaard, T. Incineration and Co-Combustion of Waste: Accounting of Greenhouse Gases and Global Warming Contributions. Waste Manag. Res. 2009, 27, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Poretti, F.; Stengler, E. The Climate Roadmap of the European Waste-to-Energy Sector|The Path to Carbon Negative. SSRN J. 2022. [Google Scholar] [CrossRef]
- Cucchiella, F.; D’Adamo, I.; Gastaldi, M. Sustainable Management of Waste-to-Energy Facilities. Renew. Sustain. Energy Rev. 2014, 33, 719–728. [Google Scholar] [CrossRef]
- Wang, D.; Tang, Y.-T.; Long, G.; Higgitt, D.; He, J.; Robinson, D. Future Improvements on Performance of an EU Landfill Directive Driven Municipal Solid Waste Management for a City in England. Waste Manag. 2020, 102, 452–463. [Google Scholar] [CrossRef]
- Christensen, T.H.; Gentil, E.; Boldrin, A.; Larsen, A.W.; Weidema, B.P.; Hauschild, M. C Balance, Carbon Dioxide Emissions and Global Warming Potentials in LCA-Modelling of Waste Management Systems. Waste Manag. Res. 2009, 27, 707–715. [Google Scholar] [CrossRef]
- Creutzig, F.; Ravindranath, N.H.; Berndes, G.; Bolwig, S.; Bright, R.; Cherubini, F.; Chum, H.; Corbera, E.; Delucchi, M.; Faaij, A.; et al. Bioenergy and Climate Change Mitigation: An Assessment. GCB Bioenergy 2015, 7, 916–944. [Google Scholar] [CrossRef]
- Gough, C.; Upham, P. Biomass Energy with Carbon Capture and Storage (BECCS or Bio-CCS). Greenh. Gases 2011, 1, 324–334. [Google Scholar] [CrossRef]
- Materazzi, M.; Chari, S.; Sebastiani, A.; Lettieri, P.; Paulillo, A. Waste-to-Energy and Waste-to-Hydrogen with CCS: Methodological Assessment of Pathways to Carbon-Negative Waste Treatment from an LCA Perspective. Waste Manag. 2024, 173, 184–199. [Google Scholar] [CrossRef]
- Pour, N.; Webley, P.A.; Cook, P.J. Potential for Using Municipal Solid Waste as a Resource for Bioenergy with Carbon Capture and Storage (BECCS). Int. J. Greenh. Gas. Control 2018, 68, 1–15. [Google Scholar] [CrossRef]
- C2ES. CCUS Technology Is Essential to the Success of the Paris Agreement—Center for Climate and Energy Solutions; C2ES: Arlington, VA, USA, 2016. [Google Scholar]
- IEA. Energy Technology Perspectives—Special Report on Carbon Capture Utilisation and Storage—CCUS in Clean Energy Transitions; International Energy Agency: Paris, France, 2020. [Google Scholar]
- Metz, B.; Davidson, O.; de Coninck, H.; Loos, M.; Meyer, L. Special Report on Carbon Dioxide Capture and Storage—The Intergovernmental Panel on Climate Change (IPCC); Cambridge University Press: Cambrige, UK; New York, NY, USA, 2005. [Google Scholar]
- Paris Agreement International Agreements. Council Decision 2016/1841 of 5 October 2016 on the Conclusion, on Behalf of the European Union, of the Paris Agreement Adopted under the United Nations Framework Convention on Climate Change. Off. J. Eur. Union 2016. [Google Scholar]
- Golombek, R.; Kverndokk, S.; Greaker, M.; Ma, L. The Transition to Carbon Capture and Storage Technologies. SSRN J. 2021. [Google Scholar] [CrossRef]
- IEA. Putting CO2 to Use: Creating Value from Emissions; International Energy Agency: Paris, France, 2019; Available online: https://www.iea.org/Reports/Putting-Co2-to-Use (accessed on 8 April 2024).
