Nutrient Effects on Motor Neurons and the Risk of Amyotrophic Lateral Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
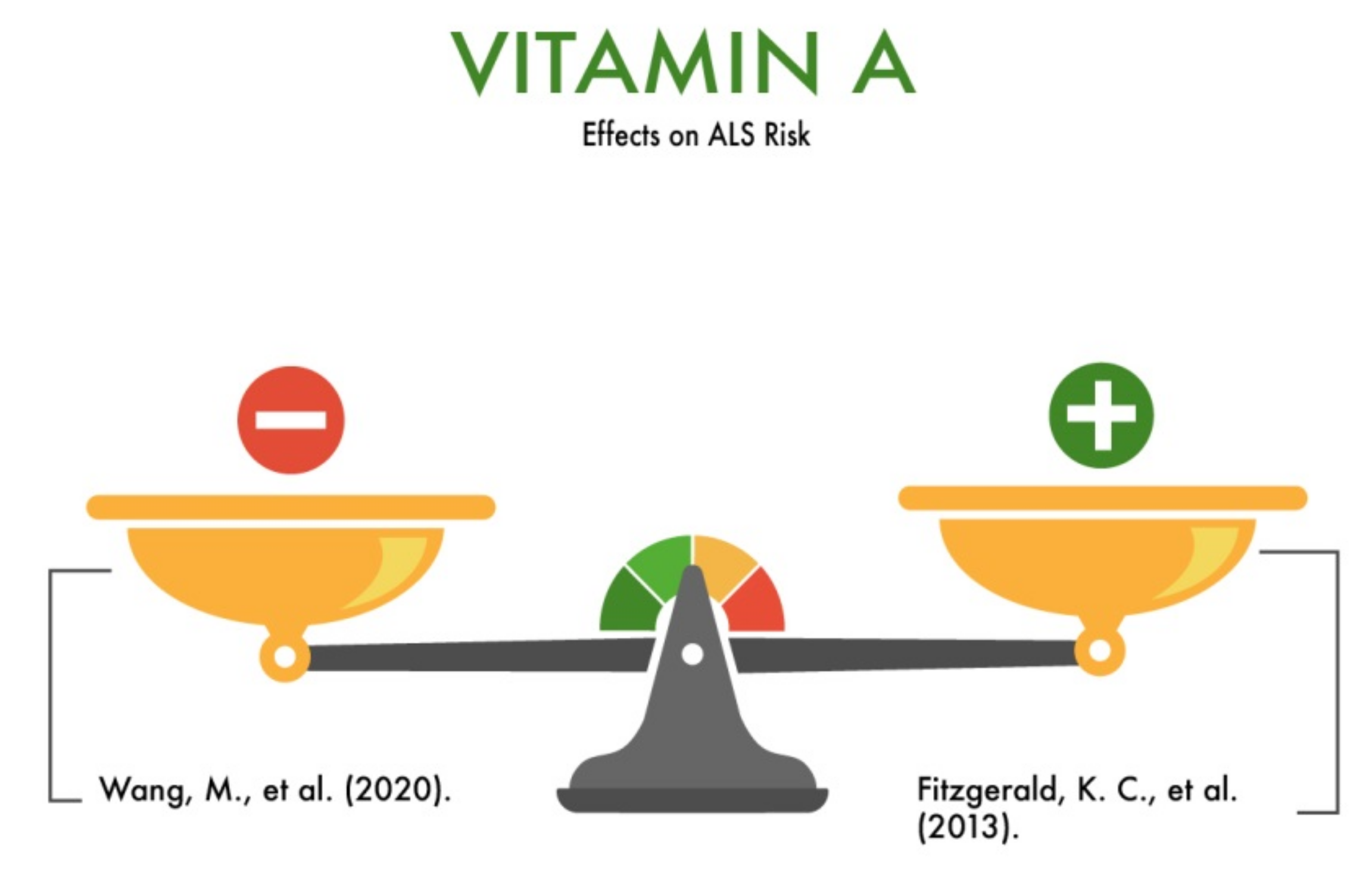
3.1. Vitamin A
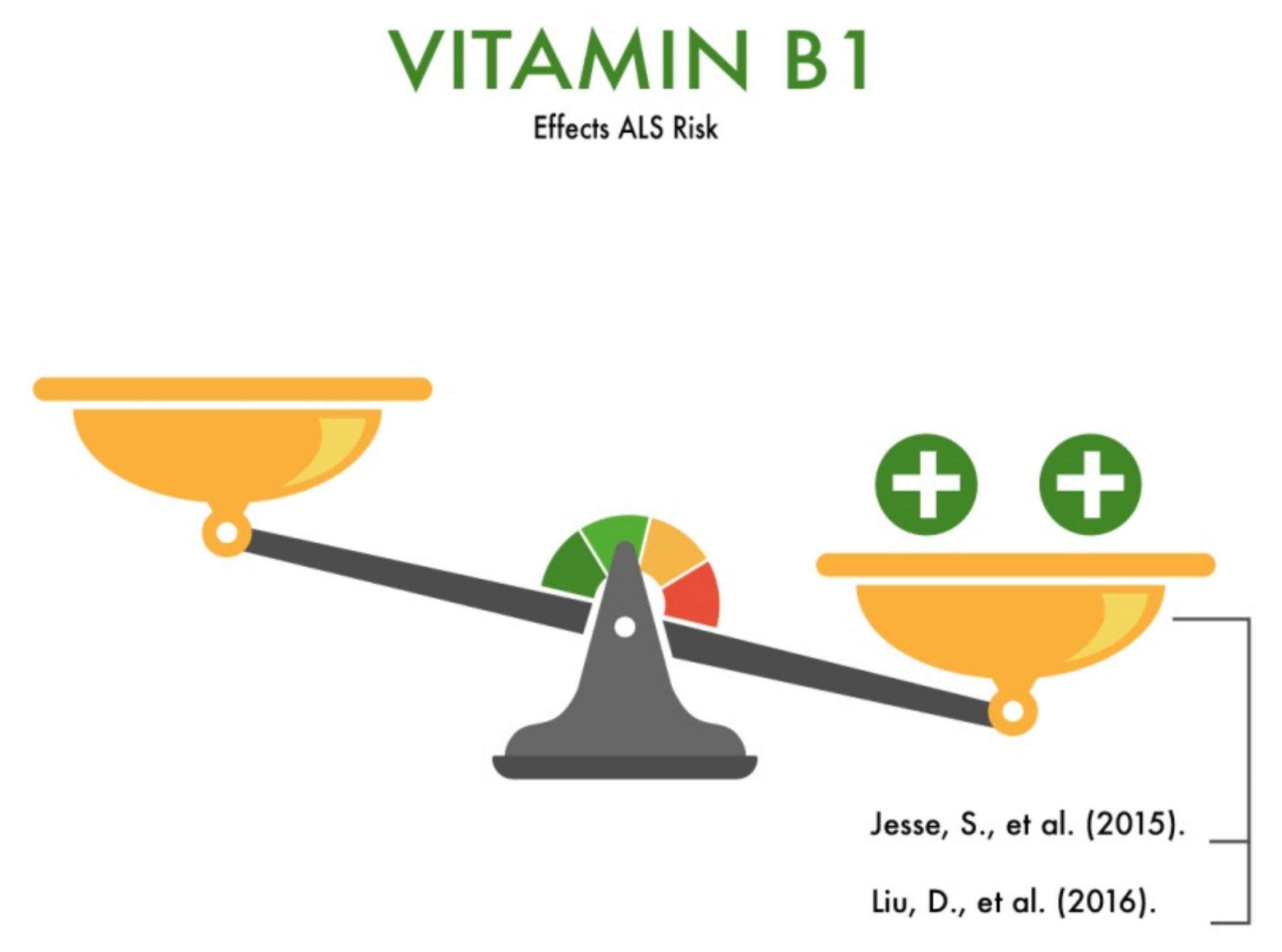
3.2. Vitamin B1
3.3. Vitamin B2
3.4. Vitamin B6
3.5. Vitamin B7
3.6. Vitamin B9
3.7. Vitamin B12
3.8. Vitamin C
3.9. Vitamin E
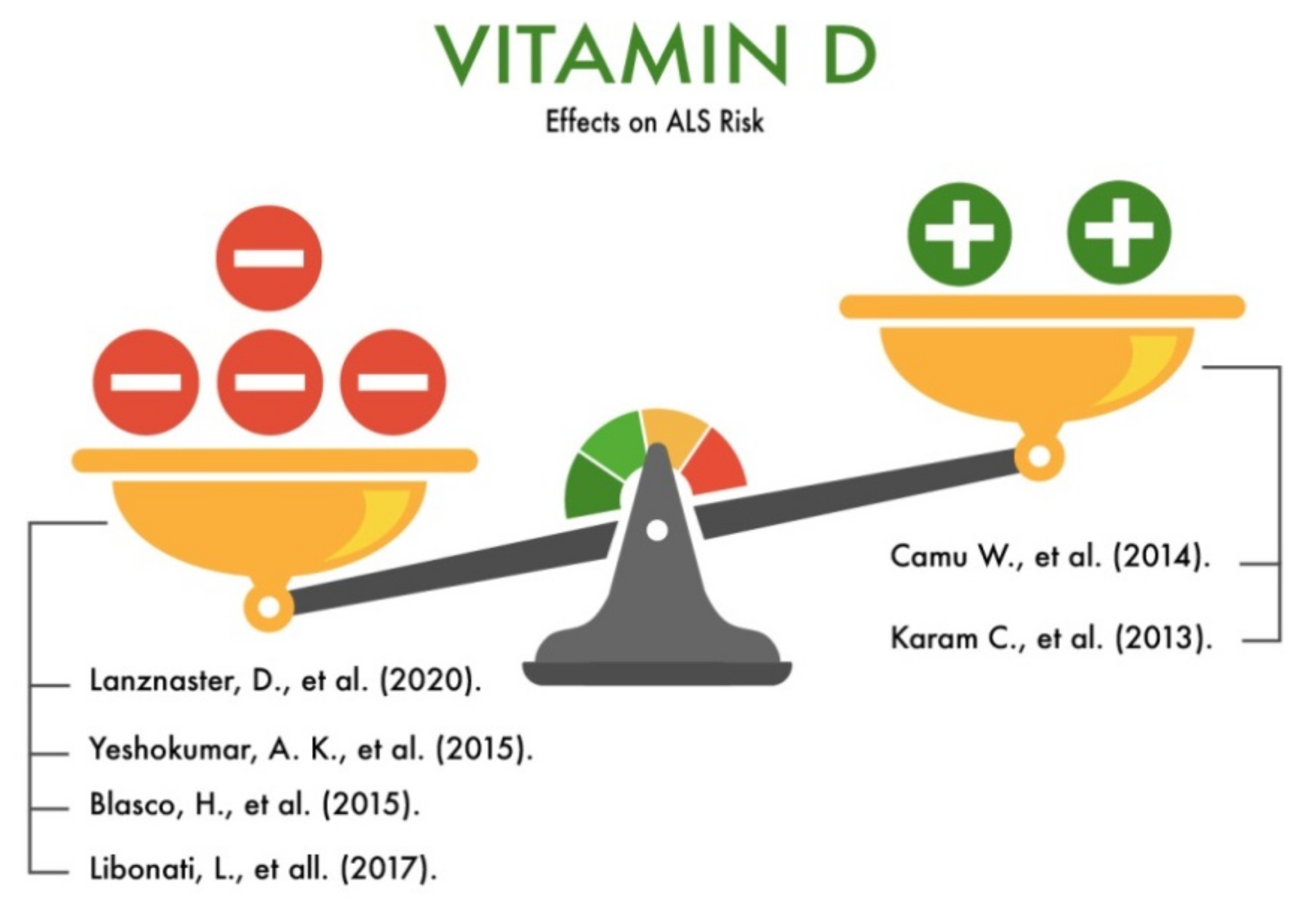
3.10. Vitamin D
3.11. Cholesterol
3.12. Polyunsaturated Fatty Acids
3.13. Urates and Purines
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riancho, J.; Delgado-Alvarado, M.; Andreu, M.D.; Paz-Fajardo, L.; Arozamena, S.; Gil-Bea, F.J.; de Munaín, A.L. Amyotrophic lateral sclerosis (ALS), cancer, autoimmunity and metabolic disorders: An unsolved tantalizing challenge. Br. J. Pharmacol. 2020, 178, 1269–1278. [Google Scholar] [CrossRef]
- Xu, L.; Liu, T.; Liu, L.; Yao, X.; Chen, L.; Fan, D.; Zhan, S.; Wang, S. Global variation in prevalence and incidence of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. 2019, 267, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, D.; Bresolin, N.; Comi, G.P.; Corti, S. Extracellular vesicles and amyotrophic lateral sclerosis: From misfolded protein vehicles to promising clinical biomarkers. Cell. Mol. Life Sci. 2021, 78, 561–572. [Google Scholar] [CrossRef]
- Nowicka, N.; Juranek, J.; Juranek, J.K.; Wojtkiewicz, J. Risk Factors and Emerging Therapies in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2019, 20, 2616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medical News Today. Available online: https://www.medicalnewstoday.com/articles/160774 (accessed on 16 July 2021).
