Multiple Metabolites Derived from Mushrooms and Their Beneficial Effect on Alzheimer’s Diseases
Abstract
:1. Introduction
2. The Main Targets for Treating Alzheimer’s Disease
2.1. Deposition of Aggregated Proteins
2.2. Modulation of Oxidative Stress
2.3. Alleviation of Neuroinflammation
2.4. Mitochondrial Dysfunction
2.5. Cell Apoptosis, Necrosis, and Autophagy
2.6. Other Factors
3. Diversity of Mushroom-Derived Metabolites Beneficial to AD and Their Possible Mechanisms
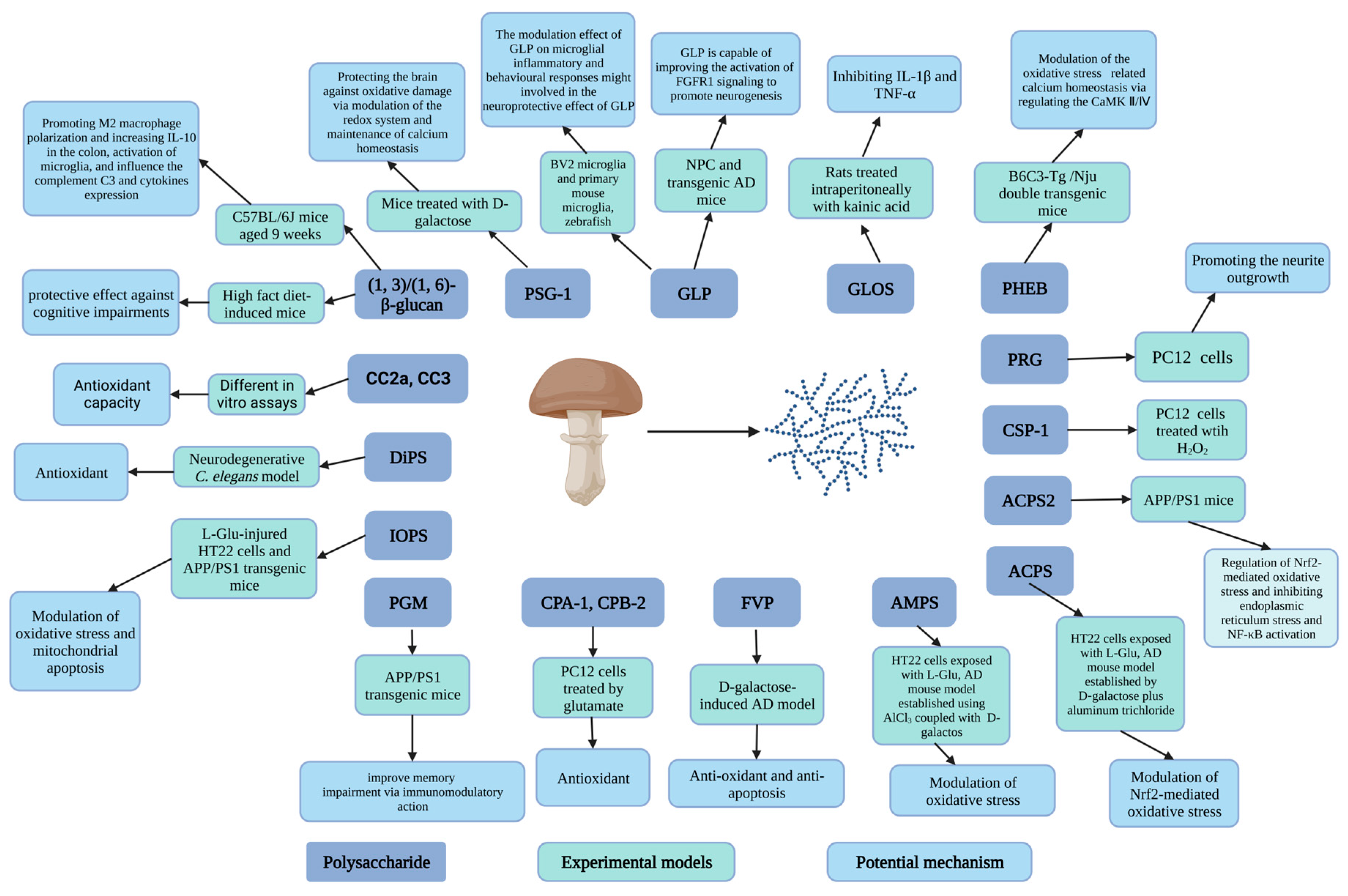
3.1. Carbohydrates
| Mushroom Species | Name | Molecule Weight (kDa) | Experimental Models | Dose and Periods | Effect | Potential Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| Amanita caesarea | Polysaccharide (ACPS) | 18.620, 33.500 | HT22 cells exposed to L-Glu, AD mouse model established by D-galactose plus aluminum trichloride | In vitro test: 2.5 or 5 μg/mL for 3 h. In vivo test: 2.5 or 5 mg/kg for 42 days | In vitro test: Cell viability, MMP ↑ Apoptotic rate, ROS levels, intracellular Ca2+ ↓ The expression of Bcl-2, HO-1, SOD1, GCLC and the Nrf2 levels in nucleus ↑ The expression of Bax, cleaved caspase-3, Keap-1, cytochrome C and the Nrf2 levels in cytoplasm ↓ In vivo test: AD-like behavior, Aβ1-42 level in brain, Aβ plaque, Ach and choline ChAT, SOD ↓ Aβ1-42 level in serum, AChE, GSH-Px, SOD ↑ | Modulation of Nrf2-mediated oxidative stress | [96] |
| Polysaccharide (ACPS2) | 16.6 | APP/PS1 mice | 6 weeks | Cognition ability and anxious behavior ↑ Tumor necrosis factor-α, interleukin-1β ↓ Brain injury, Aβ deposition, tau hyperphosphorylation↓ | Regulation of Nrf2-mediated oxidative stress and further inhibiting endoplasmic reticulum stress and nuclear factor-kappa B (NF-κB) activation | [97] | |
| Armillaria mellea | Mycelium polysaccharides (AMPS) | HT22 cells exposed to L-Glu, AD mouse model established using AlCl3 coupled with D-galactos | In vitro test: 10, 20, 40, and 80 μg/mL for 3 h In vivo test: 25, 100 mg/kg/day, 4 weeks | In vitro test: Cell viability, Mitochondrial membrane potential (MMP) depolarization ↑ Nuclear apoptosis, ROS, Caspase-3 activity ↓ In vivo test: AD-like behavior, TUNEL-positive apoptotic neurons, AchE level, ROS, the expression of Aβ in the hippocampus, 4-NHE levels, and p-Tau aggregation ↓ Ach level, ChAT level, SOD and GSH-Px level, serum Aβ1-42 concentrations ↑ | Modulation of oxidative stress and antiapoptosis | [103] | |
| Cantharellus cibarius | Polysaccharide fractions (CC2a, CC3) | Different in vitro assays | 10, 25, 50, 100 μg/mL, 48 h | Neurons viability and neurite outgrowth ↑ LDH level in cell culture medium ↓ Mitochondrial dehydrogenase activity ↑ Lactate dehydrogenase activity ↓ Neurite outgrowth ↑ DCF ↓ | Antioxidant capacity | [108] | |
| Cordyceps cicadae | Polysaccharides (CPA-1, CPB-2) | PC12 (pheochromocytoma) cells treated with glutamate | 25, 50, 100, and 200 μg/mL, 24 h | Cell viability, GSH-Px activity, SOD activity ↑ LDH breakage, ROS production, intracellular Ca2+ level, MDA level ↓ | Antioxidant | [104] | |
| Cordyceps sinensis | Polysaccharide (CSP-1) | 210 | PC12 cells treated with H2O2 | 25, 50, 100 μg/mL | Survival of cells, the activity of SOD and GSH-P ↑ MDA level ↓ | [109] | |
| Dictyophora indusiata | Polysaccharides (DiPS) | Neurodegenerative C. elegans model | 0.5–4.0 mg/mL, various times | Survival rate, SOD activity, mitochondrial membrane potential, and ATP content ↑ ROS and MDA levels ↓ DAF-16/FOXO ↑ polyQ- and Aβ-mediated behavior disorders ↓ | Antioxidant | [90] | |
| Flammulina velutipes | Polysaccharide (FVP) | D-galactose-induced AD model | 400 mg/kg/d, 30 days | Cognitive ability ↑ SOD, CAT, and GSH-Px activities, Bcl-2 expression ↑ Apoptosis rate, Bax, cytochrome C, caspase-3, caspase-9, apoptosis-inducing factor expression levels, MDA level ↓ | Anti-oxidant and anti-apoptosis | [95] | |
| Hericium erinaceus | Polysaccharide (PHEB) | 36.1 | B6C3-Tg (APPswePSEN1d E9)/Nju double transgenic mice | 25 and 100 mg/kg body weight, 6 weeks | Cognitive behavior, ChAT, and Ach level, serum levels of Aβ1-42, SOD and GSH-Px activity, the levels of Nrf2, the expression of mTOR, SHANK3, Akt, GABBR1, PKA, GluT1, Neurogranin ↑ Inflammation in brains, AChE, Aβ plaque area, phosphorylated tau plaques, and neurofibrillary tangles in hippocampus, MDA and ROS levels, the levels of Keap1 ↓ P-Ca2+/calmodulin-dependent kinase Ⅳ (CaMKⅣ), P-CaMK Ⅱ, ERK 1/2, Ras, P-GluR2 ↓ | Modulation of the oxidative stress-related calcium homeostasis via regulating the CaMK Ⅱ/Ⅳ | [110] |
| Ganoderma atrum | Polysaccharide (PSG-1) | 1013 | Mice treated with D-galactose | 50, 100, or 150 mg/kg body weight, 4 weeks, once a day | SOD, CAT, GPx, and GSH-Rd activities, GSH content ↑ GSSG and MDA level, apoptosis, ROS production, and calcium levels ↓ | Protecting the brain against oxidative damage via modulation of the redox system and maintenance of calcium homeostasis | [111,112] |
| Ganoderma lucidum | Polysaccharide (GLP) | 15 | Neural progenitor cell (NPC) and transgenic AD mice | In vivo test: 30 mg/kg body weight, once per day, 90 days; In vitro test: 10, 30, 100, 300 μg/mL | Cognitive function ↑ Double-positive cells (BrdU/NeuN) number in the hippocampus ↑ The number of Ki67 and SOX2 double-positive proliferation NPC, Phosphorylation of FGFR1, ERK, AKT ↑ 6E10-postitive Aβ area ↓ | GLP is capable of improving the activation of fibroblast growth factor receptor 1 (FGFR1) signaling to promote neurogenesis | [102] |
| BV2 microglia and primary mouse microglia, zebrafish | In vitro assays: 2 h, 1–1000 ng/mL for BV2, 0.3–100 ng/mL for primary microglia In Zebrafish, 1 μg/mL, 12 h-5 d postfertilisation | IL-1β, IL-6 and iNOS expression ↓ The expression of TGFβ ↑ MCP-1 and C1q expressions ↓ Microglial migration, morphological alterations, and phagocytosis probabilities ↓ | The modulation effect of GLP on microglial inflammatory and behavioral responses might be involved in the neuroprotective effect of GLP | [93] | |||
| Oligosaccharide fraction (GLOS) | 0.8–1.3 | Rats treated intraperitoneally with kainic acid (10 mg/kg body weight) | 10, 40, 80 mg/kg body weight | Mortality, neuronal loss, staining for (GFAP), the expression of IL-1β and TNF-α ↓ | Inhibiting the production of glia-derived toxic factors (IL-1β and TNF-α) | [107] | |
| Grifola frondosa | Proteo-β-glucan (PGM) | APPswe/PS1ΔE9 (APP/PS1) transgenic mice (AD model) | intraperitoneal injection of PGM (5, 10, 20 mg/kg body weight per day) for 3 months | Learning and memory capability, the number of Nissl bodies and neurons, the expression of astrocyte marker (GFAP) and microglial marker (Iba1), microglial recruitment to the Aβ plaques, Aβ phagocytosis ↑ Histopathological abnormalities and necrotic neurons, the mean area containing Aβ1-42-positive plaques ↓ | PGM could improve memory impairment via immunomodulatory action | [101] | |
| Inonotus obliquus | Polysaccharide (IOPS) | 111.9 | L-glutamic acid (L-Glu)-injured HT22 cells and amyloid precursor protein/presenilin 1 (APP/PS1) transgenic mice | In vitro test: 5 or 10 μg/mL for 3 h In vivo test: 25 or 50 mg/kg/d (i.g.), once daily, 8 weeks | In vitro test: Cell viability ↑ Apoptosis, caspase-3 activity, release of LDH, ROS, the levels of Bax and Keap1↓ MMP, Bcl-2, Nrf2, HO-1, SOD-1 and cysteine ligase catalytic subunit (GCLC) ↑ In vivo test: Memory and cognition ability ↑ Aβ1-42 deposition, the number of neuronal fiber tangles, 4-HNE, and Keap1 levels in brain↓ SOD and GSH-Px level, Nrf2, HO-1, GCLC and SOD-1↑ | Modulation of oxidative stress and mitochondrial apoptosis | [105] |
| Lentinula edodes | (1, 3)/(1, 6)-β-glucan | High-fat diet-induced mice | Mice supplemented with β-glucan from I. edodes (500 mg/kg food) for 7 days or 15 weeks | The abundance of Proteobacteria, energy intake, the order Clostridiales, class Clostridia, family Lachnospiracease, and family Ruminococcaceae in mice short-term supplemented with β-glucan. ↑ The proportion of Firmicutes, Proteobacteria, Actinobacteria in mice long-term supplemented with β-glucan. ↓ Discrimination index, body weight ↑ Cognitive decline, serum LPS, macrophage marker F4/80 positive cells, the expression of IL-6, TNF-α and IL-1β, microglial number, the proliferation of microglia, the expression of BDNF and PSD-95 ↓ The expression of occludin ↑ | The protective effect against cognitive impairments of sample was demonstrated via colon–brain axis improvement in mice induced by the HF diet | [113] | |