- Thomas, A., II; Leila, H.; Pranav, M.; Ikenna, O. Comparison of CO2 Capture Approaches for Fossil-Based Power Generation: Review and Meta-Study. Processes 2017, 5, 44. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An Overview of Current Status of Carbon Dioxide Capture and Storage Technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Bui, M.; Gunawan, I.; Verheyen, V.; Feron, P.; Meuleman, E.; Adeloju, S. Dynamic Modelling and Optimisation of Flexible Operation in Post-Combustion CO2 Capture Plants—A Review. Comput. Chem. Eng. 2014, 61, 245–265. [Google Scholar] [CrossRef]
- Plaza, J.M.; Wagener, D.V.; Rochelle, G.T. Modeling CO2 Capture with Aqueous Monoethanolamine. Energy Procedia 2009, 1, 1171–1178. [Google Scholar] [CrossRef]
- Tan, L.S.; Shariff, A.M.; Lau, K.K.; Bustam, M.A. Factors Affecting CO2 Absorption Efficiency in Packed Column: A Review. J. Ind. Eng. Chem. 2012, 18, 1874–1883. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-Combustion CO2 Capture with Chemical Absorption: A State-of-the-Art Review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Yun, S.; Kim, J.-K. Process Integration and Design for Maximizing Energy Efficiency of a Coal-Fired Power Plant Integrated with Amine-Based CO2 Capture Process. Appl. Energy 2018, 216, 311–322. [Google Scholar] [CrossRef]
- Pettinau, A.; Ferrara, F.; Tola, V.; Cau, G. Techno-Economic Comparison between Different Technologies for CO2-Free Power Generation from Coal. Appl. Energy 2017, 193, 426–439. [Google Scholar] [CrossRef]
- Wienchol, P.; Szlęk, A.; Ditaranto, M. Waste-to-Energy Technology Integrated with Carbon Capture—Challenges and Opportunities. Energy 2020, 198, 117352. [Google Scholar] [CrossRef]
- Bringezu, S. Carbon Recycling for Renewable Materials and Energy Supply: Recent Trends, Long-Term Options, and Challenges for Research and Development. J. Ind. Ecol. 2014, 18, 327–340. [Google Scholar] [CrossRef]
- Christensen, T.H.; Bisinella, V. Climate Change Impacts of Introducing Carbon Capture and Utilisation (CCU) in Waste Incineration. Waste Manag. 2021, 126, 754–770. [Google Scholar] [CrossRef] [PubMed]
- Bisinella, V.; Hulgaard, T.; Riber, C.; Damgaard, A.; Christensen, T.H. Environmental Assessment of Carbon Capture and Storage (CCS) as a Post-Treatment Technology in Waste Incineration. Waste Manag. 2021, 128, 99–113. [Google Scholar] [CrossRef]
- Haaf, M.; Anantharaman, R.; Roussanaly, S.; Ströhle, J.; Epple, B. CO2 Capture from Waste-to-Energy Plants: Techno-Economic Assessment of Novel Integration Concepts of Calcium Looping Technology. Resour. Conserv. Recycl. 2020, 162, 104973. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, Z.; Li, H. SNG-Electricity Cogeneration through MSW Gasification Integrated with a Dual Chemical Looping Process. Chem. Eng. Process.-Process Intensif. 2019, 145, 107665. [Google Scholar] [CrossRef]
- Roussanaly, S.; Ouassou, J.A.; Anantharaman, R.; Haaf, M. Impact of Uncertainties on the Design and Cost of CCS From a Waste-to-Energy Plant. Front. Energy Res. 2020, 8, 17. [Google Scholar] [CrossRef]
