Human Milk Oligosaccharides in Maternal Serum Respond to Oral Glucose Load and Are Associated with Insulin Sensitivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anthropometrics
2.3. Oral Glucose Tolerance Test (OGTT)
2.4. Human Milk Oligosaccharides Standards
2.5. HMO Isolation and Analysis by HPLC
2.6. Analysis of Glucometabolic Parameters
2.7. Statistics
3. Results
3.1. Study Characteristics
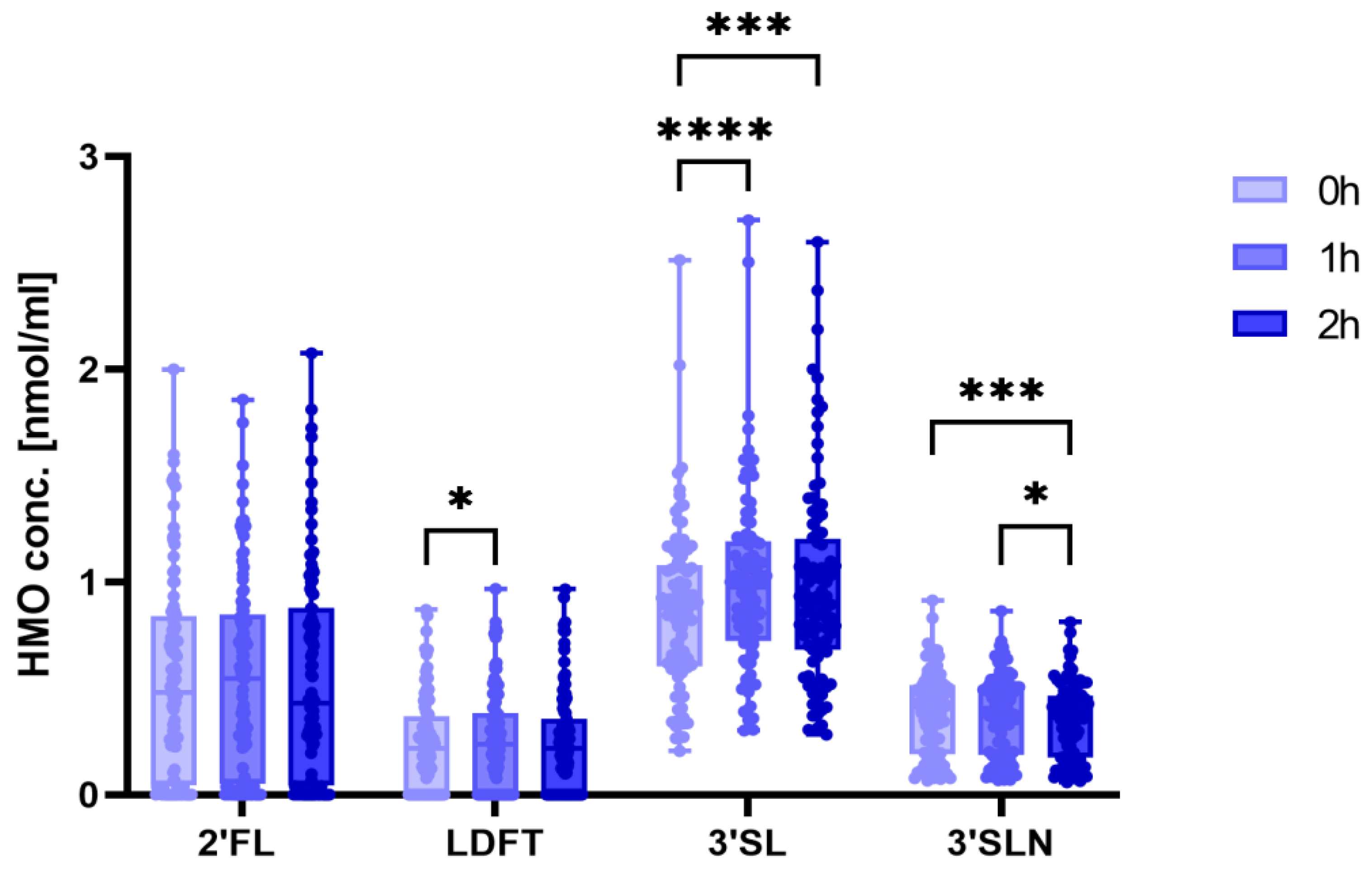
3.2. 3′SL Concentrations Increase during the OGTT
3.3. Sialylated HMOs Are Associated with Glucose Tolerance and Insulin Sensitivity
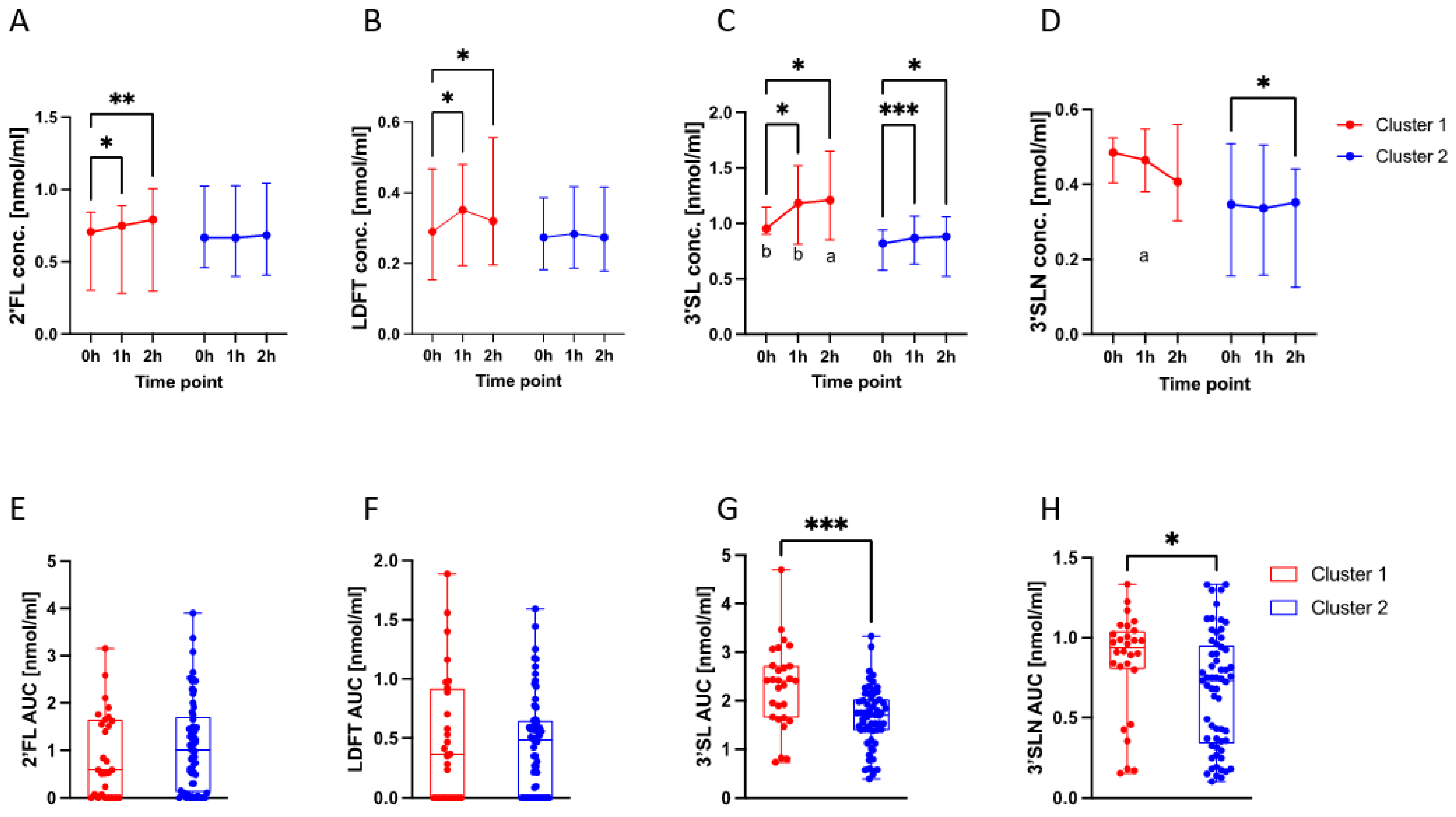
3.4. HMO Dynamics in Different Metabolic Profiles
3.5. Correlations of 3′SL and 3′SLN with Glucometabolic Indices Differed per Cluster
4. Discussion
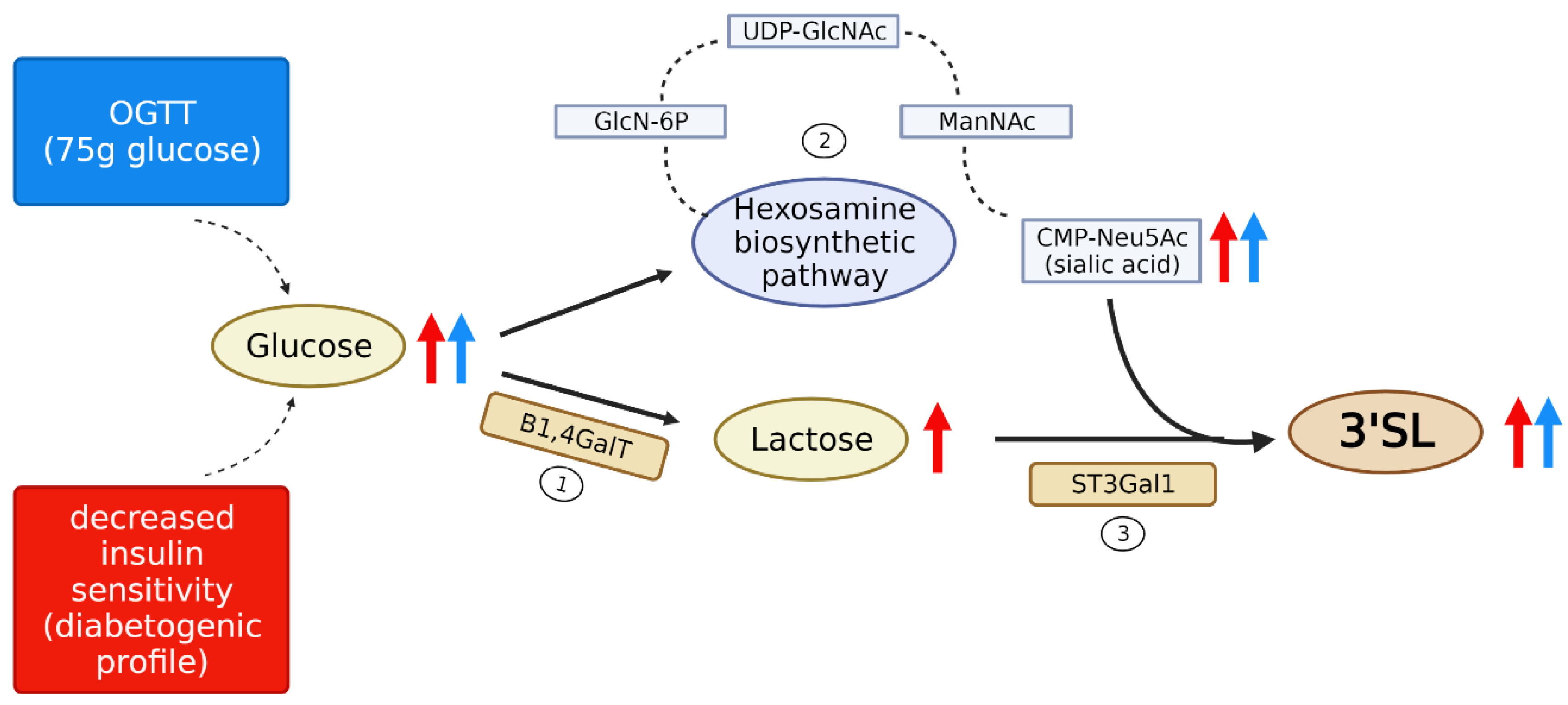
4.1. Kinetics and Mechanisms of the HMO Changes Post Glucose Load
4.2. Association of Sialylated Oligosaccharides with a Diabetogenic Metabolic Profile
4.3. Potential Roles for 3′SL as Signaling Molecules in Pregnancy
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HMO | Human Milk Oligosaccharide |
| OGTT | Oral Glucose Tolerance Test |
| GDM | Gestational Diabetes Mellitus |
| HPLC | High-performance Liquid Chromatography |
| SPE | Solid Phase Extraction |
| 2-AB | 2-aminobenzamide |
| HOMA | Homeostasis Model Assessment |
| IC | Insulin Clearance |
| ODI | Oral Disposition Index |
| AUC | Area Under the Curve |
| 2‘FL | 2′Fucosyllactose |
| LDFT | Lactodifucotetraose |
| 3′SL | 3′Sialyllactose |
| 3′SLN | 3′Sialyl-N-Acetyllactosamine |
| SAT | Subcutaneous Adipose Tissue |
| BMI | Body Mass Index |
| IQR | Interquartile Range |
| SA | Sialic Acid |
| TSA | Total Sialic Acid |
| HBP | Hexosamine Biosynthetic Pathway |
| GlcNAc | N-Acetylglucsosamine |
| Neu5Ac | N-Acetylneuraminic Acid |
| B1,4GalT | β-1,4 Galactosyltransferase |
| ST3GAL1 | Sialyltransferase 3 |
| IGT | Impaired Glucose Tolerance |
References
- Catalano, P.M.; Huston, L.; Amini, S.B.; Kalhan, S.C. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am. J. Obstet. Gynecol. 1999, 180, 903–916. [Google Scholar] [CrossRef]
- Nolan, C.J.; Proietto, J. The feto-placental glucose steal phenomenon is a major cause of maternal metabolic adaptation during late pregnancy in the rat. Diabetologia 1994, 37, 976–984. [Google Scholar] [CrossRef]