| C57BL/6J mice aged 9 weeks | 60 mg/kg body weight, 15 weeks | The discrimination index, brain-derived neurotrophic factor (BDNF), the CD206+ cell number in colon, IL-10 expression ↑ The number of Ibal1 positive cells, the expression of complement C3, IL-6, IL-1 β and TNF-α ↓ | Promoting M2 macrophage polarization and increasing IL-10 in the colon, activation of microglia, and influencing the complement C3 and cytokines expression | [114] | |||
| Phellinus ribis | Polysaccharide (PRG) | 5.16 | PC12 (pheochromocytoma) cells | 10, 50, 150 μg/mL | Mean neurite lengths of NGF-stimulated PC12 cells ↑ | Promoting the neurite outgrowth | [115] |
| Pleurotus ostreatus | Polysaccharide (POP) | 24 | D-galactose and AlCl3-induced AD rats | 400 mg/kg body weight, 30 days | Learning and memory capability ↑ SOD, GSH-Px, and CAT activities in hippocampus, liver, and serum ↑ MDA level in hippocampus, liver, and serum and hippocampal AchE activity ↓ Protein phosphatase 2A (PP2A) ↑ The expression of amyloid precursor protein (APP), Aβ, β-site APP clearing enzyme1 (BACE1), p-tau, and glycogen synthase kinase 3beta (GSK-3β) ↓ | Relieving the Aβ formation and tau phosphorylation | [94,116] |
| Polysaccharide (POP-W) | 3.034 × 103 | PC12 cells damaged by H2O2 | 0.1, 0.2, 0.4, 0.8, 1.6, 3.2 mg/mL, 24 h | Cell viability, SOD activity, GSH level, the ratio of Bcl-2/BAX, the p-Akt/Akt ratio, and PI3K expression ↑ LDH, MDA levels, Caspase-3 level ↓ | POP-W pretreatment was able to protect PC12 cells against H2O2 damage due to its capacity of antioxidant and anti-apoptosis via regulating the PI3K/AKT signaling pathway and apoptosis-related pathway proteins | [117] | |
| Pleurotus eryngii | Polysaccharide (PEP) | Aging rats and PC12 cells | In vitro test: 0.5, 1, 1.5 μM, 24 h. In vivo test: administered with PEP for 28 weeks | Cell viability ↑ Intracellular calcium, apoptosis, APP production in brain, iNOS, and COX-2 level ↓ | Modulation of calcium channels or inflammation | [91] | |
| Pleurotus sajor-caju | Polysaccharide (PSP2-1) | 44.9 | Neuronal cell HT22 induced by H2O2 and aging mice induced by D-galactose | In vitro test: 50, 100 to 150 μg/mL for 24 h In vivo test: 100, 200, and 400 mg/kg/d for 42 days | In vitro test: Cell viability, Mitochondrial membrane potential (MMP), and the expression ratio of bcl-2/bax ↑ LDH release and cytochrome c release, apoptosis rate, ROS level, and the expression of cleaved caspase-3, cleaved PARP, Erk1/2, JNK, p38 ↓ In vivo test: Learning and memory ability, CAT, and SOD ↑ MDA and ROS ↓ | The protective actions of PSP2-1 on nerve cells against oxidative damage and apoptosis induced by hydrogen peroxide were attributed to its regulating the MAPK signaling pathway | [118] |
| Sparassis crispa | Polysaccharides (SCP-1) | 13.68 | C57BL/6J mice treated with D-galactose and AlCl3 | 25 and 100 mg/kg/d, 4 weeks | Learning and recognition, GABA and Ach levels in brain ↑ Aβ deposition and Aβ1-42, Glu ↓ IL-6, TNF-α, IL-1β, serum LPS ↓ Iba1-positive microglia and GFAP-positive astrocytes in hippocampal CA1 and DG area ↓ The expression of TLR4, NF-κB, and phosphorylation of NF-κB ↓ Altering the gut microbiota | Modulation of gut microbiota and suppression of inflammation | [100] |
| HT22 cells treated by H2O2 | 10, 25, 50, 100, 200, 400, 800 μg/mL, 12 h | Cell viability, SOD, and GSH-Px activities ↑ ROS, MDA, chromatin condensation and apoptotic bodies, apoptotic rate ↓ | Antioxidant and inhibiting apoptosis | [99] | |||
| Tremella fuciformis | Polysaccharide (TL04) | 2.033 | Glutamate-induced neurotoxicity in DPC12 cells | 5 and 20 μg, 3 h | Cell viability ↑ LDH release, ROS, apoptotic nuclei ↓ Bcl-2 level and Cyto C level ↑ Bax expression, the levels of cleaved caspase-8, caspase-9 and caspase-3 ↓ | The underlying mechanism for protective effect of TL04 against glutamate-induced neurotoxicity was proved to be associated with the caspase-dependent mitochondrial pathway | [106] |
3.2. Proteins and Peptides
3.3. Phenolic Compounds
3.4. Terpenes
3.5. Vitamins
3.6. Nucleosides
3.7. Alkaloids
3.8. Sterols
3.9. Other Constituents
4. Conclusions and Future Research Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, Z.; Brunner, B.; Csilla, A. Beneficial effects of exogenous ketogenic supplements on aging processes and age-related neurodegenerative diseases. Nutrients 2021, 13, 2197. [Google Scholar] [CrossRef] [PubMed]
- Vaquer-Alicea, J.; Diamond, M.I. Propagation of protein aggregation in neurodegenerative diseases. Annu. Rev. Biochem. 2019, 88, 785–810. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.J.; Lin, C.L.; Hsu, C.Y.; Shae, Z.Y.; Kao, C.H. Association between neurodegenerative diseases and pneumonia: A retrospective population-based study. Curr. Med. Res. Opin. 2019, 35, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Chokroverty, S. Hypersomnia in neurodegenerative diseases. Sleep Med. Clin. 2017, 12, 443–460. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R. What causes neurodegenerative disease? Folia Neuropathol. 2020, 58, 93–112. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2022 Alzheimer’s disease facts and figures. Alzheimers Derment. 2022, 18, 700–789. [Google Scholar] [CrossRef]
- Fu, Y.W.; Xu, H.S.; Liu, S.J. COVID-19 and neurodegenerative diseases. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4535–4544. [Google Scholar] [CrossRef]
- Xia, X.H.; Wang, Y.; Zheng, J.L. COVID-19 and Alzheimer’s disease: How one crisis worsens the other. Transl. Neurodegener. 2021, 10, 15. [Google Scholar] [CrossRef]
- Amiri, A.; Barreto, G.; Sathyapalan, T.; Sahebkar, A. siRNA therapeutics: Future promise for neurodegenerative diseases. Curr. Neuropharmacol. 2021, 19, 1896–1911. [Google Scholar] [CrossRef]
- Peden, A.H.; Ironside, J.W. Molecular pathology in neurodegenerative diseases. Curr. Drug Targets 2012, 13, 1548–1549. [Google Scholar] [CrossRef] [PubMed]
- Mensah-Kane, P.; Sumien, N. The potential of hyperbaric oxygen as a therapy for neurodegenerative diseases. GeroScience 2023, 45, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Solanki, I.; Parihar, P.; Mansuri, M.L.; Parihar, M.S. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. 2015, 6, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, S.K.; Ir, R.; Jeewon, R.; Doble, M.; Hyde, K.D.; Kaliappan, I.; Jeyaraman, R.; Reddi, R.N.; Krishnan, J.; Li, M.; et al. A mechanistic review on medicinal mushrooms-derived bioactive compounds: Potential mycotherapy candidates for alleviating neurological disorders. Planta Med. 2020, 86, 1161–1175. [Google Scholar] [CrossRef]
- Homer, J.A.; Sperry, J. Mushroom-derived indole alkaloids. J. Nat. Prod. 2017, 80, 2178–2187. [Google Scholar] [CrossRef]
- Rangel-Vargas, E.; Rodriguez, J.A.; Domínguez, R.; Lorenzo, J.M.; Sosa, M.E.; Andres, S.C.; Rosmini, M.; Perez-Alvarez, J.A.; Teixeira, A.; Santos, E.M. Edible mushrooms as a natural source of food ingredient/additive replacer. Food 2021, 10, 2687. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.R.S.; El-Sayed, H. Molecular identification and antimicrobial activities of some wild Egyptian mushrooms: Bjerkandera adusta as a promising source of bioactive antimicrobial phenolic compounds. J. Genet. Eng. Biotechnol. 2021, 19, 106. [Google Scholar] [CrossRef]
- Ma, G.X.; Du, H.J.; Hu, Q.H.; Yang, W.J.; Pei, F.; Xiao, H. Health benefits of edible mushroom polysaccharides and associated gut microbiota regulation. Crit. Rev. Food Sci. Nutr. 2021, 62, 6646–6663. [Google Scholar] [CrossRef]
- Thu, Z.M.; Myo, K.K.; Aung, H.T.; Clericuzio, M.; Armijos, C.; Vidari, G. Bioactive phytochemical constituents of wild edible mushrooms from southeast Asia. Molecules 2022, 25, 1972. [Google Scholar] [CrossRef] [Green Version]
- Muszyńska, B.; Grzywacz-Kisielewska, A.; Kala, K.; Gdula-Argasińska, J. Anti-inflammatory properties of edible mushrooms: A review. Food Chem. 2017, 243, 373–381. [Google Scholar] [CrossRef]