- Bertone, M.; Stabile, L.; Buonanno, G. An Overview of Waste-to-Energy Incineration Integrated with Carbon Capture Utilization or Storage Retrofit Application. Sustainability 2024, 16, 4117. [Google Scholar] [CrossRef]
- Huttenhuis, P.; Roeloffzen, A.; Versteeg, G. CO2 Capture and Re-Use at a Waste Incinerator. Energy Procedia 2016, 86, 47–55. [Google Scholar] [CrossRef]
- Fagerlund, J.; Zevenhoven, R.; Thomassen, J.; Tednes, M.; Abdollahi, F.; Thomas, L.; Nielsen, C.J.; Mikoviny, T.; Wisthaler, A.; Zhu, L.; et al. Performance of an Amine-Based CO2 Capture Pilot Plant at the Fortum Oslo Varme Waste to Energy Plant in Oslo, Norway. Int. J. Greenh. Gas Control 2021, 106, 103242. [Google Scholar] [CrossRef]
- Dal Pozzo, A.; Capecci, S.; Cozzani, V. Techno-Economic Impact of Lower Emission Standards for Waste-to-Energy Acid Gas Emissions. Waste Manag. 2023, 166, 305–314. [Google Scholar] [CrossRef]
- Vehlow, J. Air Pollution Control Systems in WtE Units: An Overview. Waste Manag. 2015, 37, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Alie, C.; Backham, L.; Croiset, E.; Douglas, P.L. Simulation of CO2 Capture Using MEA Scrubbing: A Flowsheet Decomposition Method. Energy Convers. Manag. 2005, 46, 475–487. [Google Scholar] [CrossRef]
- De Miguel Mercader, F.; Magneschi, G.; Sanchez Fernandez, E.; Stienstra, G.J.; Goetheer, E.L.V. Integration between a Demo Size Post-Combustion CO2 Capture and Full Size Power Plant. An Integral Approach on Energy Penalty for Different Process Options. Int. J. Greenh. Gas Control 2012, 11, S102–S113. [Google Scholar] [CrossRef]
- Nittaya, T.; Douglas, P.L.; Croiset, E.; Ricardez-Sandoval, L.A. Dynamic Modeling and Evaluation of an Industrial-Scale CO2 Capture Plant Using Monoethanolamine Absorption Processes. Ind. Eng. Chem. Res. 2014, 53, 11411–11426. [Google Scholar] [CrossRef]
- Seader, J.D.; Henley, E.J.; Roper, D.K. Separation Process Principles: Chemical and Biochemical Operations, 3rd ed.; Wiley: Hoboken, NJ, USA, 2011; ISBN 978-0-470-48183-7. [Google Scholar]
- Singh, D.; Croiset, E.; Douglas, P.L.; Douglas, M.A. Techno-Economic Study of CO2 Capture from an Existing Coal-Fired Power Plant: MEA Scrubbing vs. O2/CO2 Recycle Combustion. Energy Convers. Manag. 2003, 44, 3073–3091. [Google Scholar] [CrossRef]
- Sinnott, R. Coulson and Richardson’s Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 978-0-08-041865-0. [Google Scholar]
- Madeddu, C.; Baratti, R.; Errico, M. CO2 Capture by Reactive Absorption-Stripping: Modeling, Analysis and Design, 1st ed.; Springer Briefs in Energy; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-04579-1. [Google Scholar]
- Oexmann, J.; Kather, A. Post-Combustion CO2 Capture in Coal-Fired Power Plants: Comparison of Integrated Chemical Absorption Processes with Piperazine Promoted Potassium Carbonate and MEA. Energy Procedia 2009, 1, 799–806. [Google Scholar] [CrossRef]