- MedlinePlus. Available online: https://medlineplus.gov/definitions/vitaminsdefinitions.html (accessed on 16 July 2021).
- Redfern, C.P. Vitamin A and its natural derivatives. Methods Enzymol. 2020, 637, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.R.D. The neurotoxic effects of vitamin A and retinoids. An. Da Acad. Bras. De Ciências 2015, 87 (Suppl. 2), 1361–1373. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, K.; O’Reilly, E.J.; Fondell, E.; Falcone, G.J.; McCullough, M.L.; Park, Y.; Kolonel, L.N.; Ascherio, A.; ScM, K.C.F.; ScD, M.L.M. Intakes of vitamin C and carotenoids and risk of amyotrophic lateral sclerosis: Pooled results from 5 cohort studies. Ann. Neurol. 2013, 73, 236–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Liu, Z.; Sun, W.; Yuan, Y.; Jiao, B.; Zhang, X.; Shen, L.; Jiang, H.; Xia, K.; Tang, B.; et al. Association Between Vitamins and Amyotrophic Lateral Sclerosis: A Center-Based Survey in Mainland China. Front. Neurol. 2020, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- Nabokina, S.M.; Inoue, K.; Subramanian, V.S.; Valle, J.E.; Yuasa, H.; Said, H.M. Molecular Identification and Functional Characterization of the Human Colonic Thiamine Pyrophosphate Transporter. J. Biol. Chem. 2014, 289, 4405–4416. [Google Scholar] [CrossRef] [Green Version]
- Electron Medicine. Available online: http://www.elm.su/articles/vit/role_of_vit.html (accessed on 18 July 2021).
- Jannusch, K.; Jockwitz, C.; Bidmon, H.-J.; Moebus, S.; Amunts, K.; Caspers, S. A Complex Interplay of Vitamin B1 and B6 Metabolism with Cognition, Brain Structure, and Functional Connectivity in Older Adults. Front. Neurosci. 2017, 11, 596. [Google Scholar] [CrossRef]
- Jesse, S.; Thal, D.R.; Ludolph, A.C. Thiamine deficiency in amyotrophic lateral sclerosis: Figure 1. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1166–1168. [Google Scholar] [CrossRef]
- Liu, D.; Ke, Z.-J.; Luo, J. Thiamine Deficiency and Neurodegeneration: The Interplay Among Oxidative Stress, Endoplasmic Reticulum Stress, and Autophagy. Mol. Neurobiol. 2016, 54, 5440–5448. [Google Scholar] [CrossRef] [PubMed]
- García-Angulo, V.A. Overlapping riboflavin supply pathways in bacteria. Crit. Rev. Microbiol. 2017, 43, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Tomar, S.K.; De, S. Lactic acid bacteria as a cell factory for riboflavin production. Microb. Biotechnol. 2015, 9, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.T.; Zempleni, J. Riboflavin. Adv. Nutr. 2016, 7, 973–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayengbam, S.; Chleilat, F.; Reimer, R.A. Dietary Vitamin B6 Deficiency Impairs Gut Microbiota and Host and Microbial Metabolites in Rats. Biomedicines 2020, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of Dietary and Microbial Vitamin B Family in the Regulation of Host Immunity. Front. Nutr. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serriari, N.-E.; Eoche, M.; LaMotte, L.; Lion, J.; Fumery, M.; Marcelo, P.; Chatelain, D.; Barre, A.; Nguyen-Khac, E.; Lantz, O.; et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin. Exp. Immunol. 2014, 176, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.; Stahl, S.; Hellmann, H. Vitamin B6 and Its Role in Cell Metabolism and Physiology. Cells 2018, 7, 84. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, J.; Ellis, A. Nutrition and Dietary Supplements in Motor Neuron Disease. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 573–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoccolella, S.; Simone, I.L.; Lamberti, P.; Samarelli, V.; Tortelli, R.; Serlenga, L.; Logroscino, G. Elevated plasma homocysteine levels in patients with amyotrophic lateral sclerosis. Neurology 2008, 70, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Trieu, V.N.; Uckun, F.M. Genistein Is Neuroprotective in Murine Models of Familial Amyotrophic Lateral Sclerosis and Stroke. Biochem. Biophys. Res. Commun. 1999, 258, 685–688. [Google Scholar] [CrossRef]
- Zempleni, J.; Wijeratne, S.S.; Hassan, Y.I. Biotin. BioFactors 2009, 35, 36–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, S.; Agrawal, A.; Said, H.M. Biotin deficiency enhances the inflammatory response of human dendritic cells. Am. J. Physiol. Physiol. 2016, 311, C386–C391. [Google Scholar] [CrossRef] [PubMed]
- Tong, L. Structure and function of biotin-dependent carboxylases. Cell. Mol. Life Sci. 2012, 70, 863–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, M.; Nagashima, T.; Nakamura, E.; Kato, R.; Ohshita, M.; Hayashi, M.; Takeno, S. In Vivo Roles of Fatty Acid Biosynthesis Enzymes in Biosynthesis of Biotin and α-Lipoic Acid in Corynebacterium glutamicum. Appl. Environ. Microbiol. 2017, 83, e01322-17. [Google Scholar] [CrossRef] [Green Version]
- Rao, T.S.S.; Christopher, R.; Andrade, C. Biotin supplements and laboratory test results in neuropsychiatric practice and research. Indian J. Psychiatry 2017, 59, 405–406. [Google Scholar] [CrossRef]
- Mangelsdorf, I.; Walach, H.; Mutter, J. Healing of Amyotrophic Lateral Sclerosis: A Case Report. Complement. Med. Res. 2017, 24, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Juntas-Morales, R.; Pageot, N.; Bendarraz, A.; Alphandéry, S.; Sedel, F.; Seigle, S.; Camu, W. High-dose pharmaceutical grade biotin (MD1003) in amyotrophic lateral sclerosis: A pilot study. EClinicalMedicine 2020, 19, 100254. [Google Scholar] [CrossRef] [Green Version]
- Rad, A.H.; Khosroushahi, A.Y.; Khalili, M.; Jafarzadeh, S. Folate bio-fortification of yoghurt and fermented milk: A review. Dairy Sci. Technol. 2016, 96, 427–441. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate Production by Probiotic Bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, N.; Patel, A. Recent advances in biosynthesis of vitamin and enzyme from food grade bacteria. Int. J. Food Ferment. Technol. 2014, 4, 79–85. [Google Scholar] [CrossRef]
