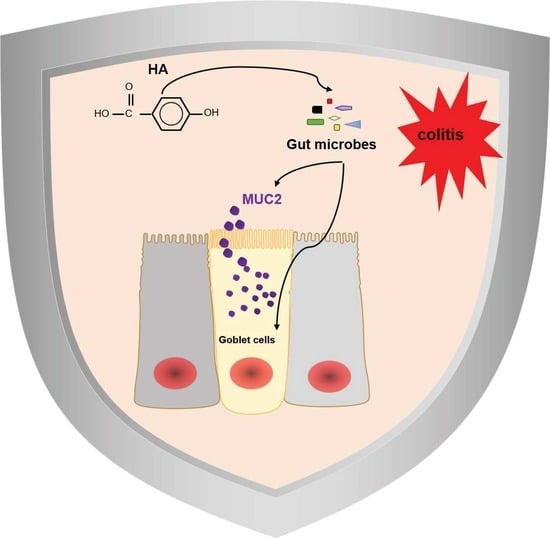
p-Hydroxybenzoic Acid Ameliorates Colitis by Improving the Mucosal Barrier in a Gut Microbiota-Dependent Manner
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Dosage Information
- (1)
- To examine the therapeutic effect of HA, mice were divided into the following three groups: the Control group, the DSS group (2.5% DSS dissolved in drinking water), and the DSS + HA group (HA purchased from TCI (H0207, Shanghai, China). HA was administrated at a dose of 100 mg/kg body weight by oral gavage for 7 days, then DSS and HA were administered for another 7 days.
- (2)
- To determine whether the effect of HA was dependent on GM, mice were pretreated with antibiotic cocktails (1 g/L ampicillin, 1 g/L neomycin, 0.5 g/L meropenem, and 0.5 g/L vancomycin dissolved in drinking water) for 7 days. Animals were subsequently fed with drinking water containing antibiotic cocktails and 2.5% DSS with or without gavage HA for 6 days.
- (3)
- For the fecal microbiota transplantation (FMT) experiment, mice were divided into the following four groups: control (Ctr, PBS gavage), DSS group (2.5% DSS dissolved in drinking water with PBS gavage), DSS + NC FMT (2.5% DSS dissolved in drinking water and FMT of DSS group feces) and DSS + HA FMT (2.5% DSS dissolved in drinking water and FMT of DSS + HA group feces). The fecal microbial solution was prepared as the supernatant after the natural settlement of 200 mg of fresh feces mashed in 5 mL sterile PBS. A total of 200 μL of fecal microbiota was transplanted to each mouse every other day. After three transplantations, 2.5% DSS was administered for 7 days to induce colitis.
2.2. Histological and Immunohistochemical Analysis
2.3. Quantitative Real-Time PCR
2.4. Flow Cytometry
2.5. Analysis of Inflammatory Cytokines
2.6. PAS-AB Staining and Analysis
2.7. 16S rRNA Gene Sequencing and Analysis
2.8. Statistics
3. Results
3.1. HA Ameliorates Colitis Induced by DSS
3.2. HA Improves Mucosal Barrier
3.3. Antibiotics Treatment Blunts the Effects of HA
3.4. Fecal Microbiota Transplants (FMT) from Colitis Mice Treated with HA Ameliorate Colitis by Increasing the Abundance of Akkermansia muciniphila
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
References
- Borren, N.Z.; van der Woude, C.J.; Ananthakrishnan, A.N. Fatigue in IBD: Epidemiology, pathophysiology and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 162, 47–259. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, A.N.; Bernstein, C.N.; Iliopoulos, D.; Macpherson, A.; Neurath, M.F.; Ali, R.A.R.; Vavricka, S.R.; Fiocchi, C. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 39–49. [Google Scholar] [CrossRef]
- Halmos, E.P.; Gibson, P.R. Dietary management of IBD–insights and advice. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 133–146. [Google Scholar] [CrossRef]
- Khalili, H.; Chan, S.S.M.; Lochhead, P.; Ananthakrishnan, A.N.; Hart, A.R.; Chan, A.T. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A. Treatment of active Crohn’s disease with an ordinary food-based diet that replicates exclusive enteral nutrition. Gastroenterology 2019, 156, 1354–1367.e1356. [Google Scholar] [CrossRef] [Green Version]
- Zamora-Ros, R.; Rothwell, J.A.; Scalbert, A.; Knaze, V.; Romieu, I.; Slimani, N.; Fagherazzi, G.; Perquier, F.; Touillaud, M.; Molina-Montes, E.; et al. Dietary intakes and food sources of phenolic acids in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2013, 110, 1500–1511. [Google Scholar] [CrossRef] [Green Version]
- Del, B.C.; Ciappellano, S.; Klimiszacas, D.; Martini, D.; Gardana, C.; Riso, P.; Porrini, M. Anthocyanin absorption, metabolism, and distribution from a wild blueberry-enriched diet (Vaccinium angustifolium) is affected by diet duration in the Sprague-Dawley rat. J. Agric. Food Chem. 2010, 58, 2491. [Google Scholar]