- Notz, R.; Mangalapally, H.P.; Hasse, H. Post Combustion CO2 Capture by Reactive Absorption: Pilot Plant Description and Results of Systematic Studies with MEA. Int. J. Greenh. Gas Control 2012, 6, 84–112. [Google Scholar] [CrossRef]
- Smith, R. Chemical Process Design and Integration; John Wiley&Sons Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Austgen, D.M.; Rochelle, G.T.; Peng, X.; Chen, C.C. Model of Vapor-Liquid Equilibria for Aqueous Acid Gas-Alkanolamine Systems Using the Electrolyte-NRTL Equation. Ind. Eng. Chem. Res. 1989, 28, 1060–1073. [Google Scholar] [CrossRef]
- Biliyok, C.; Lawal, A.; Wang, M.; Seibert, F. Dynamic Modelling, Validation and Analysis of Post-Combustion Chemical Absorption CO2 Capture Plant. Int. J. Greenh. Gas Control 2012, 9, 428–445. [Google Scholar] [CrossRef]
- Errico, M.; Madeddu, C.; Pinna, D.; Baratti, R. Model Calibration for the Carbon Dioxide-Amine Absorption System. Appl. Energy 2016, 183, 958–968. [Google Scholar] [CrossRef]
- Kvamsdal, H.M.; Rochelle, G.T. Effects of the Temperature Bulge in CO2 Absorption from Flue Gas by Aqueous Monoethanolamine. Ind. Eng. Chem. Res. 2008, 47, 867–875. [Google Scholar] [CrossRef]
- Lawal, A.; Wang, M.; Stephenson, P.; Yeung, H. Dynamic Modelling of CO2 Absorption for Post Combustion Capture in Coal-Fired Power Plants. Fuel 2009, 88, 2455–2462. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Watanasiri, S. Representing Vapor−Liquid Equilibrium for an Aqueous MEA−CO2 System Using the Electrolyte Nonrandom-Two-Liquid Model. Ind. Eng. Chem. Res. 1999, 38, 2080–2090. [Google Scholar] [CrossRef]
- Moioli, S.; Pellegrini, L.A.; Gamba, S. Simulation of CO2 Capture by MEA Scrubbing with a Rate-Based Model. Procedia Eng. 2012, 42, 1651–1661. [Google Scholar] [CrossRef]
- Posch, S.; Haider, M. Dynamic Modeling of CO2 Absorption from Coal-Fired Power Plants into an Aqueous Monoethanolamine Solution. Chem. Eng. Res. Des. 2013, 91, 977–987. [Google Scholar] [CrossRef]
- Razi, N.; Svendsen, H.F.; Bolland, O. Validation of Mass Transfer Correlations for CO2 Absorption with MEA Using Pilot Data. Int. J. Greenh. Gas Control 2013, 19, 478–491. [Google Scholar] [CrossRef]
- Lawal, A.; Wang, M.; Stephenson, P.; Koumpouras, G.; Yeung, H. Dynamic Modelling and Analysis of Post-Combustion CO2 Chemical Absorption Process for Coal-Fired Power Plants. Fuel 2010, 89, 2791–2801. [Google Scholar] [CrossRef]
- Aboudheir, A.; Tontiwachwuthikul, P.; Chakma, A.; Idem, R. Kinetics of the Reactive Absorption of Carbon Dioxide in High CO2-Loaded, Concentrated Aqueous Monoethanolamine Solutions. Chem. Eng. Sci. 2003, 58, 5195–5210. [Google Scholar] [CrossRef]
- Meldon, J.H.; Morales-Cabrera, M.A. Analysis of Carbon Dioxide Absorption in and Stripping from Aqueous Monoethanolamine. Chem. Eng. J. 2011, 171, 753–759. [Google Scholar] [CrossRef]
- Tobiesen, F.A.; Svendsen, H.F.; Juliussen, O. Experimental Validation of a Rigorous Absorber Model for CO2 Postcombustion Capture. AIChE J. 2007, 53, 846–865. [Google Scholar] [CrossRef]
