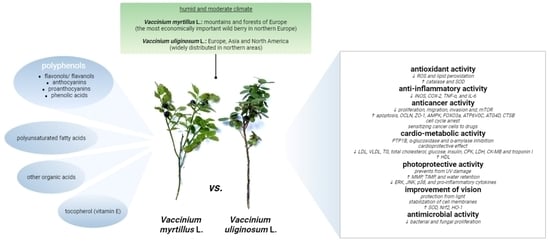
Vaccinium uliginosum and Vaccinium myrtillus—Two Species—One Used as a Functional Food
Abstract
:1. Introduction
2. Occurrence
3. V. uliginosum and V. myrtillus Composition
3.1. Polyphenols
3.1.1. Flavonols and Flavanols
3.1.2. Anthocyanins
3.1.3. Proanthocyanidins
3.1.4. Phenolic Acids
3.2. Other Organic Acids
3.3. PUFAs (Polyunsaturated Fatty Acids)
3.4. α-Tocopherol
4. Composition and Potential of Wax
5. The Use of Berries
5.1. Dermatology
5.2. Ophthalmology
5.3. Gynecology
5.4. Diabetology
5.5. Cardiology
5.6. Antimicrobial Activity
5.7. Oncology
6. Side Effects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, E.-K.; Kwon, H.-S.; Shin, S.-G.; Choi, Y.-H.; Kang, I.-J.; Chung, C.-K. Biological Effect of Vaccinium uliginosum L. on STZ-Induced Diabetes and Lipid Metabolism in Rats. J. Korean Soc. Food Sci. Nutr. 2012, 41, 1727–1733. [Google Scholar] [CrossRef]
- Fraisse, D.; Bred, A.; Felgines, C.; Senejoux, F. Stability and Antiglycoxidant Potential of Bilberry Anthocyanins in Simulated Gastrointestinal Tract Model. Foods 2020, 9, 1695. [Google Scholar] [CrossRef] [PubMed]
- Popović, D.; Đukić, D.; Katić, V.; Jović, Z.; Jović, M.; Lalić, J.; Golubović, I.; Stojanović, S.; Ulrih, N.P.; Stanković, M.; et al. Antioxidant and Proapoptotic Effects of Anthocyanins from Bilberry Extract in Rats Exposed to Hepatotoxic Effects of Carbon Tetrachloride. Life Sci. 2016, 157, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Karppinen, K.; Zoratti, L.; Nguyenquynh, N.; Häggman, H.; Jaakola, L. On the Developmental and Environmental Regulation of Secondary Metabolism in Vaccinium spp. Berries. Front. Plant Sci. 2016, 7, 655. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Wachtel-Galor, S. Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Liu, S.; Laaksonen, O.; Yang, W.; Zhang, B.; Yang, B. Pyranoanthocyanins in Bilberry (Vaccinium myrtillus L.) Wines Fermented with Schizosaccharomyces Pombe and Their Evolution during Aging. Food Chem. 2020, 305, 125438. [Google Scholar] [CrossRef] [PubMed]
- Behrends, A.; Weber, F. Influence of Different Fermentation Strategies on the Phenolic Profile of Bilberry Wine (Vaccinium myrtillus L.). J. Agric. Food Chem. 2017, 65, 7483–7490. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Wang, Q.; Li, W.; Liu, L. Effect of Vaccinium myrtillus Extract Supplement on Advanced Glycation End-Products: A Pilot Study (P06-098-19). Curr. Dev. Nutr. 2019, 3, 616. [Google Scholar] [CrossRef]
- Fraisse, D.; Bred, A.; Felgines, C.; Senejoux, F. Screening and Characterization of Antiglycoxidant Anthocyanins from Vaccinium myrtillus Fruit Using DPPH and Methylglyoxal Pre-Column HPLC Assays. Antioxidants 2020, 9, 512. [Google Scholar] [CrossRef]
- Maulik, M.; Mitra, S.; Sweeney, M.; Lu, B.; Taylor, B.E.; Bult-Ito, A. Complex Interaction of Dietary Fat and Alaskan Bog Blueberry Supplementation Influences Manganese Mediated Neurotoxicity and Behavioral Impairments. J. Funct. Foods 2019, 53, 306–317. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Chan, S.W.; Tomlinson, B. Effects of Bilberry Supplementation on Metabolic and Cardiovascular Disease Risk. Molecules 2020, 25, 1653. [Google Scholar] [CrossRef]