- Seweryn, E.; Ziala, A.; Gamian, A. Health-promoting of polysaccharides extracted from Ganoderma lucidum. Nutrients 2021, 13, 2725. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.A.; O.Elkhalifa, A.E.; Siddiqui, A.J.; Patel, M.; Awadelkareem, A.M.; Snoussi, M.; Ashraf, M.S.; Adnan, M.; Hadi, S. Cordycepin for health and wellbeing: A potent bioactive metabolites of an entomopathogenic medicinal fungus Cordyceps with its nutraceutical and therapeutic potential. Molecules 2020, 25, 2735. [Google Scholar] [CrossRef] [PubMed]
- Assemie, A.; Abaya, G. The effect of edible mushrooms on health and their biochemistry. Int. J. Microbiol. 2022, 2022, 8744788. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Y.; Lou, H.H.; Hu, J.J.; Liu, Z.J.; Chen, Q.H. Macrofungi: A review of cultivation strategies, bioactivity, and application of mushrooms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2333–2356. [Google Scholar] [CrossRef]
- Zeb, M.; Lee, C.H. Medicinal properties and bioactive compounds from wild mushrooms native to north America. Molecules 2021, 26, 251. [Google Scholar] [CrossRef]
- Bayazid, A.B.; Lim, B.O. Quercetin is an active agent in berries against neurodegenerative disease progression through modulation of Nrf2/HO1. Nutrients 2022, 14, 5132. [Google Scholar] [CrossRef]
- Jiang, X.; Li, S.Q.; Feng, X.R.; Li, L.Z.; Hao, J.; Wang, D.; Wang, Q.S. Mushroom polysaccharides as potential candidates for alleviating neurodegenerative diseases. Nutrients 2022, 14, 4833. [Google Scholar] [CrossRef]
- Khanam, H.; Ali, A.; Asif, M.; Shamsuzzaman. Neurodegenerative diseases linked to misfolded proteins and their therapeutic approaches: A review. Eur. J. Med. Chem. 2016, 124, 1121–1141. [Google Scholar] [CrossRef]
- Gao, Y.; Tan, L.; Yu, J.T.; Tan, L. Tau in Alzheimer’s disease: Mechanisms and therapeutic strategies. Neurol. Sci. 2018, 15, 283–300. [Google Scholar] [CrossRef]
- Gallardo, G.; Holtzman, D.M. Amyloid-β and Tau at the crossroads of Alzheimer’s disease. Adv. Exp. Med. Biol. 2019, 1184, 187–203. [Google Scholar] [CrossRef]
- Nonaka, T.; Masuda-Suzukake, M.; Hasegawa, M. Molecular mechanisms of the co-deposition of multiple pathological proteins in neurodegenerative diseases. Neuropathology 2018, 38, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, A.H.S.; Lopes, F.C.; John, E.B.O.; Carlini, C.R.; Ligabue-Braun, R. Modulation of disordered proteins with a focus on neurodegenerative diseases and other pathologies. Int. J. Mol. Sci. 2019, 20, 1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, D.L.; Barria, M.A. Prion diseases: A unique transmissible agent or a model for neurodegenerative diseases? Biomolecules 2021, 11, 207. [Google Scholar] [CrossRef]
- Scannevin, R.H. Therapeutic strategies for targeting neurodegenerative protein misfolding disorders. Curr. Opin. Chem. Biol. 2018, 44, 66–74. [Google Scholar] [CrossRef]
- Reiss, A.B.; Arain, H.A.; Stecker, M.M.; Siegart, N.M.; Kasselman, L.J. Amyloid toxicity in Alzheimer’s disease. Rev. Neurosci. 2018, 29, 613–627. [Google Scholar] [CrossRef]
- Sengupta, U.; Kayed, R. Amyloid β, Tau, and α-Synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog. Neurobiol. 2022, 214, 102270. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1538. [Google Scholar] [CrossRef] [Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Chong, Z.Z.; Li, F.Q.; Maiese, K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog. Neurobiol. 2005, 75, 207–246. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; Cessie, S.; Noordam, R.; Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.H.; Liu, B.D. Relationships between stress granules, oxidative stress, and neurodegenerative disease. Oxid. Med. Cell. Longev. 2017, 2017, 1809592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Pol, A.; Van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative stress: Pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative stress and cardiovascular disease: New insights. Kardiol. Pol. 2018, 76, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Floyd, R.A.; Hensley, K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging 2002, 23, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Paloczi, J.; Varga, Z.V.; Hasko, G.; Pacher, P. Neuroprotection in oxidative stress-related neurodegenerative diseases: Role of endocannabinoid system modulation. Antioxid. Redox Signal. 2018, 29, 75–108. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Salim, S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.Y.; Sun, L.; Chen, X.P.; Zhang, D.S. Oxidative stress, mitochondrial damage and neurodegenerative disease. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef]
- O’Day, D.H.; Huber, R.J. Calmodulin binding proteins and neuroinflammation in multiple neurodegenerative diseases. BMC Neurosci. 2022, 23, 10. [Google Scholar] [CrossRef]
- Fontana, L.; Ghezzi, L.; Gross, A.H.; Piccio, L. Effects of dietary restriction on neuroinflammation in neurodegenerative diseases. J. Exp. Med. 2021, 218, e20190086. [Google Scholar] [CrossRef]
- Lin, M.M.; Liu, N.; Qin, Z.H.; Wang, Y. Mitochondrial-derived damage-associated molecular patterns amplify neuroinflammation in neurodegenerative diseases. Acta Pharmacol. Sin. 2022, 43, 2439–2447. [Google Scholar] [CrossRef]
- Ruggiero, M.; Calvello, R.; Porro, C.; Messina, G.; Cianciulli, A.; Panaro, M.A. Neurodegenerative diseases: Can caffeine be a powerful ally to weaken neuroinflammation? Int. J. Mol. Sci. 2022, 23, 12958. [Google Scholar] [CrossRef]
- Hong, H.; Kim, B.S.; Im, H. Pathophysiological role of neuroinflammation in neurodegenerative diseases and psychiatric disorders. Int. Neurourol. J. 2016, 20 (Suppl. S1), S2–S7. [Google Scholar] [CrossRef] [Green Version]
- Ghavami, S.; Shojaei, S.; Yeganeh, B.; Ande, S.R.; Jangamreddy, J.R.; Mehrpour, M.; Christoffersson, J.; Chaabane, W.; Moghadam, A.R.; Kashani, H.H.; et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014, 112, 24–49. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.T.; Yu, J.; Grass, D.; Beer, F.C.; Kindy, M.S. Inflammation-dependent cerebral deposition of serum amyloid a protein in a mouse model of amyloidosis. J. Neurosci. 2002, 22, 5900–5909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.H.; Zhou, W.H.; Liu, S.C.; Deng, Y.; Cai, F.; Tone, M.; Tone, Y.; Tong, Y.G.; Song, W.H. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2012, 15, 77–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Hussain, M.D.; Yan, L.J. Microglia, neuroinflammation, and Aβ in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Golpich, M.; Amini, E.; Mohamed, Z.; Ali, R.A.; Ibrahim, N.M.; Ahmadiani, A. Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: Pathogenesis and treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef]
- Raza, C.; Anjum, R.; Shakeel, N.A. Parkinson’s disease: Mechanisms, translational models and management strategies. Life Sci. 2019, 226, 77–90. [Google Scholar] [CrossRef]
- Jurcau, A. Insights into the pathogenesis of neurodegenerative diseases: Focus on mitochondrial dysfunction and oxidative stress. Int. J. Mol. Sci. 2021, 22, 11847. [Google Scholar] [CrossRef]
- Park, J.S.; Davis, R.L.; Sue, C.M. Mitochondrial dysfunction in Parkinson’s disease: New mechanistic insights and therapeutic perspectives. Curr. Neurol. Neurosci. Rep. 2018, 18, 21. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.H.; Ho, Y.S.; Chang, R.C. Modulation of mitochondrial calcium as a pharmacological target for Alzheimer’s disease. Ageing Res. Rev. 2010, 9, 447–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, S. Apoptosis, autophagy, necrosis and their multi galore crosstalk in neurodegeneration. Neuroscience 2021, 469, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Dailah, H.G. Potential of therapeutic small molecules in apoptosis regulation in the treatment of neurodegenerative diseases: An updated review. Molecules 2022, 27, 7207. [Google Scholar] [CrossRef] [PubMed]
- Kermer, P.; Liman, J.; Weishaupt, J.H.; Bahr, M. Neuronal apoptosis in neurodegenerative diseases: From basic research to clinical application. Neurodegener. Dis. 2004, 1, 9–19. [Google Scholar] [CrossRef]
- Chi, H.; Chang, H.Y.; Sang, T.K. Neuronal cell death mechanisms in major neurodegenerative disease. Int. J. Mol. Sci. 2018, 19, 3082. [Google Scholar] [CrossRef] [Green Version]