- Zhang, Y.; Wu, S.; Qin, Y.; Liu, J.; Liu, J.; Wang, Q.; Ren, F.; Zhang, H. Interaction of phenolic acids and their derivatives with human serum albumin: Structure-affinity relationships and effects on antioxidant activity. Food Chem. 2018, 240, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Khadem, S.; Marles, R.J. Monocyclic phenolic acids; hydroxy- and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies. Molecules 2010, 15, 7985–8005. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, H.J.; Kim, Y.S.; Yeo, S.H.; Kim, S. Effects of Maclura tricuspidata (Carr.) Bur fruits and its phytophenolics on obesity-related enzymes. J. Food Biochem. 2020, 44, e13110. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.N.; Wang, K.Y.; Zhang, X.S.; Yang, C.; Li, X.Y. 4-Hydroxybenzoic acid (4-HBA) enhances the sensitivity of human breast cancer cells to adriamycin as a specific HDAC6 inhibitor by promoting HIPK2/p53 pathway. Biochem. Biophys. Res. Commun. 2018, 504, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Luo, A.; Lu, X.; Liu, M.; Wang, H.; Song, H.; Wei, C.; Wang, Y.; Duan, X. p-Hydroxybenzoic acid alleviates inflammatory responses and intestinal mucosal damage in DSS-induced colitis by activating ERβ signaling. J. Funct. Foods 2021, 87, 104835. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, W.K.; Han, D.H.; Lee, K.; Ko, G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes 2019, 10, 696–711. [Google Scholar] [CrossRef]
- Guo, J.; Han, X.; Zhan, J.; You, Y.; Huang, W. Vanillin Alleviates High Fat Diet-Induced Obesity and Improves the Gut Microbiota Composition. Front. Microbiol. 2018, 9, 2733. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Kwon, J.E.; Cho, M.L. Immunological pathogenesis of inflammatory bowel disease. Intest. Res. 2018, 16, 26–42. [Google Scholar] [CrossRef] [Green Version]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Panpetch, W.; Hiengrach, P.; Nilgate, S.; Tumwasorn, S.; Somboonna, N.; Wilantho, A.; Chatthanathon, P.; Prueksapanich, P.; Leelahavanichkul, A. Additional Candida albicans administration enhances the severity of dextran sulfate solution induced colitis mouse model through leaky gut-enhanced systemic inflammation and gut-dysbiosis but attenuated by Lactobacillus rhamnosus L34. Gut Microbes 2020, 11, 465–480. [Google Scholar] [CrossRef]
- Li, D.; Feng, Y.; Tian, M.; Ji, J.; Hu, X.; Chen, F. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARgamma signaling activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Murata, T.; Fujisawa, M.; Nagasaka, R.; Ushio, H.; Bari, A.M.; Hori, M.; Ozaki, H. Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br. J. Pharmacol. 2008, 154, 812–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, W.; Zhu, S.; Niu, Z.; Yin, Y. The protective effect of syringic acid on dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Dev. Res. 2019, 80, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Wang, C.Q.; Zeng, Z.; Ren, Y.; Li, D.Y.; Song, J.L. Ameliorative Effect of Sinapic Acid on Dextran Sodium Sulfate- (DSS-) Induced Ulcerative Colitis in Kunming (KM) Mice. Oxidative Med. Cell. Longev. 2020, 2020, 8393504. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y. Anti-inflammatory effects of sinapic acid on 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice. Arch. Pharmacal Res. 2018, 41, 243–250. [Google Scholar] [CrossRef]