- Abu-Zahra, M.R.M.; Schneiders, L.H.J.; Niederer, J.P.M.; Feron, P.H.M.; Versteeg, G.F. CO2 Capture from Power Plants. Int. J. Greenh. Gas Control 2007, 1, 37–46. [Google Scholar] [CrossRef]
- Cau, G.; Tola, V.; Deiana, P. Comparative Performance Assessment of USC and IGCC Power Plants Integrated with CO2 Capture Systems. Fuel 2014, 116, 820–833. [Google Scholar] [CrossRef]
- Lawal, A.; Wang, M.; Stephenson, P.; Obi, O. Demonstrating Full-Scale Post-Combustion CO2 Capture for Coal-Fired Power Plants through Dynamic Modelling and Simulation. Fuel 2012, 101, 115–128. [Google Scholar] [CrossRef]
- Freguia, S.; Rochelle, G.T. Modeling of CO2 Capture by Aqueous Monoethanolamine. AIChE J. 2003, 49, 1676–1686. [Google Scholar] [CrossRef]
- Davis, J.; Rochelle, G. Thermal Degradation of Monoethanolamine at Stripper Conditions. Energy Procedia 2009, 1, 327–333. [Google Scholar] [CrossRef]
- Madeddu, C.; Errico, M.; Baratti, R. Process Analysis for the Carbon Dioxide Chemical Absorption–Regeneration System. Appl. Energy 2018, 215, 532–542. [Google Scholar] [CrossRef]
- Dow Chemical Company. The Dow Chemical Company Material Safety Data Sheet: Monoethanolamine (Online); The Dow Chemical Company: Midland, MI, USA, 2023. [Google Scholar]
- Øi, L.E. Removal of CO2 from Exhaust Gas. Doctoral Thesis, Telemark University College, Porsgrunn, Norway, 2012. [Google Scholar]
- Ali, H.; Eldrup, N.H.; Normann, F.; Skagestad, R.; Øi, L.E. Cost Estimation of CO2 Absorption Plants for CO2 Mitigation—Method and Assumptions. Int. J. Greenh. Gas Control 2019, 88, 10–23. [Google Scholar] [CrossRef]
- CEIC Norway Foreign Exchange Rate: Norges Bank: Average: Euro. 2023. Available online: https://www.ceicdata.com/en/norway/foreign-exchange-rate/foreign-exchange-rate-norges-bank-average-euro (accessed on 2 March 2024).
- Ali, H.; Eldrup, N.H.; Normann, F.; Andersson, V.; Skagestad, R.; Mathisen, A.; Øi, L.E. Cost Estimation of Heat Recovery Networks for Utilization of Industrial Excess Heat for Carbon Dioxide Absorption. Int. J. Greenh. Gas Control 2018, 74, 219–228. [Google Scholar] [CrossRef]
- Husebye, J.; Brunsvold, A.L.; Roussanaly, S.; Zhang, X. Techno Economic Evaluation of Amine Based CO2 Capture: Impact of CO2 Concentration and Steam Supply. Energy Procedia 2012, 23, 381–390. [Google Scholar] [CrossRef]
- Macrotrends Inflation European Union Inflation Rate 1960–2023. Available online: https://www.macrotrends.net/ (accessed on 2 March 2024).
- Macrotrends GDP Norway 2023. Available online: https://www.macrotrends.net/global-metrics/countries/NOR/norway/gdp-gross-domestic-product (accessed on 5 March 2024).
- Macrotrends GDP Italy 2023. Available online: https://www.macrotrends.net/global-metrics/countries/ITA/italy/gdp-gross-domestic-product (accessed on 5 March 2024).
- GME Gestore Mercati Energetici. 2023. Available online: https://mercatoelettrico.org/It/Tools/Accessodati.aspx?ReturnUrl=%2fIt%2fStatistiche%2fME%2fDatiSintesi.Aspx (accessed on 15 March 2024).