- Sureda, F.X.; Junyent, F.; Verdaguer, E.; Auladell, C.; Pelegri, C.; Vilaplana, J.; Folch, J.; Canudas, A.M.; Zarate, C.B.; Pallàs, M.; et al. Antiapoptotic drugs: A therapautic strategy for the prevention of neurodegenerative diseases. Curr. Pharm. Des. 2011, 17, 230–245. [Google Scholar] [CrossRef]
- Cho, Y.S.; Challa, S.; Moquin, D.; Genga, R.; Ray, T.D.; Guildford, M.; Chan, F.K. Phosphorylation-driven assembly of the RIP1RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009, 137, 1112–1123. [Google Scholar] [CrossRef] [Green Version]
- Filippone, A.; Esposito, E.; Mannino, D.; Lyssenko, N.; Praticò, D. The contribution of altered neuronal autophagy to neurodegeneration. Pharmacol. Ther. 2022, 238, 108178. [Google Scholar] [CrossRef]
- Binvignat, O.; Olloquequi, J. Excitotoxicity as a target against neurodegenerative processes. Curr. Pharm. Des. 2020, 26, 1251–1262. [Google Scholar] [CrossRef]
- Popugaeva, E.; Vlasova, O.L.; Bezprozvanny, I. Restoring calcium homeostasis to treat Alzheimer’s disease: A future perspective. Neurodegener. Dis. Manag. 2015, 5, 395–398. [Google Scholar] [CrossRef] [Green Version]
- Molinero, N.; Antón-Fernández, A.; Hernández, F.; Ávila, J.; Bartolomé, B.; Moreno-Arribas, M.V. Gut Microbiota, an additional hallmark of human aging and neurodegeneration. Neuroscience 2023, 518, 141–161. [Google Scholar] [CrossRef]
- Fodale, V.; Quattrone, D.; Trecroci, C.; Caminiti, V.; Santamaria, L.B. Alzheimer’s disease and anaesthesia: Implications for the central cholinergic system. Neuroscience 2006, 97, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Leblanc, P.; Vorberg, I.M. Viruses in neurodegenerative diseases: More than just suspects in crimes. PLoS Pathog. 2022, 18, e1010670. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The antioxidant potential of different edible and medicinal mushrooms. Biomed. Pharmacother. 2022, 174, 112621. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Xiao, J.B.; Xu, B.J. A critical review on health promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abitbol, A.; Mallard, B.; Tiralongo, E.; Tiralongo, J. Mushroom natural products in neurodegenerative disease drug discovery. Cell 2022, 11, 3938. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Li, S.; Zhang, R.; Le, W.D. Neuroprotective effects of naturally sourced bioactive polysaccharides: An update. Neural Regen. Res. 2022, 17, 1907–1912. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chen, R.S.; Yang, Z.S.; Wen, Q.; Cao, X.; Zhao, N.H.; Yan, J.Y. Protective effects of polysaccharides in neurodegenerative diseases. Front. Aging Neurosci. 2022, 14, 917629. [Google Scholar] [CrossRef]
- Guo, S.S.; Cui, X.L.; Rausch, W.D. Ganoderma Lucidum polysaccharides protect against MPP (+) and rotenone-induced apoptosis in primary dopaminergic cell cultures through inhibiting oxidative stress. Am. J. Neurodegener. Dis. 2016, 5, 131–144. [Google Scholar]
- Zhang, J.; Shi, R.; Li, H.F.; Xiang, Y.X.; Xiao, L.Y.; Hu, M.H.; Ma, F.L.; Ma, C.W.; Huang, Z. Antioxidant and neuroprotective effects of Dictyophora indusiata polysaccharide in Caenorhabditis elegans. J. Ethnopharmacol. 2016, 192, 413–422. [Google Scholar] [CrossRef]
- Zhang, C.J.; Guo, J.Y.; Cheng, H.; Lin, L.; Liu, Y.; Shi, Y.; Xu, J.; Yu, H.T. Protective effects of the king oyster culinary-medicinal mushroom, Pleurotus eryngii (Agaricomycetes), polysaccharides on β-amyloid-induced neurotoxicity in PC12 cells and aging rats, in vitro and in vivo studies. Int. J. Med. Mushrooms 2020, 22, 325–333. [Google Scholar] [CrossRef]
- Badshah, S.L.; Riaz, A.; Muhammad, A.; Cayan, G.T.; Cayan, F.; Duru, M.E.; Ahmad, N.; Emwas, A.; Jaremko, M. Isolation, characterization, and medicinal potential of polysaccharides of Morchella esculenta. Molecules 2021, 26, 1459. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Li, Y.Y.; Pei, G. Polysaccharides from Ganoderma lucidum attenuate microglia-mediated neuroinflammation and modulate microglial phagocytosis and behavioral response. J. Neuroinflamm. 2017, 14, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yang, X.M.; Jin, G.; Yang, X.D.; Zhang, Y. Polysaccharides from Pleurotus ostreatus alleviate cognitive impairment in a rat model of Alzheimer’s disease. Int. J. Biol. Macromol. 2016, 92, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Yang, X.D.; Jin, G.; Zhang, Y. Cognitive-enhancing effect of polysaccharides from Flammulina velutipes on Alzheimer’s disease by compatibilizing with ginsenosides. Int. J. Biol. Macromol. 2018, 112, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.P.; Chen, X.; Zhang, Y.F.; Liu, X.; Wang, C.Y.; Teng, L.S.; Wang, D. Protective roles of Amanita caesarea polysaccharides against Alzheimer’s disease via Nrf2 pathway. Int. J. Biol. Macromol. 2019, 121, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.J.; Li, Z.P.; Wang, W.Q.; Song, M.K.; Dong, R.T.; Zhou, Y.L.; Li, Y.; Wang, D. Structural characterization of polysaccharide purified from Amanita caesarea and its pharmacological basis for application in Alzheimer’s disease: Endoplasmic reticulum stress. Food Funct. 2021, 12, 11009–11023. [Google Scholar] [CrossRef]
- Zhang, J.R.; An, S.S.; Hu, W.J.; Teng, M.Y.; Wang, X.; Qu, Y.D.; Liu, Y.; Yuan, Y.; Wang, D. The neuroprotective properties of Hericium erinaceus in glutamate-damaged differentiated PC12 cells and an Alzheimer’s disease mouse model. Int. J. Mol. Sci. 2016, 17, 1810. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Hu, B.; Han, M.; Guo, Y.H.; Cheng, Y.L.; Qian, H. Purification, structural characterization and neuroprotective effect of a neural polysaccharide from Sparassis crispa. Int. J. Biol. Macromol. 2022, 201, 389–399. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Guo, Y.H.; Cheng, Y.L.; Yao, W.R.; Qian, H. Neuroprotective effects of polysaccharide from Sparassis crispa on Alzheimer’s disease-like mice: Involvement of microbiota-gut-brain axis. Int. J. Biol. Macromol. 2023, 225, 974–986. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, L.L.; Chen, Y.; Chen, X.M.; Dong, Y.L.; Zheng, S.Y.; Zhang, L.; Li, W.Y.; Du, J.; Li, H.L. A Maitake (Grifola frondosa) polysaccharide ameliorates Alzheimer’s disease-like pathology and cognitive impairments by enhancing microglial amyloid-β clearance. RSC Adv. 2019, 9, 37127–37135. [Google Scholar] [CrossRef]
- Huang, S.C.; Mao, J.X.; Ding, K.; Zhou, Y.; Zeng, X.L.; Yang, W.J.; Wang, P.P.; Zhao, C.; Yao, J.; Xia, P.; et al. Polysaccharides from Ganoderma lucidum promote cognitive function and neural progenitor proliferation in mouse model of Alzheimer’s disease. Stem Cell Rep. 2017, 8, 84–94. [Google Scholar] [CrossRef] [Green Version]
- An, S.S.; Lu, W.Q.; Zhang, Y.F.; Yuan, Q.X.; Wang, D. Pharmacological basis for use of Armillaria mellea polysaccharides in Alzheimer’s disease: Antiapoptosis and antioxidation. Oxid. Med. Cell. Longev. 2017, 2017, 4184562. [Google Scholar] [CrossRef] [Green Version]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Wei, Y.; Ouyan, Z. Polysaccharides purified from Cordyceps cicadae protects PC12 cells against glutamate-induced oxidative damage. Carbohydr. Polym. 2016, 153, 187–195. [Google Scholar] [CrossRef]
- Han, Y.Q.; Nan, S.J.; Fan, J.; Chen, Q.H.; Zhang, Y.Z. Inonotus obliquus polysaccharides protect against Alzheimer’s disease by regulating Nrf2 signaling and exerting antioxidative and antiapoptotic effects. Int. J. Biol. Macromol. 2019, 131, 769–778. [Google Scholar] [CrossRef]
- Jin, Y.X.; Hu, X.Y.; Zhang, Y.; Liu, T.J. Studies on the purification of polysaccharides separated from Tremella fuciformis and their neuroprotective effect. Mol. Med. Rep. 2016, 13, 3985–3992. [Google Scholar] [CrossRef] [Green Version]