- Desoye, G.; Schweditsch, M.O.; Pfeiffer, K.P.; Zechner, R.; Kostner, G.M. Correlation of hormones with lipid and lipoprotein levels during normal pregnancy and postpartum. J. Clin. Endocrinol. Metab. 1987, 64, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Jantscher-Krenn, E.; Aigner, J.; Reiter, B.; Kofeler, H.; Csapo, B.; Desoye, G.; Bode, L.; van Poppel, M.N.M. Evidence of human milk oligosaccharides in maternal circulation already during pregnancy: A pilot study. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E347–E357. [Google Scholar] [CrossRef]
- Hirschmugl, B.; Brandl, W.; Csapo, B.; van Poppel, M.; Kofeler, H.; Desoye, G.; Wadsack, C.; Jantscher-Krenn, E. Evidence of Human Milk Oligosaccharides in Cord Blood and Maternal-to-Fetal Transport across the Placenta. Nutrients 2019, 11, 2640. [Google Scholar] [CrossRef] [PubMed]
- Jantscher-Krenn, E.; von Schirnding, L.; Trotzmuller, M.; Kofeler, H.; Kurtovic, U.; Fluhr, H.; Muller, A.; Bagci, S. Human Milk Oligosaccharides Are Present in Amniotic Fluid and Show Specific Patterns Dependent on Gestational Age. Nutrients 2022, 14, 2065. [Google Scholar] [CrossRef] [PubMed]
- Sadovnikova, A.; Garcia, S.C.; Hovey, R.C. A Comparative Review of the Cell Biology, Biochemistry, and Genetics of Lactose Synthesis. J. Mammary Gland Biol. Neoplasia 2021, 26, 181–196. [Google Scholar] [CrossRef]
- Lars Bode, E.J.-K. Structure-Function Relationships of Human Milk Oligosaccharides. Adv. Nutr. 2021, 3, 383S–391S. [Google Scholar] [CrossRef]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr. Rev. 2015, 73, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Donovan, S.M.; Comstock, S.S. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann. Nutr. Metab. 2016, 69 (Suppl. 2), 42–51. [Google Scholar] [CrossRef] [PubMed]
- Jantscher-Krenn, E.; Zherebtsov, M.; Nissan, C.; Goth, K.; Guner, Y.S.; Naidu, N.; Choudhury, B.; Grishin, A.V.; Ford, H.R.; Bode, L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012, 61, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Zuurveld, M.; van Witzenburg, N.P.; Garssen, J.; Folkerts, G.; Stahl, B.; Van’t Land, B.; Willemsen, L.E.M. Immunomodulation by Human Milk Oligosaccharides: The Potential Role in Prevention of Allergic Diseases. Front. Immunol. 2020, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Van’t Land, B.; Engen, P.A.; Naqib, A.; Green, S.J.; Nato, A.; Leusink-Muis, T.; Garssen, J.; Keshavarzian, A.; Stahl, B.; et al. Human milk oligosaccharides protect against the development of autoimmune diabetes in NOD-mice. Sci. Rep. 2018, 8, 3829. [Google Scholar] [CrossRef]