- Ojeda, L.; Nogales, F.; Murillo, L.; Carreras, O.; Murillo, M.L.O.; Bueno, F.N.; Taravillo, M.L.M.; Sánchez, O.C. The role of folic acid and selenium against oxidative damage from ethanol in early life programming: A review. Biochem. Cell Biol. 2018, 96, 178–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boumenna, T.; Scott, T.M.; Lee, J.-S.; Palacios, N.; Tucker, K.L. Folate, vitamin B-12, and cognitive function in the Boston Puerto Rican Health Study. Am. J. Clin. Nutr. 2020, 113, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ebara, S. Nutritional role of folate. Congenit. Anomalies 2017, 57, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Zoccolella, S.; Bendotti, C.; Beghi, E.; Logroscino, G. Homocysteine levels and amyotrophic lateral sclerosis: A possible link. Amyotroph. Lateral Scler. 2010, 11, 140–147. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Li, L.; Wang, Q.; Le, W. Folic acid protects motor neurons against the increased homocysteine, inflammation and apoptosis in SOD1G93A transgenic mice. Neuropharmacology 2008, 54, 1112–1119. [Google Scholar] [CrossRef]
- Fang, H.; Kang, J.; Zhang, D. Microbial production of vitamin B12: A review and future perspectives. Microb. Cell Factories 2017, 16, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood 2017, 129, 2603–2611. [Google Scholar] [CrossRef] [Green Version]
- Izumi, Y.; Kaji, R. Clinical trials of ultra-high-dose methylcobalamin in ALS. Brain Nerve 2007, 59, 1141–1147. [Google Scholar]
- Rison, R.A.; Beydoun, S.R. Amyotrophic lateral sclerosis-motor neuron disease, monoclonal gammopathy, hyperparathyroidism, and B12 deficiency: Case report and review of the literature. J. Med. Case Rep. 2010, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Chen, S.; Li, L.; Wang, Q.; Le, W. Decreased level of 5-methyltetrahydrofolate: A potential biomarker for pre-symptomatic amyotrophic lateral sclerosis. J. Neurol. Sci. 2010, 293, 102–105. [Google Scholar] [CrossRef]
- Ikeda, K.; Iwasaki, Y.; Kaji, R. Neuroprotective effect of ultra-high dose methylcobalamin in wobbler mouse model of amyotrophic lateral sclerosis. J. Neurol. Sci. 2015, 354, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Kaji, R.; Imai, T.; Iwasaki, Y.; Okamoto, K.; Nakagawa, M.; Ohashi, Y.; Takase, T.; Hanada, T.; Shimizu, H.; Tashiro, K.; et al. Ultra-high-dose methylcobalamin in amyotrophic lateral sclerosis: A long-term phase II/III randomised controlled study. J. Neurol. Neurosurg. Psychiatry 2019, 90, 451–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocot, J.; Luchowska-Kocot, D.; Kiełczykowska, M.; Musik, I.; Kurzepa, J. Does Vitamin C Influence Neurodegenerative Diseases and Psychiatric Disorders? Nutrients 2017, 9, 659. [Google Scholar] [CrossRef] [Green Version]
- Blasco, H.; Corcia, P.; Moreau, C.; Veau, S.; Fournier, C.; Vourc’H, P.; Emond, P.; Gordon, P.; Pradat, P.-F.; Praline, J.; et al. 1H-NMR-Based Metabolomic Profiling of CSF in Early Amyotrophic Lateral Sclerosis. PLoS ONE 2010, 5, e13223. [Google Scholar] [CrossRef]
- Nieves, J.; Gennings, C.; Factor-Litvak, P.; Hupf, J.; Singleton, J.; Sharf, V.; Oskarsson, B.; Filho, J.A.M.F.; Sorenson, E.J.; D’Amico, E.; et al. Association Between Dietary Intake and Function in Amyotrophic Lateral Sclerosis. JAMA Neurol. 2016, 73, 1425–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pupillo, E.; Bianchi, E.; Chiò, A.; Casale, F.; Zecca, C.; Tortelli, R.; Beghi, E. Amyotrophic lateral sclerosis and food intake. Amyotroph. Lateral Scler. Front. Degener. 2017, 19, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Spasojević, I.; Stević, Z.; Nikolić-Kokić, A.; Jones, D.R.; Blagojević, D.; Spasić, M. Different roles of radical scavengers-ascorbate and urate in the cerebrospinal fluid of amyotrophic lateral sclerosis patients. Redox Rep. 2010, 15, 81–86. [Google Scholar] [CrossRef]
- Netzahualcoyotzi, C.; Tapia, R. Degeneration of spinal motor neurons by chronic AMPA-induced excitotoxicity in vivo and protection by energy substrates. Acta Neuropathol. Commun. 2015, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagano, S.; Fujii, Y.; Yamamoto, T.; Taniyama, M.; Fukada, K.; Yanagihara, T.; Sakoda, S. The efficacy of trientine or ascorbate alone compared to that of the combined treatment with these two agents in familial amyotrophic lateral sclerosis model mice. Exp. Neurol. 2003, 179, 176–180. [Google Scholar] [CrossRef]
- Okamoto, K.; Kihira, T.; Kobashi, G.; Washio, M.; Sasaki, S.; Yokoyama, T.; Miyake, Y.; Sakamoto, N.; Inaba, Y.; Nagai, M. Fruit and Vegetable Intake and Risk of Amyotrophic Lateral Sclerosis in Japan. Neuroepidemiology 2009, 32, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienol: The Natural Vitamin E to Defend the Nervous System? Ann. N. Y. Acad. Sci. 2004, 1031, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Head, B.; La Du, J.; Tanguay, R.L.; Kioussi, C.; Traber, M.G. Vitamin E is necessary for zebrafish nervous system development. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Fang, F.; Ingre, C.; Roos, P.M.; Kamel, F.; Piehl, F. Risk factors for amyotrophic lateral sclerosis. Clin. Epidemiol. 2015, 7, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascherio, A.; Weisskopf, M.G.; O’Reilly, E.J.; Jacobs, E.J.; McCullough, M.L.; Calle, E.E.; Cudkowicz, M.; Thun, M.J. Vitamin E intake and risk of amyotrophic lateral sclerosis. Ann. Neurol. 2004, 57, 104–110. [Google Scholar] [CrossRef]