- Neuwahl, F.; Cusano, G.; Benavides, J.G.; Holbrook, S.; Roudier, S. Best Available Techniques (BAT) Reference Document for Waste Inceneration—JRC Science for Policy Report; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Tobiesen, F.A.; Haugen, G.; Hartono, A. A Systematic Procedure for Process Energy Evaluation for Post Combustion CO2 Capture: Case Study of Two Novel Strong Bicarbonate-Forming Solvents. Appl. Energy 2018, 211, 161–173. [Google Scholar] [CrossRef]
- Deng, H.; Roussanaly, S.; Skaugen, G. Techno-Economic Analyses of CO2 Liquefaction: Impact of Product Pressure and Impurities. Int. J. Refrig. 2019, 103, 301–315. [Google Scholar] [CrossRef]
- European Parliament Directive (EU) 2023/959 of the European Parliament and of the Council of 10 May 2023, Which Modifies the Directive 2003/87/EC Establishing a System for Greenhouse Gas Emission Allowance Trading within the Union and Decision (EU) 2015/1814 Concerning the Establishment and Operation of a Market Stability Reserve for the Union Greenhouse Gas Emission Trading System, Is Also Known as EU ETS Directive. Off. J. Eur. Union 2023.
- Sandbag Carbon Price for the EU Emissions Trading System. 2024. Available online: https://sandbag.be/Carbon-Price-Viewer/ (accessed on 19 March 2024).