- Bhargava, P.; Li, C.; Stanya, K.J.; Jacobi, D.; Dai, L.; Liu, S.; Gangl, M.R.; Harn, D.A.; Lee, C.H. Immunomodulatory glycan LNFPIII alleviates hepatosteatosis and insulin resistance through direct and indirect control of metabolic pathways. Nat. Med. 2012, 18, 1665–1672. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Hoch, D.; Brandl, W.; Strutz, J.; Kofeler, H.C.; van Poppel, M.N.M.; Bode, L.; Hiden, U.; Desoye, G.; Jantscher-Krenn, E. Human Milk Oligosaccharides in Cord Blood Are Altered in Gestational Diabetes and Stimulate Feto-Placental Angiogenesis In Vitro. Nutrients 2021, 13, 4257. [Google Scholar] [CrossRef]
- Jantscher-Krenn, E.; Treichler, C.; Brandl, W.; Schonbacher, L.; Kofeler, H.; van Poppel, M.N.M. The association of human milk oligosaccharides with glucose metabolism in overweight and obese pregnant women. Am. J. Clin. Nutr. 2019, 110, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- De Biasio, P.; Siccardi, M.; Volpe, G.; Famularo, L.; Santi, F.; Canini, S. First-trimester screening for Down syndrome using nuchal translucency measurement with free beta-hCG and PAPP-A between 10 and 13 weeks of pregnancy—The combined test. Prenat. Diagn. 1999, 19, 360–363. [Google Scholar] [CrossRef]
- Moller, R.; Tafeit, E.; Pieber, T.R.; Sudi, K.; Reibnegger, G. Measurement of subcutaneous adipose tissue topography (SAT-Top) by means of a new optical device, LIPOMETER, and the evaluation of standard factor coefficients in healthy subjects. Am. J. Hum. Biol. 2000, 12, 231–239. [Google Scholar] [CrossRef]
- Gelaye, B.; Clish, C.B.; Denis, M.; Larrabure, G.; Tadesse, M.G.; Deik, A.; Pierce, K.; Bullock, K.; Dennis, C.; Enquobahrie, D.A.; et al. Metabolomics signatures associated with an oral glucose challenge in pregnant women. Diabetes Metab. 2019, 45, 39–46. [Google Scholar] [CrossRef]
- Yang, W.; Braun, J.M.; Vuong, A.M.; Percy, Z.; Xu, Y.; Xie, C.; Deka, R.; Calafat, A.M.; Ospina, M.; Yolton, K.; et al. Maternal urinary organophosphate ester metabolite concentrations and glucose tolerance during pregnancy: The HOME Study. Int. J. Hyg. Environ. Health 2022, 245, 114026. [Google Scholar] [CrossRef]
- Haslam, D.E.; Li, J.; Liang, L.; Martinez, M.; Palacios, C.; Trak-Fellermeier, M.A.; Franks, P.W.; Joshipura, K.; Bhupathiraju, S.N. Changes in Metabolites During an Oral Glucose Tolerance Test in Early and Mid-Pregnancy: Findings from the PEARLS Randomized, Controlled Lifestyle Trial. Metabolites 2020, 10, 284. [Google Scholar] [CrossRef]