- Tello, I.; Campos-Pena, V.; Montiel, E.; Rodriguez, V.; Aguirre-Moreno, A.; Leon-Rivera, I.; Rio-Portilla, F.D.; Herrera-Ruiz, M.; Villeda-Hernandez, J. Anticonvulsant and neuroprotective effects of oligosaccharides from Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (Higher Basidiomycetes). Int. J. Med. Mushrooms 2013, 15, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Lemieszek, M.K.; Nunes, F.M.; Cardoso, C.; Marques, G.; Rzeski, W. Neuroprotective properties of Cantharellus cibarius polysaccharide fractions in different in vitro models of neurodegeneration. Carbohydr. Polym. 2018, 197, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Zhao, K.J.; Ji, Z.N.; Song, Z.H.; Dong, T.T.X.; Lo, C.K.; Cheung, J.K.H.; Zhu, S.Q.; Tsim, W.K. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003, 73, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.J.; Song, M.K.; Wang, C.Y.; Guo, Z.; Li, Y.; Wang, D. Structural characterization of polysaccharide purified from Hericium erinaceus fermented mycelium and its pharmacological basis for application in Alzheimer’s disease: Oxidative stress related calcium homeostasis. Int. J. Biol. Macromol. 2021, 193 Pt A, 358–369. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.Y.; Nie, S.P.; Li, C.; Wang, Y.X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Li, W.J.; Nie, S.P.; Xie, M.Y.; Yu, Q.; Chen, Y.; He, M. Ganoderma atrum polysaccharide attenuates oxidative stress induced by d-galactose in mouse. Life Sci. 2011, 88, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Jiang, P.F.; Zhao, J.X.; Shi, H.L.; Zhang, P.; Yang, X.Y.; Biazik, J.; Hu, M.; Hua, H.; Ge, X.; et al. β-Glucan from Lentinula edodes prevents cognitive impairments in high-fat diet-induced obese mice: Involvement of colon-brain axis. J. Transl. Med. 2021, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.M.; Zhang, P.; Wang, R.Q.; Zhou, M.L.; Pang, N.; Cui, X.Y.; Ge, X.; Liu, X.M.; Huang, X.F.; Yu, Y.H. Three different types of β-glucans enchance cognition: The role of the gut-brain axis. Front. Nutr. 2022, 9, 848930. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Liu, C.H.; Jiang, H.Q.; Zhou, H.L.; Li, P.L.; Wang, F.S. Isolation, structural characterization and neurotrophic activity of a polysaccharide from Phellinus ribis. Carbohydr. Polym. 2015, 127, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Liu, J.C. Purification, structure and immunobiological activity of a water-soluble polysaccharide from the fruiting body of Pleurotus ostreatus. Bioresour. Technol. 2009, 100, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Li, K.; Cui, Y.D.; Peng, H.H.; Hu, Y.; Zhu, Z.Y. Preparation, structural characterization and neuroprotective effects to against H2O2-induced oxidative damage in PC12 cells of polysaccharides from Pleurotus ostreatus. Food Res. Int. 2023, 163, 112146. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.D.; Dai, Y.D.; Song, C.M.; Wang, J.; Liu, Y.; Wang, D. Structural characterization of a Pleurotus sajor-caju polysaccharide and its neuroprotection related to the inhibition of oxidative stress. Nutrients 2022, 14, 4047. [Google Scholar] [CrossRef]
- Zhang, K.R.; Tang, Y.Z.; Chen, Q.; Liu, Y. The screening of therapeutic peptides for anti-inflammation through phage display technology. Int. J. Mol. Sci. 2022, 23, 8554. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Saeed, M.; Alharbi, A.M.; Barreto, G.E.; Ashraf, G.M.; Choi, I. Peptide based therapeutics and their use for the treatment of neurodegenerative and other diseases. Biomed. Pharmacother. 2018, 103, 574–581. [Google Scholar] [CrossRef]
- Wu, S.J.; Chen, M.F.; Liao, X.Y.; Huang, R.; Wang, J.; Xie, Y.Z.; Hu, H.P.; Zhang, J.M.; Wu, Q.P.; Ding, Y. Protein hydrolysates from Pleurotus geesteranus obtained by simulated gastrointestinal digestion exhibit neuroprotective effects in H2O2-injured PC12 cells. J. Food Biochem. 2021, 46, e13879. [Google Scholar] [CrossRef] [PubMed]
- La Manna, S.; Di Natale, C.; Florio, D.; Marasco, D. Peptides as therapeutic agents for inflammatory-related diseases. Int. J. Mol. Sci. 2018, 19, 2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.C.; Zhang, X.Y.; Gao, H.L.; Qing, G.Y. Phage display derived peptides for Alzheimer’s disease therapy and diagnosis. Theranostics 2022, 12, 2041–2062. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.M.; Cao, W.; Yao, K.W.; Liu, Z.Q.; Guo, J.Y. Anti-inflammation and antioxidant effect of Cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Metab. Brain Dis. 2012, 27, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Ng, T.B.; Wang, H.X.; Sze, S.C.W.; Zhang, K.Y.; Li, Q.; Lu, X.X. Cordymin, an antifungal peptide from the medicinal fungus Cordyceps militaris. Phytomedicine 2011, 18, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Wu, Q.P.; Wang, J.; Li, Y.F.; Chen, B.; Zhu, Z.J.; Huang, R.; Chen, M.F.; Huang, A.H.; Xie, Y.Z.; et al. Novel selenium peptides obtained from selenium-enriched Cordyceps militaris alleviate neuroinflammation and gut microbiota dysbateriosis in LPS-injured mice. J. Agric. Food Chem. 2022, 70, 3194–3206. [Google Scholar] [CrossRef] [PubMed]
- Dubost, N.; Ou, B.; Beelman, R. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem. 2007, 105, 727–735. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Martino, E.; Balestrieri, A.; Mele, L.; Cautela, D.; Castaldo, D.; Balestrieri, M.L. Diet-derived ergothioneine induces necroptosis in colorectal cancer cells by activating the SIRT3/MLKL pathway. FEBS Lett. 2022, 596, 1313–1329. [Google Scholar] [CrossRef]
- Servillo, L.; D’Onofrio, N.; Balestrieri, M.L. Ergothioneine antioxidant function: From chemistry to cardiovascular therapeutic potential. J. Cardiovasc. Pharmacol. 2017, 69, 183–191. [Google Scholar] [CrossRef]
- Cheah, I.K.; Feng, L.; Tang, R.M.Y.; Lim, K.H.C.; Halliwell, B. Ergothioneine levels in an elderly population decrease with age and incidence of cognitive decline; a risk factor for neurodegeneration? Biochem. Biophys. Res. Commun. 2016, 478, 162–167. [Google Scholar] [CrossRef]
- Wu, L.Y.; Kan, C.N.; Cheah, I.K.; Chong, J.R.; Xu, X.; Vrooman, H.; Hilal, S.; Venketasubramanian, N.; Chen, C.P.; Halliwell, B.; et al. Low plasma ergothinoneine predicts cognitive and functional decline in an elderly cohort attending memory clinics. Antioxidants 2022, 11, 1717. [Google Scholar] [CrossRef] [PubMed]
- Roda, E.; Ratto, D.; Luca, F.D.; Desiderio, A.; Ramieri, M.; Goppa, L.; Savino, E.; Bottone, M.G.; Locatelli, C.A.; Rossi, P. Searching for a longevity food, we bump into Hericium erinaceus primordium rich in ergothioneine: The “longevity vitamin” improves locomotor performances during aging. Nutrients 2022, 14, 1177. [Google Scholar] [CrossRef] [PubMed]
- Moncaster, J.; Walsh, D.T.; Gentleman, S.M.; Jen, L.S.; Aruoma, O.I. Ergothioneine treatment protects neurons against N-methyl-D-aspartate excitotoxicity in an in vivo rat retinal model. Neurosci. Lett. 2002, 328, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Duda, J.E. Dietary modifications in Parkinson’s disease: A neuroprotective intervention? Med. Hypotheses 2015, 85, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Kato, Y. Ergothioneine in the brain. FEBS Lett. 2022, 596, 1290–1298. [Google Scholar] [CrossRef]
- Nakamichi, N.; Tsuzuku, S.; Shibagaki, F. Ergothioneine and central nervous system diseases. Neurochem. Res. 2022, 47, 2513–2521. [Google Scholar] [CrossRef]
- Cheah, I.K.; Ng, L.T.; Lam, V.Y.; Gruber, J.; Huang, C.Y.W.; Goh, F.Q.; Lim, K.H.C.; Halliwell, B. Inhibition of amyloid-induced toxicity by ergothioneine in a transgenic Caenorhabditis elegans model. FEBS Lett. 2019, 593, 2139–2150. [Google Scholar] [CrossRef]
- Whitmore, C.A.; Haynes, J.R.; Behof, W.J.; Rosenberg, A.J.; Tantawy, M.N.; Hachey, B.C.; Wadzinski, B.E.; Spiller, B.W.; Peterson, T.E.; Paffenroth, K.C.; et al. Longitudinal consumption of ergothioneine reduces oxidative stress and amyloid plaques and restores glucose metabolism in the 5XFAD mouse model of Alzheimer’s disease. Pharmaceuticals 2022, 15, 742. [Google Scholar] [CrossRef]
- Hung, P.V. Phenolic compounds of cereals and their antioxidant capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef]
- Abdelshfy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.S.; Li, L. A comprehensive review on phenolic compounds from edible mushrooms: Occurrence, biological activity, application and further prospective. Crit. Rev. Food Sci. Nutr. 2021, 62, 6204–6224. [Google Scholar] [CrossRef]
- Khumlianlal, J.; Sharma, K.C.; Singh, L.M.; Mukherjee, P.K.; Indira, S. Nutritional profiling and antioxidant property of three wild edible mushrooms from north east India. Molecules 2022, 27, 5423. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lee, H.S.; Kim, S.H.; Moon, B.; Lee, C. Antioxidant and anti-inflammatory activities of methanol extracts of Tremella fuciformis and its major phenolic acids. J. Food Sci. 2014, 79, C460–C468. [Google Scholar] [CrossRef]