- Wang, H.; O’Reilly, J.; Weisskopf, M.G.; Logroscino, G.; McCullough, M.L.; Schatzkin, A.; Kolonel, L.N.; Ascherio, A. Vitamin E Intake and Risk of Amyotrophic Lateral Sclerosis: A Pooled Analysis of Data From 5 Prospective Cohort Studies. Am. J. Epidemiol. 2011, 173, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Veldink, J.H.; Kalmijn, S.; Groeneveld, G.-J.; Wunderink, W.; Koster, A.; de Vries, J.H.M.; van der Luyt, J.; Wokke, J.H.J.; Berg, L.H.V.D. Intake of polyunsaturated fatty acids and vitamin E reduces the risk of developing amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2006, 78, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Wechsler, I. Treatment of amyotrophic lateral sclerosis with Vitamin E. Am. J. Med. Sci. 1940, 200, 765–778. [Google Scholar] [CrossRef]
- Wechsler, I. Recovery in ALS, treated with tocopherols: Preliminary Report. JAMA 1940, 114, 948–950. [Google Scholar]
- Richard, B. ALS Untangled 55: Vitamin E (α-tocopherol). Amyotroph. Lateral Scler. Front. Degener. 2020, 22, 154–160. [Google Scholar] [CrossRef]
- Wrzosek, M.; Łukaszkiewicz, J.; Wrzosek, M.; Jakubczyk, A.; Matsumoto, H.; Piątkiewicz, P.; Radziwoń-Zaleska, M.; Wojnar, M.; Nowicka, G. Vitamin D and the central nervous system. Pharmacol. Rep. 2013, 65, 271–278. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Roman, S.; Mowry, E.M. Vitamin D and the Central Nervous System. Development, Protection, and Disease. Extraskeletal Effects of Vitamin D.; Humana Press, Cham: Totowa, NJ, USA, 2018; pp. 227–247. [Google Scholar] [CrossRef]
- Bivona, G.; Gambino, C.M.; Iacolino, G.; Ciaccio, M. Vitamin D and the nervous system. Neurol. Res. 2019, 41, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Camu, W.; Tremblier, B.; Plassot, C.; Alphandery, S.; Salsac, C.; Pageot, N.; Juntas-Morales, R.; Scamps, F.; Daures, J.-P.; Raoul, C. Vitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosis. Neurobiol. Aging 2014, 35, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Karam, C.; Barrett, M.; Imperato, T.; MacGowan, D.J.; Scelsa, S. Vitamin D deficiency and its supplementation in patients with amyotrophic lateral sclerosis. J. Clin. Neurosci. 2013, 20, 1550–1553. [Google Scholar] [CrossRef]
- Lanznaster, D.; Bejan-Angoulvant, T.; Gandía, J.; Blasco, H.; Corcia, P. Is There a Role for Vitamin D in Amyotrophic Lateral Sclerosis? A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Yeshokumar, A.K.; Saylor, D.; Kornberg, M.D.; Mowry, E.M. Evidence for the Importance of Vitamin D Status in Neurologic Conditions. Curr. Treat. Options Neurol. 2015, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Blasco, H.; Hounoum, B.M.; Dufour-Rainfray, D.; Patin, F.; Maillot, F.; Beltran, S.; Gordon, P.H.; Andres, C.R.; Corcia, P. Vitamin D is Not a Protective Factor in ALS. CNS Neurosci. Ther. 2015, 21, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Libonati, L.; Onesti, E.; Gori, M.C.; Ceccanti, M.; Cambieri, C.; Fabbri, A.; Frasca, V.; Inghilleri, M. Vitamin D in amyotrophic lateral sclerosis. Funct. Neurol. 2017, 32, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.K.; Darwish, H.M.; Deluca, H.F. Molecular Biology of Vitamin D Action. Adv. Res. Appl. Steroids 1994, 49, 281–326. [Google Scholar] [CrossRef]
- Narwal, V.; Deswal, R.; Batra, B.; Kalra, V.; Hooda, R.; Sharma, M.; Rana, J. Cholesterol biosensors: A review. Steroids 2018, 143, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Pape, J.; Grose, J. The effects of diet and sex in amyotrophic lateral sclerosis. Rev. Neurol. 2020, 176, 301–315. [Google Scholar] [CrossRef]
- Huisman, M.H.B.; Seelen, M.; Van Doormaal, P.T.C.; De Jong, S.W.; De Vries, J.H.M.; Van Der Kooi, A.J.; De Visser, M.; Schelhaas, H.J.; Berg, L.H.V.D.; Veldink, J.H. Effect of Presymptomatic Body Mass Index and Consumption of Fat and Alcohol on Amyotrophic Lateral Sclerosis. JAMA Neurol. 2015, 72, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.M.; Matkin, C.; Longstreth, W.T.; McGuire, V. Population-Based Case-Control Study of Amyotrophic Lateral Sclerosis in Western Washington State. II. Diet. Am. J. Epidemiol. 2000, 151, 164–173. [Google Scholar] [CrossRef]
- Okamoto, K.; Kihira, T.; Kondo, T.; Kobashi, G.; Washio, M.; Sasaki, S.; Yokoyama, T.; Miyake, Y.; Sakamoto, N.; Inaba, Y.; et al. Nutritional status and risk of amyotrophic lateral sclerosis in Japan. Amyotroph. Lateral Scler. 2007, 8, 300–304. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, J.; Wang, H.; Weisskopf, M.G.; Fitzgerald, K.; Falcone, G.; McCullough, M.L.; Thun, M.; Park, Y.; Kolonel, L.N.; Ascherio, A. Premorbid body mass index and risk of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourre, J.-M. Dietary omega-3 fatty acids for women. Biomed. Pharmacother. 2007, 61, 105–112. [Google Scholar] [CrossRef]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef] [PubMed]
- Bruce, K.D.; Zsombok, A.; Eckel, R.H.; Bruce, K.D.; Zsombok, A.; Eckel, R.H. Lipid Processing in the Brain: A Key Regulator of Systemic Metabolism. Front. Endocrinol. 2017, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Joffre, C. Polyunsaturated fatty acid metabolism in the brain and brain cells, feed your mind-How does nutrition modulate brain function throughout life? In Corinne Joffre. In Feed Your Mind: How Does Nutrition Modulate Brain Function throughout Life? Bosch-Bouju, C., Layé, S., Pallet, V., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Alessandri, J.-M.; Guesnet, P.; Vancassel, S.; Astorg, P.; Denis, I.; Langelier, B.; Aïd, S.; Poumès-Ballihaut, C.; Champeil-Potokar, G.; Lavialle, M. Polyunsaturated fatty acids in the central nervous system: Evolution of concepts and nutritional implications throughout life. Reprod. Nutr. Dev. 2004, 44, 509–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-J.; Nakatomi, R.; Akagi, T.; Hashikawa, T.; Takahashi, R. Unsaturated Fatty Acids Induce Cytotoxic Aggregate Formation of Amyotrophic Lateral Sclerosis-linked Superoxide Dismutase 1 Mutants. J. Biol. Chem. 2005, 280, 21515–21521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, K.; O’Reilly, J.; Falcone, G.J.; McCullough, M.L.; Park, Y.; Kolonel, L.N.; Ascherio, A. Dietary ω-3 Polyunsaturated Fatty Acid Intake and Risk for Amyotrophic Lateral Sclerosis. JAMA Neurol. 2014, 71, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- De Aguilar, J.-L.G. Lipid Biomarkers for Amyotrophic Lateral Sclerosis. Front. Neurol. 2019, 10, 284. [Google Scholar] [CrossRef]