- Lan, H.; Zhang, L.Y.; He, W.; Li, W.Y.; Zeng, Z.; Qian, B.; Wang, C.; Song, J.L. Sinapic Acid Alleviated Inflammation-Induced Intestinal Epithelial Barrier Dysfunction in Lipopolysaccharide- (LPS-) Treated Caco-2 Cells. Mediators Inflamm. 2021, 2021, 5514075. [Google Scholar] [CrossRef]
- Gao, W.; Wang, C.; Yu, L.; Sheng, T.; Wu, Z.; Wang, X.; Zhang, D.; Lin, Y.; Gong, Y. Chlorogenic Acid Attenuates Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice through MAPK/ERK/JNK Pathway. Biomed. Res. Int. 2019, 2019, 6769789. [Google Scholar] [CrossRef]
- Lee, Y.M.; Shin, D.W.; Lim, B.O. Chlorogenic Acid Improves Symptoms of Inflammatory Bowel Disease in Interleukin-10 Knockout Mice. J. Med. Food 2020, 23, 1043–1053. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Mohebali, N.; Esa, N.M.; Looi, C.Y.; Ismail, S.; Saadatdoust, Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int. Immunopharmacol. 2015, 28, 1034–1043. [Google Scholar] [CrossRef]
- Shree, A.; Islam, J.; Vafa, A.; Mohammad Afzal, S.; Sultana, S. Gallic acid prevents 1, 2-Dimethylhydrazine induced colon inflammation, toxicity, mucin depletion, and goblet cell disintegration. Environ. Toxicol. 2020, 35, 652–664. [Google Scholar] [CrossRef]
- Zhu, L.; Gu, P.; Shen, H. Gallic acid improved inflammation via NF-kappaB pathway in TNBS-induced ulcerative colitis. Int. Immunopharmacol. 2019, 67, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Moorthy, B.; Haribabu, B.; Jala, V.R. Cytochrome P450 1A1 is essential for the microbial metabolite, Urolithin A-mediated protection against colitis. Front. Immunol. 2022, 13, 1004603. [Google Scholar] [CrossRef]
- Rudiansyah, M.; Abdalkareem Jasim, S.; Azizov, S.B.; Samusenkov, V.; Kamal Abdelbasset, W.; Yasin, G.; Mohammad, H.J.; Jawad, M.A.; Mahmudiono, T.; Hosseini-Fard, S.R.; et al. The emerging microbiome-based approaches to IBD therapy: From SCFAs to urolithin A. J. Dig. Dis. 2022, 23, 412–434. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Ray Chaudhuri, S. The opportunistic nature of gut commensal microbiota. Crit. Rev. Microbiol. 2022, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Dey, P. Targeting gut barrier dysfunction with phytotherapies: Effective strategy against chronic diseases. Pharmacol. Res. 2020, 161, 105135. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geerlings, S.Y.; Kostopoulos, I.; de Vos, W.M.; Belzer, C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms 2018, 6, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res. 2019, 147, 104367. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Chaudhuri, S.R.; Efferth, T.; Pal, S. The intestinal 3M (microbiota, metabolism, metabolome) zeitgeist–from fundamentals to future challenges. Free Radic. Biol. Med. 2021, 176, 265–285. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Li, M.; Sun, L.; Liu, X.; Yin, Y.; Hao, J.; Zhang, W. p-Hydroxybenzoic Acid Ameliorates Colitis by Improving the Mucosal Barrier in a Gut Microbiota-Dependent Manner. Nutrients 2022, 14, 5383. https://doi.org/10.3390/nu14245383
Han X, Li M, Sun L, Liu X, Yin Y, Hao J, Zhang W. p-Hydroxybenzoic Acid Ameliorates Colitis by Improving the Mucosal Barrier in a Gut Microbiota-Dependent Manner. Nutrients. 2022; 14(24):5383. https://doi.org/10.3390/nu14245383
Chicago/Turabian StyleHan, Xue, Miaomiao Li, Lijun Sun, Xinjuan Liu, Yue Yin, Jianyu Hao, and Weizhen Zhang. 2022. "p-Hydroxybenzoic Acid Ameliorates Colitis by Improving the Mucosal Barrier in a Gut Microbiota-Dependent Manner" Nutrients 14, no. 24: 5383. https://doi.org/10.3390/nu14245383
APA StyleHan, X., Li, M., Sun, L., Liu, X., Yin, Y., Hao, J., & Zhang, W. (2022). p-Hydroxybenzoic Acid Ameliorates Colitis by Improving the Mucosal Barrier in a Gut Microbiota-Dependent Manner. Nutrients, 14(24), 5383. https://doi.org/10.3390/nu14245383