- Bujor, O.C.; Tanase, C.; Popa, M.E. Phenolic Antioxidants in Aerial Parts of Wild Vaccinium Species: Towards Pharmaceutical and Biological Properties. Antioxidants 2019, 8, 649. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Caleja, C.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Vaccinium myrtillus L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications—A Review. Curr. Pharm. Des. 2020, 26, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Szakiel, A.; Pa̧czkowski, C.; Huttunen, S. Triterpenoid Content of Berries and Leaves of Bilberry Vaccinium myrtillus from Finland and Poland. J. Agric. Food Chem. 2012, 60, 11839–11849. [Google Scholar] [CrossRef] [PubMed]
- Vrancheva, R.; Ivanov, I.; Dincheva, I.; Badjakov, I.; Pavlov, A. Triterpenoids and Other Non-Polar Compounds in Leaves of Wild and Cultivated Vaccinium Species. Plants 2021, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Anadon-Rosell, A.; Palacio, S.; Nogués, S.; Ninot, J.M. Vaccinium myrtillus Stands Show Similar Structure and Functioning under Different Scenarios of Coexistence at the Pyrenean Treeline. Plant Ecol. 2016, 217, 1115–1128. [Google Scholar] [CrossRef]
- Ştefanescu, B.E.; Szabo, K.; Mocan, A.; Crisan, G. Phenolic Compounds from Five Ericaceae Species Leaves and Their Related Bioavailability and Health Benefits. Molecules 2019, 24, 2046. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Sánchez-Juanes, F.; Meirinho, S.; Silva, L.R.; Alves, G.; Flores-Félix, J.D. Insight into the Taxonomic and Functional Diversity of Bacterial Communities Inhabiting Blueberries in Portugal. Microorganisms 2022, 10, 2193. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Karppinen, K.; Klavins, L.; Kviesis, J.; Sundqvist, P.; Nguyen, N.; Heinonen, E.; Klavins, M.; Jaakola, L.; Väänänen, J.; et al. Compositional and Morphological Analyses of Wax in Northern Wild Berry Species. Food Chem. 2019, 295, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Diaconeasa, Z. Time-Dependent Degradation of Polyphenols from Thermally-Processed Berries and Their In Vitro Antiproliferative Effects against Melanoma. Molecules 2018, 23, 2534. [Google Scholar] [CrossRef]
- Bujor, O.C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal Variations of the Phenolic Constituents in Bilberry (Vaccinium myrtillus L.) Leaves, Stems and Fruits, and Their Antioxidant Activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. A Comparison of Fruit Quality Parameters of Wild Bilberry (Vaccinium myrtillus L.) Growing at Different Locations. J. Sci. Food Agric. 2015, 95, 776–785. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Bobinaitė, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viškelis, P.; Ferrari, G. Application of Pulsed Electric Field in the Production of Juice and Extraction of Bioactive Compounds from Blueberry Fruits and Their By-Products. J. Food Sci. Technol. 2015, 52, 5898. [Google Scholar] [CrossRef] [PubMed]
- Raudonė, L.; Liaudanskas, M.; Vilkickytė, G.; Kviklys, D.; Žvikas, V.; Viškelis, J.; Viškelis, P. Phenolic Profiles, Antioxidant Activity and Phenotypic Characterization of Lonicera caerulea L. Berries, Cultivated in Lithuania. Antioxidants 2021, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Urbonaviciene, D.; Bobinaite, R.; Viskelis, P.; Bobinas, C.; Petruskevicius, A.; Klavins, L.; Viskelis, J. Geographic Variability of Biologically Active Compounds, Antioxidant Activity and Physico-Chemical Properties in Wild Bilberries (Vaccinium myrtillus L.). Antioxidants 2022, 11, 588. [Google Scholar] [CrossRef]