- Lam, Y.S.; Okello, E.J. Determination of Lovastatin, β-glucan, total polyphenols, and antioxidant activity in raw and processed Oyster culinary-medicinal mushroom, Pleurotus ostreatus (higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Mišković, J.; Rašeta, M.; Čapelja, E.; Krsmanović, N.; Novakovićc, A.; Karaman, M. Mushroom species Stereum hirsutum as natural source of phenolics and fatty acids as antioxidants and acetylcholinesterase inhibitors. Chem. Biodivers. 2021, 18, e2100409. [Google Scholar] [CrossRef]
- Chen, W.; Feng, L.N.; Huang, Z.Y.; Su, H.M. Hispidin produced from Phellinus linteus against peroxynitrite-mediated DNA damage and hydroxyl radical generation. Chem. Biol. Interact. 2012, 30, 137–142. [Google Scholar] [CrossRef]
- Guo, J.J.; Liu, X.X.; Li, Y.J.; Ji, H.Y.; Liu, C.; Zhou, L.; Huang, Y.; Bai, C.C.; Jiang, Z.B.; Wu, X.L. Screening for proteins related to the biosynthesis of hispidin and its derivatives in Phellinus igniarius using iTRAQ proteomic analysis. BMC Microbiol. 2021, 21, 81. [Google Scholar] [CrossRef] [PubMed]
- Smolskaite, L.; Slapsyte, G.; Mierauskiene, J.; Dedonyte, V.; Venskutonis, P.R. Antioxidant and genotoxic properties of hispidin isolated from the Velvet-Top Mushroom, Phaeolus schweinitzii (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.H.; Wu, C.Y.; Li, P.H.; Liang, Z.C. Optimal liquid inoculum conditions and grain medium enhanced hispidin production by species of genus Phellinus (Agaricomycetes) in solid-state fermentation. Int. J. Med. Mushrooms 2022, 24, 77–90. [Google Scholar] [CrossRef]
- Jin, M.H.; Chen, D.Q.; Jin, Y.H.; Han, Y.H.; Sun, H.N.; Kwon, T. Hispidin inhibits LPS-induced nitric oxide production in BV-2 microglial cells via ROS-dependent MAPK signaling. Exp. Ther. Med. 2021, 22, 970. [Google Scholar] [CrossRef]
- Park, I.H.; Jeon, S.Y.; Lee, H.J.; Kim, S.I.; Song, K.S. A beta-secretase (BACE1) inhibitor hispidin from the mycelial cultures of Phellinus linteus. Planta Med. 2004, 70, 143–146. [Google Scholar] [CrossRef]
- Mori, K.; Kikuchi, H.; Obara, Y.; Iwashita, M.; Azumi, Y.; Kinugasa, S.; Inatomi, S.; Oshima, Y.; Nakahata, N. Inhibitory effect of hericenone B from Hericium erinaceus on collagen-induced platelet aggregation. Phytomedicine 2010, 17, 1082–1085. [Google Scholar] [CrossRef]
- Mori, K.; Obara, Y.; Hirota, M.; Azumi, Y.; Kinugasa, S.; Inatomi, S.; Nakahata, N. Nerve growth factor-inducing activity of Hericium erinaceus in 1321N1 human astrocytoma cells. Biol. Pharm. Bull. 2008, 31, 1727–1732. [Google Scholar] [CrossRef] [Green Version]
- Saitsu, Y.; Nishide, A.; Kikushima, K.; Shimizu, K.; Ohnuki, K. Improvement of cognitive functions by oral intake of Hericium erinaceus. Biomed. Res. 2019, 40, 125–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratto, D.; Corana, F.; Mannucci, B.; Priori, E.C.; Cobelli, F.; Roda, E.; Ferrari, B.; Occhinegro, A.; Di Iorio, C.; De Luca, F.; et al. Hericium erinaceus improves recognition memory and induces hippocampal and cerebellar neurogenesis in frail mice during aging. Nutrients 2019, 11, 715. [Google Scholar] [CrossRef] [Green Version]
- Phan, C.W.; Lee, G.S.; Hong, S.L.; Wong, Y.T.; Brkljaca, R.; Urban, S.; Malek, S.N.A.; Sabaratnam, V. Hericium erinaceus (Bull.: Fr) Pers. cultivated under tropical conditions: Isolation of hericenones and demonstration of NGF-mediated neurite outgrowth in PC12 cells via MEK/ERK and PI3K-Akt signaling pathways. Food Funct. 2014, 5, 3160–3169. [Google Scholar] [CrossRef]
- Ueda, K.; Tsujimori, M.; Kodani, S.; Chiba, A.; Kubo, M.; Masuno, K.; Sekiya, A.; Nagai, K.; Kawagishi, H. An endoplasmic reticulum (ER) stress-suppressive compound and its analogues from the mushroom Hericium erinaceum. Bioorg. Med. Chem. 2008, 16, 9467–9470. [Google Scholar] [CrossRef]
- Lin, S.L.; Ching, L.T.; Ke, X.X.; Cheung, P.C.K. Comparison of the composition and antioxidant activities of phenolics from the fruiting bodies of cultivated Asian culinary-medicinal mushrooms. Int. J. Med. Mushrooms 2016, 18, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Loureiro, J.A.; Pereira, M.D.C. Influence of in vitro neuronal membranes on the anti-amloidogenic activity of gallic acid: Implication for the therapy of Alzheimer’s disease. Arch. Biochem. Biophys. 2021, 711, 109022. [Google Scholar] [CrossRef]
- Bindhu, J.; Arunava, D.; Sakthivel, K.M. Anthraquinone from edible fungi Pleurotus ostreatus protects human SH-SY5Y neuroblastoma cells against 6-hydroxydopamine-induced cell death-preclinical validation of gene knockout possibilities of PARK7, PINK1, and SNCA1 using CRISPR SpCas9. Appl. Biochem. Biotechnol. 2020, 191, 555–566. [Google Scholar]
- Sgarbossa, A.; Giacomazza, D.; di Carlo, M. Ferulic acid: A hope for Alzheimer’s disease therapy from plants. Nutrients 2015, 7, 5764–5782. [Google Scholar] [CrossRef] [Green Version]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef]
- Dhakal, S.; Kushairi, N.; Phan, C.W.; Adhikari, B.; Sabaratnam, V.; Macreadie, I. Dietary polyphenols: A multifactorial strategy to target Alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 5090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Jiang, Q.D.; Chai, Y.P.; Zhang, H.; Peng, P.; Yang, X.X. Natural terpenes as penetration enhancers for transdermal drug delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Guo, W.H.; Cao, C.Y.; Kou, R.W.; Xu, Y.Z.; Górecki, M.; Bari, L.D.; Pescitelli, G.; Gao, J.M. Polyoxygenated cyathane diterpenoids from the mushroom Cyathus africanus, and their neurotrophic and anti-neuroinflammatory activities. Sci. Rep. 2018, 8, 2175. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.D.; Yong, T.Q.; Xiao, C.; Gao, X.; Xie, Y.Z.; Hu, H.P.; Li, X.M.; Chen, D.L.; Pan, H.H.; Wu, Q.P. Inhibitory effect of triterpenoids from the mushroom Inonotus obliquus against α-glucosidase and their interaction: Inhibition kinetics and molecular stimulations. Bioorg. Chem. 2021, 115, 105276. [Google Scholar] [CrossRef]
- Zeng, M.; Qi, L.K.; Guo, Y.R.; Zhu, X.X.; Tang, X.C.; Yong, T.Q.; Xie, Y.Z.; Wu, Q.P.; Zhang, M.; Chen, D.L. Long-term administration of triterpenoids from Ganoderma lucidum mitigates age-associated brain physiological decline via regulating sphingolipid metabolism and enhancing autophagy in mice. Front. Aging Neurosci. 2021, 13, 628860. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, D.D.; Yin, H.; Li, H.R.; Du, J.; Bao, H.K. Ganoderic acid A attenuates LPS-induced neuroinflammation in BV2 microglia by activating farnesoid X receptor. Neurochem. Res. 2021, 46, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.Y.; Yang, X.M.; Yang, X.D.; Xue, J.F.; Yang, Y.J. Ganoderic acid A to alleviate neuroinflammation of Alzheimer’s disease in mice by regulating the imbalance of the Th17/Tregs axis. J. Agric. Food Chem. 2021, 69, 14204–14214. [Google Scholar] [CrossRef]
- Qi, L.F.R.; Liu, S.; Liu, Y.C.; Li, P.; Xu, X.J. Ganoderic acid A promotes amyloid-β clearance (in vitro) and ameliorates cognitive deficiency in Alzheimer’s disease (mouse model) through autophagy induced by activating Axl. Int. J. Mol. Sci. 2021, 22, 5559. [Google Scholar] [CrossRef]
- Shen, S.H.; Wang, X.M.; Lv, H.; Shi, Y.; Xiao, L.W. PADI4 mediates autophagy and participates in the role of ganoderic acid A monomers in delaying the senescence of Alzheimer’s cells through the Akt / mTOR pathway. Biosci. Biotechnol. Biochem. 2021, 85, 1818–1829. [Google Scholar] [CrossRef]