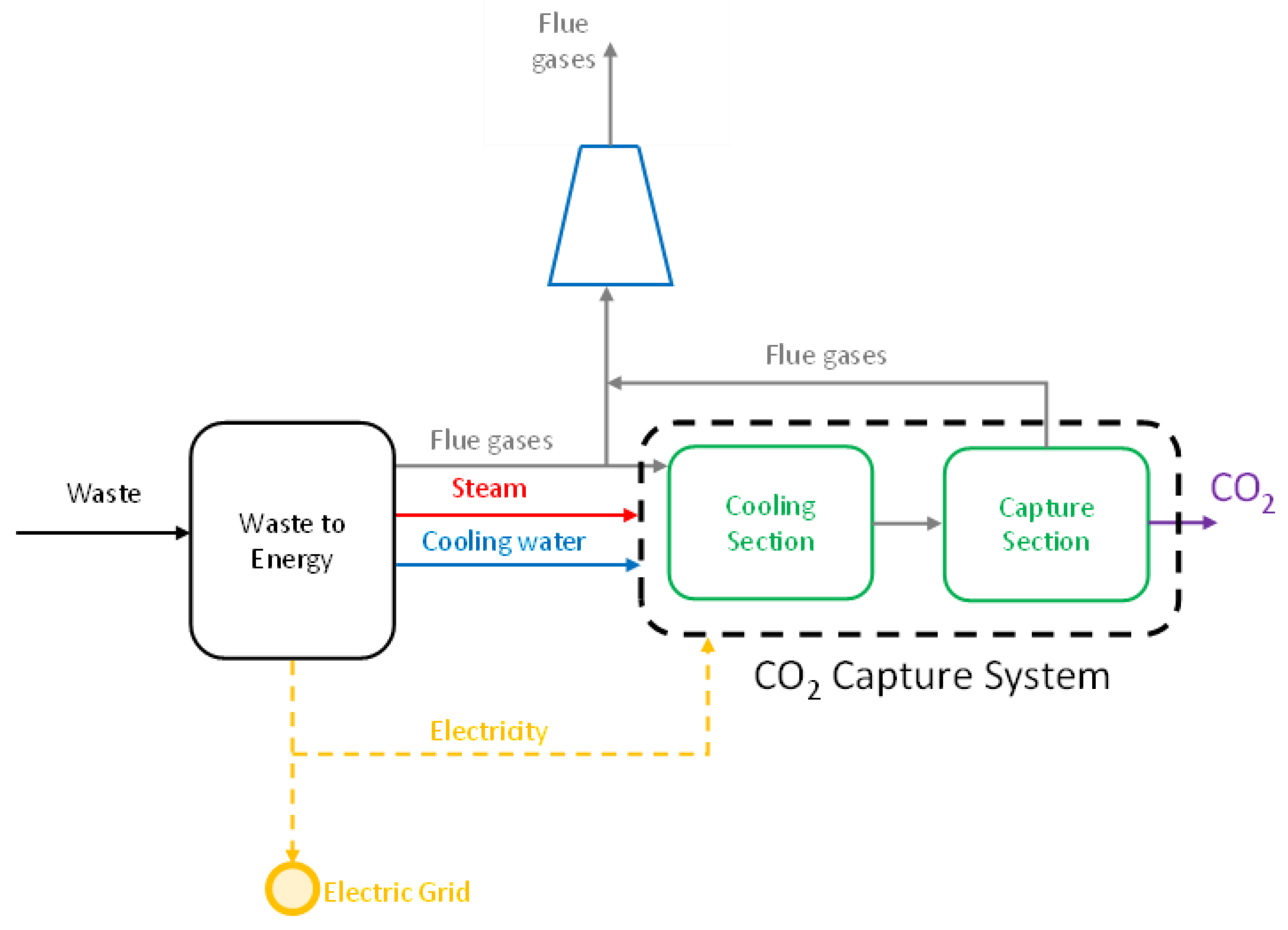


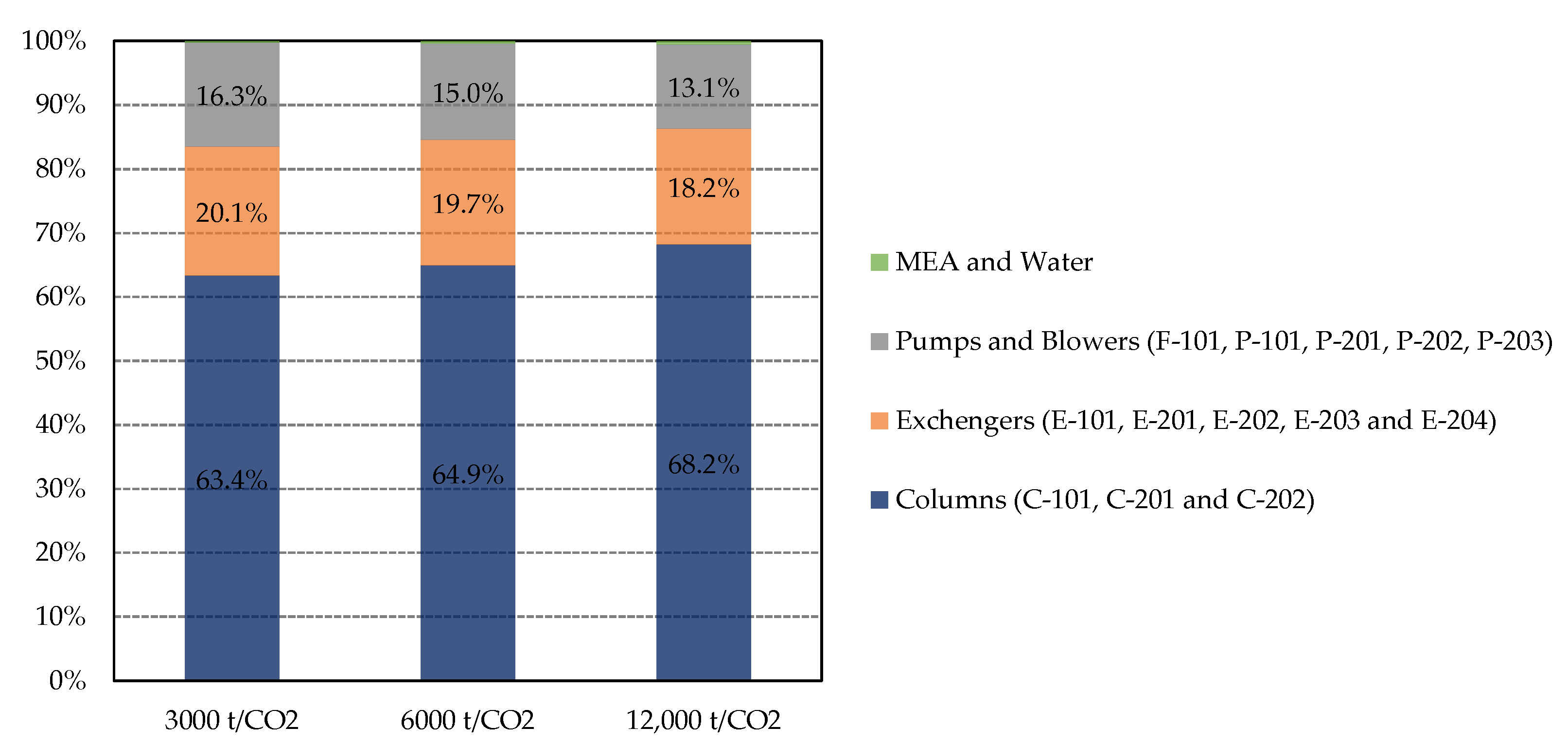
| Parameter | Unit | Value |
|---|---|---|
| Operative time | h/y | 8000 |
| CO2 emission | t/y | 119,747 |
| Flue gas temperature | °C | 131.5 |
| Flue gas pressure | bar | 1.006 |
| Biomass-derived | % | 58 |
| SRF (Solid Refuse Fuel) | t/h | 12.5 |
| Net electrical power | MW | 10.8 |
| Flow rate | Nm3/h | 86,027 |
| CO2 | vol.% | 8.9 |
| O2 | vol.% | 10.3 |
| H2O | vol.% | 14.0 |
| N2 | vol.% | 66.8 |
| Simulation Parameter | Unit | Value |
|---|---|---|
| Flue gas temperature from the process | °C | 131.5 |
| Absorber: inlet flue gases temperature | °C | 52 |
| Absorber: inlet flue gases pressure | bar | 1.13 |
| Lean solvent: temperature | °C | 50 |
| Lean solvent: pressure | bar | 1.1 |
| Lean Solvent: MEA Content | Mass-% | 30 |
| Lean Solvent: CO2 Content | Mass-% | 6.5 |
| Stripper: inlet rich temperature | °C | 98 |
| Stripper: pressure | bar | 1.8 |
| Reboiler: temperature | °C | 120 |
| Exchanger: ΔT | °C | 10 |
| Parameter | Unit | Value |
|---|---|---|
| Plant life | year | 20 |
| Interest rate | % | 7.5 |
| Currency conversion | NOK/€ | 9.6 |
| Operating hours per year | h | 8000 |