- Liudvinaviciute, D.; Rutkaite, R.; Bendoraitiene, J.; Klimaviciute, R.; Dagys, L. Formation and Characteristics of Alginate and Anthocyanin Complexes. Int. J. Biol. Macromol. 2020, 164, 726–734. [Google Scholar] [CrossRef]
- Szakiel, A.; Pa̧czkowski, C.; Koivuniemi, H.; Huttunen, S. Comparison of the Triterpenoid Content of Berries and Leaves of Lingonberry Vaccinium vitis-Idaea from Finland and Poland. J. Agric. Food Chem. 2012, 60, 4994–5002. [Google Scholar] [CrossRef]
- Mi, J.C.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid Glycosides and Antioxidant Capacity of Various Blackberry, Blueberry and Red Grape Genotypes Determined by High-Performance Liquid Chromatography/Mass Spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Može, Š.; Polak, T.; Gašperlin, L.; Koron, D.; Vanzo, A.; Poklar Ulrih, N.; Abram, V. Phenolics in Slovenian Bilberries (Vaccinium myrtillus L.) and Blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Systematic Identification and Characterization of Anthocyanins by HPLC-ESI-MS/MS in Common Foods in the United States: Fruits and Berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Lätti, A.K.; Jaakola, L.; Riihinen, K.R.; Kainulainen, P.S. Anthocyanin and Flavonol Variation in Bog Bilberries (Vaccinium uliginosum L.) in Finland. J. Agric. Food Chem. 2010, 58, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Bederska-Łojewska, D.; Pieszka, M.; Marzec, A.; Rudzińska, M.; Grygier, A.; Siger, A.; Cieślik-Boczula, K.; Orczewska-Dudek, S.; Migdał, W. Physicochemical Properties, Fatty Acid Composition, Volatile Compounds of Blueberries, Cranberries, Raspberries, and Cuckooflower Seeds Obtained Using Sonication Method. Molecules 2021, 26, 7446. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Simoes, A.; Domingues, M.R. Fruit Seeds and Their Oils as Promising Sources of Value-Added Lipids from Agro-Industrial Byproducts: Oil Content, Lipid Composition, Lipid Analysis, Biological Activity and Potential Biotechnological Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 1305–1339. [Google Scholar] [CrossRef]
- Michalska, A.; Łysiak, G. Bioactive Compounds of Blueberries: Post-Harvest Factors Influencing the Nutritional Value of Products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef] [PubMed]
- Frum, A.; Dobrea, C.M.; Rus, L.L.; Virchea, L.I.; Morgovan, C.; Chis, A.A.; Arseniu, A.M.; Butuca, A.; Gligor, F.G.; Vicas, L.G.; et al. Valorization of Grape Pomace and Berries as a New and Sustainable Dietary Supplement: Development, Characterization, and Antioxidant Activity Testing. Nutrients 2022, 14, 3065. [Google Scholar] [CrossRef] [PubMed]
- Colak, N.; Torun, H.; Gruz, J.; Strnad, M.; Hermosín-Gutiérrez, I.; Hayirlioglu-Ayaz, S.; Ayaz, F.A. Bog Bilberry Phenolics, Antioxidant Capacity and Nutrient Profile. Food Chem. 2016, 201, 339–349. [Google Scholar] [CrossRef]
- Kalt, W.; Forney, C.F.; Martin, A.; Prior, R.L. Antioxidant Capacity, Vitamin C, Phenolics, and Anthocyanins after Fresh Storage of Small Fruits. J. Agric. Food Chem. 1999, 47, 4638–4644. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, Y.; Li, Z.; Xie, X.; Gong, E.S.; Tian, J.; Si, X.; Wang, Y.; Gao, N.; Shu, C.; et al. Effects of High Hydrostatic Pressure and Thermal Processing on Anthocyanin Content, Polyphenol Oxidase and β-Glucosidase Activities, Color, and Antioxidant Activities of Blueberry (Vaccinium spp.) Puree. Food Chem. 2021, 342, 128564. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Palma, A.; Cefola, M.; Pace, B.; D’Aquino, S.; Carboni, C.; Baruzzi, F. Effect of Modified Atmosphere Packaging (MAP) and Gaseous Ozone Pre-Packaging Treatment on the Physico-Chemical, Microbiological and Sensory Quality of Small Berry Fruit. Food Packag. Shelf Life 2020, 26, 100573. [Google Scholar] [CrossRef]