- Madon, R.J.; Martin, S.; Davies, A.; Fawcett, H.A.; Flint, D.J.; Baldwin, S.A. Identification and characterization of glucose transport proteins in plasma membrane- and Golgi vesicle-enriched fractions prepared from lactating rat mammary gland. Biochem. J. 1990, 272, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Q. Biology of glucose transport in the mammary gland. J. Mammary Gland Biol. Neoplasia 2014, 19, 3–17. [Google Scholar] [CrossRef]
- Neville, M.C.; Sawicki, V.S.; Hay, W.W., Jr. Effects of fasting, elevated plasma glucose and plasma insulin concentrations on milk secretion in women. J. Endocrinol. 1993, 139, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Meledeo, M.A.; Wang, Z.; Khanna, H.S.; Paruchuri, V.D.; Yarema, K.J. Metabolic glycoengineering: Sialic acid and beyond. Glycobiology 2009, 19, 1382–1401. [Google Scholar] [CrossRef] [PubMed]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Akella, N.M.; Ciraku, L.; Reginato, M.J. Fueling the fire: Emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 2019, 17, 52. [Google Scholar] [CrossRef]
- Inafuku, S.; Noda, K.; Amano, M.; Ohashi, T.; Yoshizawa, C.; Saito, W.; Murata, M.; Kanda, A.; Nishimura, S.; Ishida, S. Alteration of N-Glycan Profiles in Diabetic Retinopathy. Invest. Ophthalmol. Vis. Sci. 2015, 56, 5316–5322. [Google Scholar] [CrossRef]
- Selma-Royo, M.; Gonzalez, S.; Gueimonde, M.; Chang, M.; Furst, A.; Martinez-Costa, C.; Bode, L.; Collado, M.C. Maternal Diet Is Associated with Human Milk Oligosaccharide Profile. Mol. Nutr. Food Res. 2022, 66, e2200058. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, M.D.; Mohammad, M.; Pace, R.M.; Engevik, M.; Versalovic, J.; Bode, L.; Haymond, M.; Aagaard, K.M. Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci. Rep. 2020, 10, 22092. [Google Scholar] [CrossRef]
- Berger, P.K.; Hampson, H.E.; Schmidt, K.A.; Alderete, T.L.; Furst, A.; Yonemitsu, C.; Demerath, E.; Goran, M.I.; Fields, D.A.; Bode, L. Stability of Human-Milk Oligosaccharide Concentrations Over 1 Week of Lactation and Over 6 Hours Following a Standard Meal. J. Nutr. 2023, 152, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, K.; Liu, J. Effects of glucose availability on expression of the key genes involved in synthesis of milk fat, lactose and glucose metabolism in bovine mammary epithelial cells. PLoS ONE 2013, 8, e66092. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Chen, Z.; Zhang, L.; Wang, J.; Gao, H.; Wu, X.; Natarajan, R. RNA-sequencing analysis of high glucose-treated monocytes reveals novel transcriptome signatures and associated epigenetic profiles. Physiol. Genomics 2013, 45, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, G.; Rastam, L.; Gullberg, B.; Eklund, G.A. Serum sialic acid concentration predicts both coronary heart disease and stroke mortality: Multivariate analysis including 54,385 men and women during 20.5 years follow-up. Int. J. Epidemiol. 1992, 21, 253–257. [Google Scholar] [CrossRef]