- Lee, K.F.; Tung, S.Y.; Teng, C.C.; Shen, C.H.; Hsieh, M.C.; Huang, C.Y.; Lee, K.C.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; et al. Post-treatment with erinacine A, a derived diterpenoid of H. erinaceus, attenuates neurotoxicity in MPTP model of Parkinson’s disease. Antioxidants 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzeng, T.T.; Chen, C.C.; Lee, L.Y.; Chen, W.P.; Lu, C.K.; Shen, C.C.; Huang, F.C.Y.; Chen, C.C.; Shiao, Y.J. Erinacine A-enriched Hericium erinaceus mycelium ameliorates Alzheimer’s disease-related pathologies in APPswe/PS1dE9 transgenic mice. J. Biomed. Sci. 2016, 23, 49. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.L.; Chen, S.C.; Chang, J.C.; Lin, W.Y.; Chen, C.C.; Li, C.C.; Hsieh, M.L.; Chen, H.W.; Chang, T.Y.; Liu, C.S.; et al. The protective effect of erinacine A-enriched Hericium erinaceus mycelium ethanol extract on oxidative stress-induced neurotoxicity in cell and Drosophila models of spinocerebellar ataxia type 3. Free Radic. Biol. Med. 2023, 195, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; Chou, W.; Chen, W.P.; Wang, M.F.; Chen, Y.J.; Chen, C.C.; Tung, K.C. Erinacine A-enriched Hericium erinaceus mycelium delays progression of age-related cognitive decline in senescence accelerated mouse prone 8 (SAMP8) mice. Nutrients 2021, 13, 3659. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Lin, Y.C.; Huang, C.C.; Villaflores, O.B.; Wu, T.Y.; Huang, S.M.; Chin, T.Y. Hericium erinaceus mycelium and its isolated compound, erinacine A, ameliorate high-fat high-sucrose diet-induced metabolic dysfunction and spatial learning deficits in aging mice. J. Med. Food 2019, 22, 469–478. [Google Scholar] [CrossRef]
- Li, I.C.; Chang, H.H.; Lin, C.H.; Chen, W.P.; Lu, T.H.; Lee, L.Y.; Chen, Y.W.; Chen, Y.P.; Chen, C.C.; Lin, D.C. Prevention of early Alzheimer’s disease by erinacine A-enriched Hericium erinaceus mycelia pilot double-blind placebo-controlled study. Front. Aging Neurosci. 2020, 12, 155. [Google Scholar] [CrossRef]
- Lee, S.L.; Hsu, J.Y.; Chen, T.C.; Huang, C.C.; Wu, T.Y.; Chin, T.Y. Erinacine A prevents lipopolysaccharide-mediated glial cell activation to protect dopaminergic neurons against inflammatory factor-induced cell death in vitro and in vivo. Int. J. Mol. Sci. 2022, 23, 810. [Google Scholar] [CrossRef]
- Lee, K.F.; Chen, J.H.; Teng, C.C.; Shen, C.H.; Hsieh, M.C.; Lu, C.C.; Lee, K.C.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; et al. Protective effects of Hericium erinaceus mycelium and its isolated erinacine A against ischemia-injury-induced neuronal cell death via the inhibition of iNOS/p38 MAPK and nitrotyrosine. Int. J. Mol. Sci. 2014, 15, 15073–15089. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.C.; Cao, C.Y.; Kubo, M.; Harada, K.; Yan, X.T.; Yan, X.T.; Fukuyama, Y.; Gao, J.M. Chemical constituents from Hericium erinaceus promote neuronal survival and potentiate neurite outgrowth via the TrkA/Erk1/2 pathway. Int. J. Mol. Sci. 2017, 18, 1659. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.C.; Tzeng, T.T.; Chen, C.C.; Ni, C.L.; Lee, L.Y.; Chen, W.P.; Shiao, Y.J.; Shen, C.C. Erinacine S, a rare sesterterpene from the mycelia of Hericium erinaceus. J. Nat. Prod. 2016, 79, 438–441. [Google Scholar] [CrossRef]
- Marcotullio, M.C.; Pagiotti, R.; Maltese, F.; Mwankie, G.N.O.M.; Hoshino, T.; Obara, Y.; Nakahata, N. Cyathane diterpenes from Sarcodon cyrneus and evaluation of their activities of neuritegenesis and nerve growth factor production. Bioorg. Med. Chem. 2007, 15, 2878–2882. [Google Scholar] [CrossRef]
- Liu, L.; Shi, X.W.; Zong, S.C.; Tang, J.J.; Gao, J.M. Scabronine M, a novel inhibitor of NGF-induced neurite outgrowth from PC12 cells from the fungus Sarcodon scabrosus. Bioorg. Med. Chem. Lett. 2012, 22, 2401–2406. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.Y.; Zhang, C.C.; Shi, X.W.; Li, D.; Cao, W.; Yin, X.; Gao, J.M. Sarcodonin G derivatives exhibit distinctive effects on neurite outgrowth by modulating NGF signaling in PC12 cells. ACS Chem. Neurosci. 2018, 9, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Ishiyama, D.; Mori, H.; Sakamoto, H.; Ishiguro, Y.; Furukawa, S.; Li, J. Dictyophorines A and B, two stimulators of NGF-synthesis from the mushroom Dictyophora indusiata. Phytochemistry 1997, 45, 1203–1205. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.M.; Wang, X.; Song, C.G. Neuroprotective and antioxidant lanostanoid triterpenes from the fruiting bodies of Ganoderma atrum. Fitoterapia 2016, 109, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Zhang, C.C.; Yin, X.; Wei, J.; Gao, J.M. Striatoids A-F, cyathane diterpenoids with neurotrophic activity from cultures of the fungus Cyathus striatus. J. Nat. Prod. 2015, 78, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.R.; Luo, R.C.; Su, H.G.; Zhou, L.; Ran, X.Q.; Guo, Y.R.; Yao, Y.G.; Qiu, M.H. (±)-Spiroganoapplanin A, a complex polycyclic meroterpenoid dimer from Ganoderma applanatum displaying potential against Alzheimer’s disease. Org. Chem. Front. 2022, 9, 3093–3101. [Google Scholar] [CrossRef]
- Janjusevic, M.; Gagno, G.; Fluca, A.L.; Padoan, L.; Beltrami, A.P.; Sinagra, G.; Moretti, R.; Aleksova, A. The peculiar role of vitamin D in the pathophysiology of cardiovascular and neurodegenerative diseases. Life Sci. 2022, 289, 120193. [Google Scholar] [CrossRef]
- Mavraki, E.; Loannidis, P.; Tripsianis, G.; Gioka, T.; Kolousi, M.; Vadikolias, K. Vitamin D in mild cognitive impairment and Alzheimer’s disease. A study in older Greek adults. Hippokratia 2020, 24, 120–126. [Google Scholar]
- Popescu, A.; German, M. Vitamin K2 holds promise for Alzheimer’s prevention and treatment. Nutrients 2021, 13, 2206. [Google Scholar] [CrossRef]
- Ono, K.; Yamada, M. Vitamin A and Alzheimer’s disease. Geriatr. Gerontol. Int. 2012, 12, 180–188. [Google Scholar] [CrossRef]
- Lloret, A.; Esteve, D.; Monllor, P.; Cervera-Ferri, A.; Lloret, A. The effectiveness of vitamin E treatment in Alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 879. [Google Scholar] [CrossRef] [Green Version]
- Keflie, T.S.; Nolle, N.; Lambert, C.; Nöhr, D.; Biesalski, H.K. Impact of the natural resource of UVB on the content of viatmin D2 in oyster mushroom (Pleurotus ostreatus) under subtropical settings. Saudi J. Biol. Sci. 2019, 26, 1724–1730. [Google Scholar] [CrossRef]
- Mattila, P.; Könkö, K.; Eurola, M.; Pihlava, J.M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; Piironen, V. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A review of mushrooms as a potential source of dietary vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.C.; Cai, W.X.; Xu, B.J. Vitamin D2, ergosterol, and vitamin B2 content in commercially dried mushrooms marketed in China and increased vitamin D2 content following UV-C irradiation. Int. J. Vitam. Nutr. Res. 2017, 87, 237–246. [Google Scholar] [CrossRef]
- Ahlborn, J.; Calzolari, N.; Spielmeyer, A.; Avci, S.S.; Zimmer, M.; Rühl, M. Enrichment of vitamin D2 in mycelium from submerged cultures of the agaric mushroom Pleurotus sapidus. J. Food Sci. Technol. 2018, 55, 3833–3839. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Karen Ildico, H.E.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of vitamin D2 mushroom powder as a Novel food pursuant to Regulation (EU) 2015/2283 (NF 2019/1471). EFSA J. 2022, 20, e07326. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; O’Mahony, L.; O’Sullivan, A.; Collier, J.; Frase, W.D.; Gibney, M.J.; Nugent, A.P.; Brennan, L. Effect of supplementation with vitamin D2-enhanced mushrooms on vitamin D status in healthy adults. J. Nutr. Sci. 2013, 2, e29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, J.A.; Lee, B.H.; Lee, J.S.; Park, H.J. Effect of UV-B exposure on the concentration of vitamin D2 in sliced shiitake mushroom (Lentinus edodes) and white button mushroom (Agaricus bisporus). J. Agric. Food Chem. 2008, 56, 3671–3674. [Google Scholar] [CrossRef]
- Bennett, L.; Kersaitis, C.; Macaulay, S.L.; Munch, G.; Niedermayer, G.; Nigro, J.; Payne, M.; Sheean, P.; Vallotton, P.; Zabaras, D.; et al. Vitamin D2-enriched button mushroom (Agricus bisporus) improves memory in both wild type and APPswe/PS1dE9 treansgenic mice. PLoS ONE 2013, 8, e76362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, Z.M.; Li, C.H.; Song, Y.L.; Zhou, M.X.; Li, W.J. Rapid determination of adenosine in Cordyceps by online extraction HPLC. J. Chromatogr. Sci. 2019, 57, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.W.; Wang, J.K.; Cheah, S.C.; Naidu, M.; David, P.; Sabaratnam, V. A review on the nucleic acid constituents in mushrooms: Nucleobases, nucleosides and nucleotides. Crit. Rev. Biotechnol. 2017, 38, 762–777. [Google Scholar] [CrossRef]