- Muñoz-Fariña, O.; López-Casanova, V.; García-Figueroa, O.; Roman-Benn, A.; Ah-Hen, K.; Bastias-Montes, J.M.; Quevedo-León, R.; Ravanal-Espinosa, M.C. Bioaccessibility of Phenolic Compounds in Fresh and Dehydrated Blueberries (Vaccinium corymbosum L.). Food Chem. Adv. 2023, 2, 100171. [Google Scholar] [CrossRef]
- Maryam, A.; Anwar, R.; Malik, A.U.; Raheem, M.I.U.; Khan, A.S.; Hasan, M.U.; Hussain, Z.; Siddique, Z. Combined Aqueous Ozone and Ultrasound Application Inhibits Microbial Spoilage, Reduces Pesticide Residues and Maintains Storage Quality of Strawberry Fruits. J. Food Meas. Charact. 2021, 15, 1437–1451. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of Cold Plasma on Blueberry Juice Quality. Food Chem. 2019, 290, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, P.; Lante, A. Environmentally Friendly Techniques for the Recovery of Polyphenols from Food By-Products and Their Impact on Polyphenol Oxidase: A Critical Review. Appl. Sci. 2022, 12, 1923. [Google Scholar] [CrossRef]
- Cesa, S.; Carradori, S.; Bellagamba, G.; Locatelli, M.; Casadei, M.A.; Masci, A.; Paolicelli, P. Evaluation of Processing Effects on Anthocyanin Content and Colour Modifications of Blueberry (Vaccinium spp.) Extracts: Comparison between HPLC-DAD and CIELAB Analyses. Food Chem. 2017, 232, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda, J.; Alemán, M.D.; Gironés-Vilaplana, A.; Pérez, A.; Caravaca, G.; Figueroa, F.; Mulero, J.; Zafrilla, P. Phenolic Composition, Antioxidant Activity, and in Vitro Availability of Four Different Berries. J. Chem. 2016, 2016, 5194901. [Google Scholar] [CrossRef]
- Prencipe, F.P.; Bruni, R.; Guerrini, A.; Rossi, D.; Benvenuti, S.; Pellati, F. Metabolite Profiling of Polyphenols in Vaccinium Berries and Determination of Their Chemopreventive Properties. J. Pharm. Biomed. Anal. 2014, 89, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Su, S.; Wu, J.; Du, H.; Li, S.S.; Huo, J.W.; Zhang, Y.; Wang, L.S. Variation of Anthocyanins and Flavonols in Vaccinium uliginosum Berry in Lesser Khingan Mountains and Its Antioxidant Activity. Food Chem. 2014, 160, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Guo, Z.; Sun, B.; Zhao, Y. Identification of Anthocyanins from Four Kinds of Berries and Their Inhibition Activity to α-Glycosidase and Protein Tyrosine Phosphatase 1B by HPLC-FT-ICR MS/MS. J. Agric. Food Chem. 2017, 65, 6211–6221. [Google Scholar] [CrossRef] [PubMed]
- Hajazimi, E.; Landberg, R.; Zamaratskaia, G. Simultaneous Determination of Flavonols and Phenolic Acids by HPLC-CoulArray in Berries Common in the Nordic Diet. LWT 2016, 74, 128–134. [Google Scholar] [CrossRef]
- Levaj, B.; Dragović-Uzelac, V.; Delonga, K.; Kovačević Ganić, K.; Banović, M.; Bursać Kovačević, D. Polyphenols and Volatiles in Fruits of Two Sour Cherry Cultivars, Some Berry Fruits and Their Jams. Food Technol. Biotechnol. 2010, 48, 538–547. [Google Scholar]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC-MSn Identification and Quantification of Flavonol Glycosides in 28 Wild and Cultivated Berry Species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef]