- Lindberg, G.; Eklund, G.A.; Gullberg, B.; Rastam, L. Serum sialic acid concentration and cardiovascular mortality. BMJ 1991, 302, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Crook, M.A.; Tutt, P.; Simpson, H.; Pickup, J.C. Serum sialic acid and acute phase proteins in type 1 and type 2 diabetes mellitus. Clin. Chim. Acta 1993, 219, 131–138. [Google Scholar] [CrossRef]
- Crook, M.A.; Tutt, P.; Pickup, J.C. Elevated serum sialic acid concentration in NIDDM and its relationship to blood pressure and retinopathy. Diabetes Care 1993, 16, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Abdella, N.; Akanji, A.O.; Mojiminiyi, O.A.; Al Assoussi, A.; Moussa, M. Relation of serum total sialic acid concentrations with diabetic complications and cardiovascular risk factors in Kuwaiti Type 2 diabetic patients. Diabetes Res. Clin. Pract. 2000, 50, 65–72. [Google Scholar] [CrossRef]
- Gavella, M.; Lipovac, V.; Car, A.; Vucic, M.; Sokolic, L.; Rakos, R. Serum sialic acid in subjects with impaired glucose tolerance and in newly diagnosed type 2 diabetic patients. Acta Diabetol. 2003, 40, 95–100. [Google Scholar] [CrossRef]
- Findik, R.B.; Yilmaz, F.M.; Yilmaz, G.; Yilmaz, H.; Karakaya, J. Sialic acid levels in the blood in pregnant women with impaired glucose tolerance test. Arch. Gynecol. Obstet. 2012, 286, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Abdulkadir, A.; Onojah, A.; Sani, L.; Adamu, A.; Abdullahi, H. Modulation of sialic acid levels among some organs during insulin resistance or hyperglycemic states. Mol. Cell Biochem. 2016, 411, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.N.; Nath, N. Sialic acid content and sialidase activity of polymorphonuclear leucocytes in diabetes mellitus. Am. J. Med. Sci. 1984, 288, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Pessentheiner, A.; Spann, N.; Collins, C.; Ramms, B.; Chiang, A.; Wang, Y.; Quach, A.; Booshehri, L.; Hammond, A.; Gordts, P.; et al. The Human Milk Oligosaccharide 3′Sialyllactose Promotes Inflammation Resolution and Reduces Atherosclerosis Development in Mice. Atherosclerosis 2023, 331, e31. [Google Scholar] [CrossRef]