- Henriques, A.; Blasco, H.; Fleury, M.-C.; Corcia, P.; Echaniz-Laguna, A.; Robelin, L.; Rudolf, G.; Lequeu, T.; Bergaentzle, M.; Gachet, C.; et al. Blood Cell Palmitoleate-Palmitate Ratio Is an Independent Prognostic Factor for Amyotrophic Lateral Sclerosis. PLoS ONE 2015, 10, e0131512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Reilly, É.J.; Bjornevik, K.; Furtado, J.D.; Kolonel, L.N.; Le Marchand, L.; McCullough, M.L.; McCullough, M.L.; Stevens, V.L.; Shadyab, A.H.; Snetselaar, L.; et al. Prediagnostic plasma polyunsaturated fatty acids and the risk of amyotrophic lateral sclerosis. Neurology 2019, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.; Bradley, W.; Chen, C.; Pioro, E.; Stommel, E.; Andrew, A. Amyotrophic Lateral Sclerosis Risk, Family Income, and Fish Consumption Estimates of Mercury and Omega-3 PUFAs in the United States. Int. J. Environ. Res. Public Health 2021, 18, 4528. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Q.; Ke, Y.; Hao, J.; Lu, L.; Lu, N.; Chen, X. Serum uric acid levels in patients with amyotrophic lateral sclerosis: A meta-analysis. Sci. Rep. 2018, 8, 1–6. [Google Scholar] [CrossRef]
- O’Reilly, J.; Bjornevik, K.; Schwarzschild, M.A.; McCullough, M.L.; Kolonel, L.N.; Le Marchand, L.; Manson, J.E.; Ascherio, A. Pre-diagnostic plasma urate and the risk of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2017, 19, 194–200. [Google Scholar] [CrossRef]
- Paganoni, S.; Nicholson, K.; Chan, J.; Shui, A.; Schoenfeld, D.; Sherman, A.; Berry, J.; Cudkowicz, M.; Atassi, N. Urate levels predict survival in amyotrophic lateral sclerosis: Analysis of the expanded Pooled Resource Open-Access ALS clinical trials database. Muscle Nerve 2017, 57, 430–434. [Google Scholar] [CrossRef]
- Food Composition Tables for Japan: International Network of Food Data Systems (INFOODS). Food and Agriculture Organization of the Unation Nations. Available online: https://www.fao.org/infoods/infoods/tables-and-databases/japan/en/ (accessed on 3 January 2017).
- Kephart, W.C.; Pledge, C.D.; Roberson, P.A.; Mumford, P.; Romero, M.A.; Mobley, C.B.; Martin, J.S.; Young, K.C.; Lowery, R.P.; Wilson, J.M.; et al. The Three-Month Effects of a Ketogenic Diet on Body Composition, Blood Parameters, and Performance Metrics in CrossFit Trainees: A Pilot Study. Sports 2018, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McSwiney, F.T.; Wardrop, B.; Hyde, P.N.; Lafountain, R.A.; Volek, J.S.; Doyle, L. Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism 2018, 81, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 309–319. [Google Scholar] [CrossRef]
- Bueno, N.; De Melo, I.S.V.; De Oliveira, S.L.; Ataide, T.D.R. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parry, H.A.; Kephart, W.C.; Mumford, P.; Romero, M.A.; Mobley, C.B.; Zhang, Y.; Roberts, M.D.; Kavazis, A.N. Ketogenic diet increases mitochondria volume in the liver and skeletal muscle without altering oxidative stress markers in rats. Heliyon 2018, 4, e00975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, W.; Hu, F. Cellular and physiological functions of C9ORF72 and implications for ALS/FTD. J. Neurochem. 2021, 157, 334–350. [Google Scholar] [CrossRef]
- Neznanov, N.G. A paradigm shift to treat psychoneurological disorders. Pers. Psychiatry Neurol. 2021, 1, 1–2. [Google Scholar]











| Nutrient | Function in the CNS | Role in the Development of ALS | Authors |
|---|---|---|---|
| Vitamin A (retinol) | Regulation of oxidation-reduction processes; regulation of protein synthesis; participation in redox processes (neutralization of free oxygen radicals); participation in the development of cells, providing sensitivity to hormones and growth stimuli; regulation of normal growth and differentiation of cells of the embryo and young organism; regulation of division and differentiation of rapidly dividing tissues, including cells of the immune system. | Is likely to reduce the risk of ALS | [7,8,9,10] |
| Vitamin B1 (thiamine) | Participation in oxidative metabolism; neuroprotection (reduction of neuroinflammation and neurodegeneration); participation in carbohydrate metabolism and associated energy, fat, protein, and water-salt metabolism; regulation of the activity of the central nervous system; optimization of the impact on cognitive activity; participation in the neutralization of xenobiotics (protection from the toxic effects of alcohol and nicotine); slowing down the aging process; imitation of the action of acetylcholine on neurons; participation in the exchange of zinc and manganese. | Is likely to reduce the risk of ALS | [11,12,13,14,15] |
| Vitamin В2 (riboflavin) | Participation in the oxidation of fatty acids, succinic acid, and amino acids; participation in the regulation of tissue respiration and cell growth processes; participation in redox reactions as an antioxidant; participation in energy exchange; participation in the processes of assimilation of iron; protection of the retina from the harmful effects of ultraviolet radiation; neuroprotection; participation in tissue regeneration; participation in the formation of red blood cells and antibodies; influence on pain and tactile sensitivity; excitability of the CNS and PNS. | Is likely to reduce the risk of ALS | [10,12,16,17,18] |
| Vitamin В6 (pyridoxine) | Participation in the synthesis of serotonin; participation in the circadian cycle regulation; vitamin B12 cofactor; coenzyme of a large group of pyridoxal enzymes (transfer of amino groups, decarboxylation of amino acids, and hydroxylation); decrease in the excitability of the CNS. | Is likely to reduce the risk of ALS | [12,13,19,20,21,22,23,24,25] |