- Chan, J.S.L.; Barseghyan, G.S.; Asatiani, M.D.; Wasser, S.P. Chemical composition and medicinal value of fruiting bodies and submerged cultured mycelia of caterpillar medicinal fungus Corydceps militaris CBS-132098 (Ascomycetes). Int. J. Med. Mushrooms 2015, 17, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.F.; Cao, Z.P.; Huang, S.Y.; Yan, W.W.; Huang, J.N.; Wu, B.Y.; Li, C.H. The metaplastic effects of cordycepin in hippocampal CA1 area of rats. Eur. J. Pharmacol. 2021, 897, 173946. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Wang, J.X.; Liu, C.; Wei, S.S.; Li, G.Y.; Wang, S.H.; Meng, W.; Liu, Z.B.; Huang, L.P. Cordycepin protects against β-amyloid and ibotenic acid-induced hippocampal CA1 pyramidal neuronal hyperactivity. Korean J. Physiol. Pharmacol. 2019, 23, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Ouyang, Z. Cordycepin protects PC12 cells against 6-hydroxydopamine induced neurotoxicity via its antioxidant properties. Biomed. Pharmacother. 2016, 81, 7–14. [Google Scholar] [CrossRef]
- Yan, Q.B.; Zhang, H.D.; Hui, K.; Shao, Q. Cordycepin ameliorates intracerebral hemorrhage induced neurological and cognitive impairment through reducing anti-oxidative stress in a mouse model. J. Stroke Cerebrovasc. Dis. 2022, 31, 106199. [Google Scholar] [CrossRef]
- Wei, P.J.; Wang, K.; Luo, C.; Huang, Y.C.; Misilimu, D.; Wen, H.M.; Jin, P.; Li, C.H.; Gong, Y.; Gao, Y.Q. Cordycepin confers long-term neuroprotection via inhibiting neutrophil infiltration and neuroinflammation after traumatic brain injury. J. Neuroinflamm. 2021, 18, 137. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Wei, Y.X.; Yang, W.L.; Song, Y.Y.; Shang, H.B.; Cai, Y.; Wu, Z.B.; Zhao, W.G. Cordycepin confers neuroprotection in mice models of intracerebral hemorrhage via suppressing NLRP3 inflammasome activation. Metab. Brain Dis. 2017, 32, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.; Feng, Y.; Olatunji, O.O.; Tang, J.; Ouyang, Z.; Su, Z.L.; Wang, D.J.; Yu, X.F. Neuroprotective effects of adenosine isolated from Cordyceps cicadae against oxidative and ER stress damages induced by glutamate in PC12 cells. Environ. Toxicol. Pharmacol. 2016, 44, 53–61. [Google Scholar] [CrossRef]
- Phan, C.W.; David, P.; Wong, K.H.; Naidu, M.; Sabaratnam, V. Uridine from Pleurotus giganteus and its neurite outgrowth stimulatory effects with underlying mechanism. PLoS ONE 2015, 10, e0143004. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, N.; Obuchi, T.; Tamai, M.; Araki, H.; Omura, S.; Yang, J.S.; Yu, D.Q.; Liang, X.T.; Huan, J.H. A novel N6-substituted adenosine isolated from mi huan jun (Armillaria mellea) as a cerebral-protecting compound. Planta Med. 1990, 56, 48–52. [Google Scholar] [CrossRef]
- Lowe, H.; Toyang, N.; Steele, B.; Valentine, H.; Grant, J.; Ali, A.; Ngwa, W.; Gordon, L. The therapeutic potential of psilocybin. Molecules 2021, 26, 2948. [Google Scholar] [CrossRef]
- Zorrilla, J.G.; Evidente, A. Structures and biological activities of alkaloids produced by mushrooms, a fungal subgroup. Biomolecules 2022, 12, 1025. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Hui, S.; Zhang, A.H.; Xu, H.Y.; Yan, G.L.; Han, Y.; Wang, X.J. Natural alkaloids: Basic aspects, biological roles, and future perspectives. Chin. J. Nat. Med. 2014, 12, 401–406. [Google Scholar] [CrossRef]
- Wittstein, K.; Rascher, M.; Rupcic, Z.; Löwen, E.; Winter, B.; Köster, R.W.; Stadler, M. Corallocins A-C, nerve growth and brain-derived neurotrophic factor inducing metabolites from the mushroom Hericium coralloides. J. Nat. Prod. 2016, 79, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.C.; Hu, M.G.; Sun, Z.H.; Zhu, N.L.; Yang, J.S.; Ma, G.X.; Xu, X.D. Pyrrole alkaloids from the edible mushroom Phlebopus portentosus with their bioactive activities. Molecules 2018, 23, 1198. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.H.; Hong, S.M.; Khan, Z.; Lee, S.K.; Vishwanath, M.; Turk, A.; Yeon, S.W.; Jo, Y.H.; Lee, D.H.; Lee, J.K. Neurotrophic isoindolinones from the fruiting bodies of Hericium erinaceus. Bioorg. Med. Chem. Lett. 2021, 31, 127714. [Google Scholar] [CrossRef]
- Lee, I.K.; Yun, B.S.; Han, G.; Cho, D.H.; Kim, Y.H.; Yoo, I.D. Dictyoquinazaols A, B, and C, new neuroprotective compounds from mushroom Dictyophora indusiata. J. Nat. Prod. 2002, 65, 1769–1772. [Google Scholar] [CrossRef]
- Geissler, T.; Brandt, W.; Porzel, A.; Schlenzing, D.; Kehlen, A.; Wessjohann, L.; Arnold, N. Acetylcholinesterase inhibitors from the toadstool Cortinarius infractus. Bioorg. Med. Chem. 2010, 18, 2173–2177. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, Y.; Qin, F.Y.; Yan, Y.M.; Cheng, Y.X. Meroterpenoids and alkaloids from Ganoderma australe. Nat. Prod. Res. 2019, 35, 3226–3232. [Google Scholar] [CrossRef]
- Vishwanath, M.; Chaudhary, C.L.; Park, Y.; Viji, M.; Jung, C.; Lee, K.; Sim, J.; Hong, S.M.; Yoon, D.H.; Lee, D.H.; et al. Total synthesis of isohericerinol A and its analogues to access their potential neurotrophic effects. J. Org. Chem. 2022, 87, 10836–10847. [Google Scholar] [CrossRef]
- Lizarme, Y.; Wangsahardja, J.; Marcolin, G.M.; Morris, J.C.; Jones, N.M.; Hunter, L. Synthesis and neuroprotective activity of dictyoquinazol A and analogues. Bioorg. Med. Chem. 2016, 24, 1480–1487. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Chen, H.P.; Wu, B.; Zhang, L.; Li, Z.H.; Feng, T.; Liu, J.K. Matsutakone and matsutoic acid, two (Nor) steroids with unusual skeletons from the edible mushroom Tricholoma matsutake. J. Org. Chem. 2017, 82, 7974–7979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Yin, X.; Cao, C.Y.; Wei, J.; Zhang, Q.; Gao, J.M. Chemical constituents from Hericium erinaceus and their ability to stimulate NGF-mediated neurite outgrowth on PC12 cells. Bioorg. Med. Chem. Lett. 2015, 25, 5078–5082. [Google Scholar] [CrossRef] [PubMed]
- Meza-Menchaca, T.; Poblete-Naredo, I.; Albores-Medina, A.; Pedraza-Chaverri, J.; Quiroz-Figueroa, F.R.; Cruz-Gregorio, A.; Zepeda, R.C.; Melgar-Lalanne, G.; Lagunes, I.; Trigos, Á. Ergosterol peroxide isolated from oyster medicinal mushroom, Pleurotus ostreatus (Agaricomycetes), potentially induces radiosensitivity in cervical cancer. Int. J. Med. Mushrooms 2020, 22, 1109–1119. [Google Scholar] [CrossRef]
- Kang, J.H.; Jang, J.E.; Mishra, S.K.; Lee, H.J.; Nho, C.W.; Shin, D.; Jin, M.; Kim, M.K.; Choi, C.; Oh, S.H. Ergosterol peroxide from Chaga mushroom (Inonotus obliquus) exhibits anticancer activity by down-regulation of the β-catenin pathway in colorectal cancer. J. Ethnopharmacol. 2015, 173, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.U.; Park, Y.J. Ergosterol peroxide from the medicinal mushroom Ganoderma lucidum inhibits differentiation and lipid accumulation of 3T3-L1 adipocytes. Int. J. Mol. Sci. 2020, 21, 460. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.Y.; Zhang, Y.; Jiang, H.Q.; Ren, W.J.; Xu, L.C.; Zhang, Y.Q.; Liu, Y.H. Benzofuran derivatives with nerve growth factor-potentiating activity from Phellinus ribis. Nat. Prod. Res. 2021, 35, 5145–5152. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Di, X.; Bian, B.; Li, K.; Guo, J. Neuroprotective effects of Poria cocos (Agaricomycetes) essential oil on Aβ1-40-induced learning and memory deficit in rats. Int. J. Med. Mushrooms 2022, 24, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, R.; Sun, K.Y.; Bai, Y.Y.; Zhang, Z.; Zhou, L.B.; Qi, Z.; Qi, J.H.; Chen, L. Cerebroside-A provides potent neuroprotection after cerebral ischaemia through reducing glutamate release and Ca2+ influx of NMDA receptors. Int. J. Neuropasychopharmacol. 2012, 15, 497–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Z.; Chu, G.; Wan, C.; Wang, Q.; Yang, J.; Meng, Z.; Du, L.; Yang, J.; Ma, H. Multiple Metabolites Derived from Mushrooms and Their Beneficial Effect on Alzheimer’s Diseases. Nutrients 2023, 15, 2758. https://doi.org/10.3390/nu15122758
Tong Z, Chu G, Wan C, Wang Q, Yang J, Meng Z, Du L, Yang J, Ma H. Multiple Metabolites Derived from Mushrooms and Their Beneficial Effect on Alzheimer’s Diseases. Nutrients. 2023; 15(12):2758. https://doi.org/10.3390/nu15122758
Chicago/Turabian StyleTong, Zijian, Guodong Chu, Chenmeng Wan, Qiaoyu Wang, Jialing Yang, Zhaoli Meng, Linna Du, Jing Yang, and Hongxia Ma. 2023. "Multiple Metabolites Derived from Mushrooms and Their Beneficial Effect on Alzheimer’s Diseases" Nutrients 15, no. 12: 2758. https://doi.org/10.3390/nu15122758
APA StyleTong, Z., Chu, G., Wan, C., Wang, Q., Yang, J., Meng, Z., Du, L., Yang, J., & Ma, H. (2023). Multiple Metabolites Derived from Mushrooms and Their Beneficial Effect on Alzheimer’s Diseases. Nutrients, 15(12), 2758. https://doi.org/10.3390/nu15122758