- Angeloni, S.; Ridet, J.L.; Kusy, N.; Gao, H.; Crevoisier, F.; Guinchard, S.; Kochhar, S.; Sigrist, H.; Sprenger, N. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology 2005, 15, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.J.; Kwon, E.S.; Lee, K.M.; Cho, C.; Lee, J.I.; Ryu, Y.B.; Youm, T.H.; Jeon, J.; Cho, M.R.; Jeong, S.Y.; et al. 3′-Sialyllactose as an inhibitor of p65 phosphorylation ameliorates the progression of experimental rheumatoid arthritis. Br. J. Pharmacol. 2018, 175, 4295–4309. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.J.; Oh, E.; Cho, C.; Kwon, H.; Lee, C.G.; Jeon, J.; Lee, H.; Choi, S.; Han, S.J.; Nam, J.; et al. 3’-Sialyllactose prebiotics prevents skin inflammation via regulatory T cell differentiation in atopic dermatitis mouse models. Sci. Rep. 2020, 10, 5603. [Google Scholar] [CrossRef]
- Hedo, J.A.; Kasuga, M.; Van Obberghen, E.; Roth, J.; Kahn, C.R. Direct demonstration of glycosylation of insulin receptor subunits by biosynthetic and external labeling: Evidence for heterogeneity. Proc. Natl. Acad. Sci. USA 1981, 78, 4791–4795. [Google Scholar] [CrossRef]
- Sparrow, L.G.; Lawrence, M.C.; Gorman, J.J.; Strike, P.M.; Robinson, C.P.; McKern, N.M.; Ward, C.W. N-linked glycans of the human insulin receptor and their distribution over the crystal structure. Proteins 2008, 71, 426–439. [Google Scholar] [CrossRef]
- Duncan, B.B.; Schmidt, M.I.; Pankow, J.S.; Ballantyne, C.M.; Couper, D.; Vigo, A.; Hoogeveen, R.; Folsom, A.R.; Heiss, G.; Atherosclerosis Risk in Communities Study. Low-grade systemic inflammation and the development of type 2 diabetes: The atherosclerosis risk in communities study. Diabetes 2003, 52, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Chassard, C.; Hausmann, M.; von Itzstein, M.; Hennet, T. Sialic acid catabolism drives intestinal inflammation and microbial dysbiosis in mice. Nat. Commun. 2015, 6, 8141. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, V.S.; Pillai, S. Sialic acids and autoimmune disease. Immunol. Rev. 2016, 269, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Fougerat, A.; Pan, X.; Smutova, V.; Heveker, N.; Cairo, C.W.; Issad, T.; Larrivee, B.; Medin, J.A.; Pshezhetsky, A.V. Neuraminidase 1 activates insulin receptor and reverses insulin resistance in obese mice. Mol. Metab. 2018, 12, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Pietzner, M.; Kaul, A.; Henning, A.K.; Kastenmuller, G.; Artati, A.; Lerch, M.M.; Adamski, J.; Nauck, M.; Friedrich, N. Comprehensive metabolic profiling of chronic low-grade inflammation among generally healthy individuals. BMC Med. 2017, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y. Postprandial C-Peptide to Glucose Ratio as a Marker of beta Cell Function: Implication for the Management of Type 2 Diabetes. Int. J. Mol. Sci. 2016, 17, 744. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Huston-Presley, L.; Kalhan, S.C.; Catalano, P.M. Clinically useful estimates of insulin sensitivity during pregnancy: Validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care 2001, 24, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, T.; Farrell, K.A.; Huston-Presley, L.; Amini, S.B.; Kirwan, J.P.; McIntyre, H.D.; Catalano, P.M. Estimates of insulin sensitivity using glucose and C-Peptide from the hyperglycemia and adverse pregnancy outcome glucose tolerance test. Diabetes Care 2010, 33, 490–494. [Google Scholar] [CrossRef]
- Patarrão, R.S.; Lautt, W.W.; Macedo, M.P. Assessment of methods and indexes of insulin sensitivity. Rev. Port. Endocrinol. Diabetes Metab. 2014, 9, 65–73. [Google Scholar] [CrossRef]
- Kim, J.D.; Kang, S.J.; Lee, M.K.; Park, S.E.; Rhee, E.J.; Park, C.Y.; Oh, K.W.; Park, S.W.; Lee, W.Y. C-Peptide-Based Index Is More Related to Incident Type 2 Diabetes in Non-Diabetic Subjects than Insulin-Based Index. Endocrinol. Metab. 2016, 31, 320–327. [Google Scholar] [CrossRef]
- Álvarez-Nava, F.; Bastidas, D.; Racines-Orbe, M.; Guarderas, J. Insulin Sensitivity and Pancreatic beta-Cell Function in Ecuadorian Women with Turner Syndrome. Front. Endocrinol. 2020, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- De Fronzo, R.A.; Matsuda, M. Reduced time points to calculate the composite index. Diabetes Care 2010, 33, e93. [Google Scholar] [CrossRef] [PubMed]
- Semnani-Azad, Z.; Johnston, L.W.; Lee, C.; Retnakaran, R.; Connelly, P.W.; Harris, S.B.; Zinman, B.; Hanley, A.J. Determinants of longitudinal change in insulin clearance: The Prospective Metabolism and Islet Cell Evaluation cohort. BMJ Open Diabetes Res. Care 2019, 7, e000825. [Google Scholar] [CrossRef] [PubMed]

| Maternal Parameters | N | Mean ± SD |
|---|---|---|
| Age (years) | 99 | 35.3 ± 4.2 |
| Height (cm) | 99 | 167.5 ± 6.7 |
| Weight (kg, pre-pregnancy) | 99 | 65.3 ± 12.5 |
| BMI (kg/m2, pre-pregnancy) | 99 | 23.3 ± 4.7 |
| Weight (kg, delivery) | 86 | 79.9 ± 13.2 |
| BMI (kg/m2, delivery) | 87 | 28.4 ± 4.8 |
| Weight gain (kg) | 84 | 14.2 ± 4.9 |
| Parity (% nulliparous) | 97 | 6.2 |
| Metabolic Parameters | N | Mean ± SD |
|---|---|---|
| Fasting Glucose (mg/dL) | 98 | 77.7 ± 5.7 |
| 1 h Glucose (mg/dL) | 97 | 119.5 ± 28.1 |
| 2 h Glucose (mg/dL) | 95 | 105.6 ± 24.3 |
| AUC 1 Glucose 0–2 h | 94 | 209.0 ± 38.4 |