| Vitamin В7 (biotin) | Energy function and regulation of energy metabolism (adenosine triphosphate (ATP) production); participation in carboxylation reactions; participation in the synthesis of purines; participation in the metabolism of fatty acids; participation in the neutralization of xenobiotics (detoxification); participation in the circadian cycle regulation; influence on cognitive functions and attention; participation in regeneration processes; reduction of neuroinflammation processes; coenzyme participating in the reaction of the CO2 transfer to organic compounds; interaction with insulin (stabilization of blood glucose levels); participation in the production of glucokinase; slowing down the aging process; participation in the neutralization of xenobiotics (detoxification). | Is likely to reduce the risk of ALS | [12,26,27,28,29,30,31,32] |
| Vitamin В9 (folic acid) | Cyanocobalamin cofactor; participation in the formation of erythrocytes and leukocytes; participation in the processes of iron metabolism; participation in the synthesis of nucleotides and DNA; participation in the resynthesis of methionine from homocysteine (together with vitamin B12); participation in the synthesis of choline, creatine, and adrenaline; influence on lipid metabolism and blood cholesterol level; regulation of cell division and participation in fetal development; participation in neuroimmune reactions and neuroprotection; participation in the synthesis of purines and pyrimidines necessary for the formation of the genetic code (DNA, RNA-replication processes); participation in the exchange of glycine and serine, methionine, and histidine; participation in the biosynthesis of dopamine, norepinephrine, and serotonin; slowing down the aging process and protection against oncopathology. | Is likely to reduce the risk of ALS | [10,12,23,24,33,34,35,36,37,38,39,40] |
| Vitamin В12 (cyanocobalamin) | Participation in the regulation of the folate cycle (regulation of homocysteine levels); cofactor of vitamin B9 (folic acid); participation in the regulation of amino acids and fatty acids (pro-pionic acid); participation in the regulation of growth and differentiation of neurons (active influence on cell division); participation in the regulation of the formation of the myelin sheath; influence on cognitive and emotional-volitional functions; participation in the regulation of the balance function; participation in the conversion of folic acid derivatives necessary for the synthesis of DNA and RNA nucleotides; participation in the regeneration of methionine; participation in the metabolism of polyunsaturated fatty acids with an odd number of carbon atoms; influence on the exchange of amino acids with a branched side chain (methionine, isoleucine, trionine, and valine); participation in the synthesis of adrenaline, acetylcholine; influence on the level of cholesterol in the blood; regulation of CNS excitability; participation in the formation of erythrocytes; slowing down the aging process and protection against oncopathology. | Reduces the risk of ALS | [12,23,36,37,41,42,43,44,45,46,47] |
| Vitamin C (ascorbic acid) | Participation in redox processes (protection from oxygen free radicals); participation in the synthesis of proteins (amidation of peptides); participation in the synthesis of myelin; synaptic potentiation; neuroprotection (protection from the action of excitatory neurotransmitters such as glutamate); participation in regeneration processes; participation in energy processes; participation in the absorption of calcium and iron; participation in the regulation of the neuroimmune response (influence on resistance to viruses, bacteria, and parasites); slowing down the aging process and protection against oncopathology; enhancing the effect of adrenaline (anti-stress effect); participation in the regulation of emotional reactions, cognitive functions; participation in the exchange of cholesterol; participation in the synthesis of collagen; impact on mental and physical performance; influence on the function of equilibrium; increasing resistance to unfavorable environmental factors (infections, exposure to low doses of chemicals, ionizing radiation, and reduction of undesirable reactions of a number of drugs). | Reduces the risk of ALS | [9,10,12,48,49,50,51,52,53,54,55] |
| Vitamin D (ergocalciferol, cholecalciferol) | Regulation of blood calcium phosphate levels; participation in the regulation of the neuroimmune response; influence on the proliferation and differentiation of neurons; influence on synaptic transmission of nerve impulses mediated by calcium current; neurotrophic function; neuroprotective function; influence on neurotransmission and synaptic plasticity; influence on synaptogenesis; participation in the regulation of aging processes, including the death of neurons (apoptosis). | Is likely to reduce the risk of ALS | [56,57,58,59,60,61,62,63,64,65,66] |
| Vitamin E (alphatocopherol) | Decrease in neuroinflammation and oxidative stress; participation in redox processes (antioxidant function, preventing lipid peroxidation, and reducing free radical reactions in rapidly dividing cells); protection of vitamin A from oxidation, which contributes to the growth of the stimulating activity of vitamin A; participation in the regulation of tissue regeneration; participation in the regulation of neuronal excitability and neuroinflammation; participation in the regulation of aging processes; participation in the synthesis of hormones; slowing down the aging process and protection against oncopathology; participation in maintaining the normal functioning of skeletal muscles; reduction of muscle tissue degeneration processes; participation in energy metabolism and thrombogenesis; participation in the formation of collagen and elastin fibers (strengthening the walls of cerebral vessels); participation in the formation of hemoglobin; reduction of muscle tissue degeneration processes. | Reduces the risk of ALS | [55,67,68,69,70,71,72,73,74,75] |