| CO2 removal efficiency | % | 85 |
| Maintenance | % of EIC | 4 |
| Electricity | €/MWh | 128 |
| Steam | €/t | 12.1 |
| Cooling water | €/m3 | 0.038 |
| Demineralized water | €/m3 | 2 |
| MEA | €/m3 | 1866 |
| Size | Auxiliary [MW] | Steam [MW] | Cooling Water [MW] |
|---|---|---|---|
| 3000 t CO2 captured | 0.03 | 0.08 | 0.01 |
| 6000 t CO2 captured | 0.05 | 0.15 | 0.02 |
| 12,000 t CO2 captured | 0.10 | 0.31 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertone, M.; Stabile, L.; Cortellessa, G.; Arpino, F.; Buonanno, G. Techno-Economic Assessment of Amine-Based Carbon Capture in Waste-to-Energy Incineration Plant Retrofit. Sustainability 2024, 16, 8468. https://doi.org/10.3390/su16198468
Bertone M, Stabile L, Cortellessa G, Arpino F, Buonanno G. Techno-Economic Assessment of Amine-Based Carbon Capture in Waste-to-Energy Incineration Plant Retrofit. Sustainability. 2024; 16(19):8468. https://doi.org/10.3390/su16198468
Chicago/Turabian StyleBertone, Michele, Luca Stabile, Gino Cortellessa, Fausto Arpino, and Giorgio Buonanno. 2024. "Techno-Economic Assessment of Amine-Based Carbon Capture in Waste-to-Energy Incineration Plant Retrofit" Sustainability 16, no. 19: 8468. https://doi.org/10.3390/su16198468