- Sezer, E.D.; Oktay, L.M.; Karadadaş, E.; Memmedov, H.; Selvi Gunel, N.; Sözmen, E. Assessing Anticancer Potential of Blueberry Flavonoids, Quercetin, Kaempferol, and Gentisic Acid, Through Oxidative Stress and Apoptosis Parameters on HCT-116 Cells. J. Med. Food 2019, 22, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Ancillotti, C.; Ciofi, L.; Pucci, D.; Sagona, E.; Giordani, E.; Biricolti, S.; Gori, M.; Petrucci, W.A.; Giardi, F.; Bartoletti, R.; et al. Polyphenolic Profiles and Antioxidant and Antiradical Activity of Italian Berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. Subsp. Gaultherioides (Bigelow) S.B. Young. Food Chem. 2016, 204, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Wang, S.; Gu, P.; Ouyang, X.; Liu, S.; Li, Y.; Zhang, B.; Zhu, B. Comparison of Physicochemical Indexes, Amino Acids, Phenolic Compounds and Volatile Compounds in Bog Bilberry Juice Fermented by Lactobacillus plantarum under Different PH Conditions. J. Food Sci. Technol. 2018, 55, 2240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.; Chen, J.Z.; Tian, X.; Zheng, Y.H.; Hao, J.; Xue, Y.J.; Ding, S.Y.; Zong, C.W. The Variation of Total Flavonoids, Anthocyanins and Total Phenols in Vaccinium uliginosum Fruits in Changbai Mountain of China Is Closely Related to Spatial Distribution. J. Berry Res. 2022, 12, 463–481. [Google Scholar] [CrossRef]
- Kraujalyte, V.; Venskutonis, P.R.; Pukalskas, A.; Česoniene, L.; Daubaras, R. Antioxidant Properties, Phenolic Composition and Potentiometric Sensor Array Evaluation of Commercial and New Blueberry (Vaccinium corymbosum) and Bog Blueberry (Vaccinium uliginosum) Genotypes. Food Chem. 2015, 188, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Bayazid, A.B.; Chun, E.M.; Al Mijan, M.; Park, S.H.; Moon, S.K.; Lim, B.O. Anthocyanins Profiling of Bilberry (Vaccinium myrtillus L.) Extract That Elucidates Antioxidant and Anti-Inflammatory Effects. Food Agric. Immunol. 2021, 32, 713–726. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, Y.; Liu, D.; Liu, D.; Zhang, C.; Qi, H.; Gu, H.; Yang, L.; Zhou, Z. Efficient Homogenization-Ultrasound-Assisted Extraction of Anthocyanins and Flavonols from Bog Bilberry (Vaccinium uliginosum L.) Marc with Carnosic Acid as an Antioxidant Additive. Molecules 2019, 24, 2537. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P. Berry Fruits: Compositional Elements, Biochemical Activities, and the Impact of Their Intake on Human Health, Performance, and Disease. J. Agric. Food Chem. 2008, 56, 627–629. [Google Scholar] [CrossRef]
- Holkem, A.T.; Robichaud, V.; Favaro-Trindade, C.S.; Lacroix, M. Chemopreventive Properties of Extracts Obtained from Blueberry (Vaccinium myrtillus L.) and Jabuticaba (Myrciaria cauliflora Berg.) in Combination with Probiotics. Nutr. Cancer 2021, 73, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, G.; González-Burgos, E.; Smith, C.; López, V.; Gómez-Serranillos, M.P. Regulation of Redox Status in Neuronal SH-SY5Y Cells by Blueberry (Vaccinium myrtillus L.) Juice, Cranberry (Vaccinium macrocarpon A.) Juice and Cyanidin. Food Chem. Toxicol. 2018, 118, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lee, H.J. Anti-Inflammatory Activity of Bilberry (Vaccinium myrtillus L.). Curr. Issues Mol. Biol. 2022, 44, 4570–4583. [Google Scholar] [CrossRef]