| LDL Cholesterol | Proatherogenic activity; transport of cholesterol from the liver to the nervous tissue; transfer of unsaturated fatty acids (diene, triene) and polyunsaturated fatty acids as part of cholesterol and triglyceride esters. | Is likely to increase the risk of ALS | [51,72,76,77,78,79,80,81] |
| HDL Cholesterol | Antiatherogenic effect; removal of excess cholesterol from nerve tissue cells and from the surface of other lipoproteins; the supply of proteins and esterified cholesterol to lipoproteins (increasing their stability); antioxidant effect on low density lipoproteins; capture of cholesterol from macrophages (prevention of atherosclerotic vascular lesions); reverse transport of cholesterol from the nervous tissue to the liver (excretion of cholesterol from the body as part of bile acids); prevention of the capture of particles saturated with cholesterol by cells (high affinity with apolipoprotein E- receptors and apolipoprotein B-receptors). | Is likely to reduce the risk of ALS | [51,72,76,77,78,79,80,81] |
| Polyunsaturated fatty acids (PUFAs) | Plastic function (substrate for the formation of phospholipids, glycoproteins, the formation of a cell membrane, a sheath of nerve fibers); elimination of cholesterol from the body; protective action; participation in the exchange of vitamins B1 and B6; increased elasticity and decreased vascular permeability (antiatherogenic function); biosynthesis of prostaglandins; acceleration of the transmission of nerve impulses. | Are likely to reduce the risk of ALS | [72,78,82,83,84,85,86,87,88,89,90,91,92] |
| Urates and purines | Participation in the synthesis of nucleic acids (participation of pyrimidine and purine in the composition of pyrimidine and purine bases). | Are unlikely to have a role in increasing/reducing the risk of ALS | [51,93,94,95] |
| Nutrients | Foods |
|---|---|
| Vitamin А | Carrots, pumpkin, parsley, peas, broccoli, beef liver, egg yolk, red caviar, butter, milk, cottage cheese, and cheese. |
| Vitamin В1 | Pork, beef, wheat germ and whole grains, organ meats, eggs, fish, legumes, and nuts. |
| Vitamin В2 | Chicken eggs, milk, cottage cheese, cheese, and liver. |
| Vitamin В6 | Pistachios, marjoram, beans, sea buckthorn, salmon, tuna, mackerel, walnuts, liver, hazelnuts, sardines, horseradish, garlic, chili peppers, sweet peppers, millet, chicken, pomegranate, and pine nuts. |
| Vitamin В7 (В8 and Н) | Meat products, egg yolk, yeast, nuts and seeds, salmon, dairy products, avocados, sweet potatoes, and cauliflower. |
| Vitamin В9 | Liver (chicken, beef, and pork), peanuts; sunflower seeds, lentils, orange juice, parsley, raw beans, avocado, walnuts, spinach, beets; hazelnuts, peas, broccoli, cauliflower, almonds, porcini mushrooms, wild garlic, papaya, and strawberry. |
| Vitamin В12 | Liver (beef, pork, and chicken), octopus, mackerel, sardine, rabbit, beef, sea bass, pork, lamb, cod, carp, Dutch cheese, crab, chicken egg, and sour cream. |
| Vitamin С | Rowan, strawberry, orange, radish, black currant, apple, lemon, sea buckthorn, cherry, shea, tomato, cabbage, and potatoes. |
| Vitamin D | Salmon species, herring, cod liver, egg yolk, and cow’s milk. |
| Vitamin Е | Almonds, hazelnuts, peanuts, pistachios, cashews, dried apricots, sea buckthorn, eel, rose hips, wheat, walnuts, spinach, squid, viburnum, sorrel, salmon, pike perch, prunes, oatmeal, and barley groats. |
| LDL cholesterol | Red meat, sausages, hard cheeses, bacon, flour confectionery, cream, and hydrogenated vegetable fats. |
| HDL cholesterol | Olive oil, flaxseed oil, fish oil, nuts, and whole grain wheat products. |
| PUFAs | Fish oil, sunflower oil, wheat germ oil, peanut oil, soybean oil, olive oil, red caviar, fresh salmon, fresh herring, mackerel, chicken eggs, flax seeds, pine nuts, walnuts, and sprouted wheat grains. |
| Nutrients | Daily Requirement in Adults |
|---|---|
| Vitamin А | 1.0 μ |
| Vitamin В 1 | 1.6–1.9 μ |
| Vitamin В2 | 2.5–3.5 μ |
| Vitamin В6 | 2.0 μ |
| Vitamin В7 | 150—200 μ |
| Vitamin В9 | 200 μ |
| Vitamin В12 | 3 μ |
| Vitamin С | 50–60 mg |
| Vitamin D | 1000–2000 IU |
| Vitamin Е | 12–15 IU |
| PUFAs | Omega-3—1–3 g Omega-6—5–9 g Omega-9—20–30% of daily ration |
| HDL cholesterol | 300 mg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goncharova, P.S.; Davydova, T.K.; Popova, T.E.; Novitsky, M.A.; Petrova, M.M.; Gavrilyuk, O.A.; Al-Zamil, M.; Zhukova, N.G.; Nasyrova, R.F.; Shnayder, N.A. Nutrient Effects on Motor Neurons and the Risk of Amyotrophic Lateral Sclerosis. Nutrients 2021, 13, 3804. https://doi.org/10.3390/nu13113804
Goncharova PS, Davydova TK, Popova TE, Novitsky MA, Petrova MM, Gavrilyuk OA, Al-Zamil M, Zhukova NG, Nasyrova RF, Shnayder NA. Nutrient Effects on Motor Neurons and the Risk of Amyotrophic Lateral Sclerosis. Nutrients. 2021; 13(11):3804. https://doi.org/10.3390/nu13113804
Chicago/Turabian StyleGoncharova, Polina S., Tatiana K. Davydova, Tatiana E. Popova, Maxim A. Novitsky, Marina M. Petrova, Oksana A. Gavrilyuk, Mustafa Al-Zamil, Natalia G. Zhukova, Regina F. Nasyrova, and Natalia A. Shnayder. 2021. "Nutrient Effects on Motor Neurons and the Risk of Amyotrophic Lateral Sclerosis" Nutrients 13, no. 11: 3804. https://doi.org/10.3390/nu13113804
APA StyleGoncharova, P. S., Davydova, T. K., Popova, T. E., Novitsky, M. A., Petrova, M. M., Gavrilyuk, O. A., Al-Zamil, M., Zhukova, N. G., Nasyrova, R. F., & Shnayder, N. A. (2021). Nutrient Effects on Motor Neurons and the Risk of Amyotrophic Lateral Sclerosis. Nutrients, 13(11), 3804. https://doi.org/10.3390/nu13113804