| Fasting Insulin (mU/L) | 98 | 8.2 ± 4.0 |
| 1 h Insulin (mU/L) | 97 | 74.1 ± 44.1 |
| 2 h Insulin (mU/L) | 96 | 56.4 ± 37.3 |
| AUC 1 Insulin 0–2 h | 94 | 101.2 ± 47.2 |
| Fasting C-Peptide (ng/mL) | 98 | 1.1 ± 0.4 |
| 1 h C-Peptide (ng/mL) | 97 | 6.5 ± 2.3 |
| 2 h C-Peptide (ng/mL) | 96 | 5.9 ± 2.3 |
| AUC 1 C-Peptide 0–2 h | 93 | 9.8 ± 3.0 |
| Glucometabolic Indices 1 | N | Mean± SD |
| HOMA-IR Index | 97 | 1.6 ± 0.8 |
| HOMA-IR Index (C-Peptide) | 97 | 0.07 ± 0.03 |
| HOMA-β | 97 | 224.0 ± 127.1 |
| Matsuda Index | 93 | 7.3 ± 3.5 |
| Matsuda Index (C-Peptide) | 93 | 2819.9 ± 966.4 |
| C-Peptide Index 1 | 97 | 1.5 ± 0.5 |
| C-Peptide Index 2 | 93 | 11.0 ± 56.2 |
| Insulin Clearance (fasting) | 98 | 7.2 ± 2.0 |
| Insulin Clearance (AUC) | 93 | 0.11 ± 0.03 |
| Insulinogenic Index | 96 | 256.7 ± 246.3 |
| C-Peptidogenic Index | 96 | 1.11 ± 1.14 |
| Oral Disposition Index (Insulin) | 96 | 188.0 ± 184.9 |
| Oral Disposition Index (C-Peptide) | 96 | 16.8 ± 18.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiser-Fuchs, M.-T.; Maggauer, E.; van Poppel, M.N.M.; Csapo, B.; Desoye, G.; Köfeler, H.C.; Groselj-Strele, A.; Trajanoski, S.; Fluhr, H.; Obermayer-Pietsch, B.; et al. Human Milk Oligosaccharides in Maternal Serum Respond to Oral Glucose Load and Are Associated with Insulin Sensitivity. Nutrients 2023, 15, 4042. https://doi.org/10.3390/nu15184042
Weiser-Fuchs M-T, Maggauer E, van Poppel MNM, Csapo B, Desoye G, Köfeler HC, Groselj-Strele A, Trajanoski S, Fluhr H, Obermayer-Pietsch B, et al. Human Milk Oligosaccharides in Maternal Serum Respond to Oral Glucose Load and Are Associated with Insulin Sensitivity. Nutrients. 2023; 15(18):4042. https://doi.org/10.3390/nu15184042
Chicago/Turabian StyleWeiser-Fuchs, Marie-Therese, Elena Maggauer, Mireille N. M. van Poppel, Bence Csapo, Gernot Desoye, Harald C. Köfeler, Andrea Groselj-Strele, Slave Trajanoski, Herbert Fluhr, Barbara Obermayer-Pietsch, and et al. 2023. "Human Milk Oligosaccharides in Maternal Serum Respond to Oral Glucose Load and Are Associated with Insulin Sensitivity" Nutrients 15, no. 18: 4042. https://doi.org/10.3390/nu15184042
APA StyleWeiser-Fuchs, M. -T., Maggauer, E., van Poppel, M. N. M., Csapo, B., Desoye, G., Köfeler, H. C., Groselj-Strele, A., Trajanoski, S., Fluhr, H., Obermayer-Pietsch, B., & Jantscher-Krenn, E. (2023). Human Milk Oligosaccharides in Maternal Serum Respond to Oral Glucose Load and Are Associated with Insulin Sensitivity. Nutrients, 15(18), 4042. https://doi.org/10.3390/nu15184042