- Piberger, H.; Oehme, A.; Hofmann, C.; Dreiseitel, A.; Sand, P.G.; Obermeier, F.; Schoelmerich, J.; Schreier, P.; Krammer, G.; Rogler, G. Bilberries and Their Anthocyanins Ameliorate Experimental Colitis. Mol. Nutr. Food Res. 2011, 55, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Liu, Y.; Liu, J.; Wang, E. Stability of Blueberry Anthocyanin, Anthocyanidin and Pyranoanthocyanidin Pigments and Their Inhibitory Effects and Mechanisms in Human Cervical Cancer HeLa Cells. RSC Adv. 2019, 9, 10842. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant Genotype Affects Total Antioxidant Capacity and Phenolic Contents in Fruit. Nutrition 2005, 21, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant Capacity as Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium Species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry, and Strawberry Varies with Cultivar and Developmental Stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic Compounds and Antioxidant Capacity of Georgia-Grown Blueberries and Blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, Phenolics, and Antioxidant Capacity in Diverse Small Fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Piekarska, A.; Mrugalska, B.; Konieczka, P.; Namieśnik, J.; Bartoszek, A. Phenolic Composition and Antioxidant Properties of Polish Blue-Berried Honeysuckle Genotypes by HPLC-DAD-MS, HPLC Postcolumn Derivatization with ABTS or FC, and TLC with DPPH Visualization. J. Agric. Food Chem. 2012, 60, 1755–1763. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, M.; Wu, L.; Wang, F.; Li, L.; Zhang, S.; Sun, B. Qualitative and Quantitative Analysis of Phenolic Compounds in Blueberries and Protective Effects on Hydrogen Peroxide-Induced Cell Injury. J. Sep. Sci. 2021, 44, 2837–2855. [Google Scholar] [CrossRef] [PubMed]
- Bornsek, S.M.; Ziberna, L.; Polak, T.; Vanzo, A.; Ulrih, N.P.; Abram, V.; Tramer, F.; Passamonti, S. Bilberry and Blueberry Anthocyanins Act as Powerful Intracellular Antioxidants in Mammalian Cells. Food Chem. 2012, 134, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Vaneková, Z.; Rollinger, J.M. Bilberries: Curative and Miraculous—A Review on Bioactive Constituents and Clinical Research. Front. Pharmacol. 2022, 13, 909914. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Nguyen, N.; Klavins, L.; Kviesis, J.; Heinonen, E.; Remes, J.; Jokipii-Lukkari, S.; Klavins, M.; Karppinen, K.; Jaakola, L.; et al. Analysis of Composition, Morphology, and Biosynthesis of Cuticular Wax in Wild Type Bilberry (Vaccinium myrtillus L.) and Its Glossy Mutant. Food Chem. 2021, 354, 129517. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.; Bae, G.Y.; Cho, K.; Park, S.S.; Suh, H.J.; Hong, K.B. An Anthocyanin-Enriched Extract from Vaccinium uliginosum Improves Signs of Skin Aging in UVB-Induced Photodamage. Antioxidants 2020, 9, 844. [Google Scholar] [CrossRef] [PubMed]
- Bucci, P.; Prieto, M.J.; Milla, L.; Calienni, M.N.; Martinez, L.; Rivarola, V.; Alonso, S.; Montanari, J. Skin Penetration and UV-Damage Prevention by Nanoberries. J. Cosmet. Dermatol. 2018, 17, 889–899. [Google Scholar] [CrossRef]
- Tadić, V.M.; Nešić, I.; Martinović, M.; Rój, E.; Brašanac-Vukanović, S.; Maksimović, S.; Žugić, A. Old Plant, New Possibilities: Wild Bilberry (Vaccinium myrtillus L., Ericaceae) in Topical Skin Preparation. Antioxidants 2021, 10, 465. [Google Scholar] [CrossRef]
- Pastori, V.; Tavazzi, S.; Lecchi, M. Lactoferrin-Loaded Contact Lenses: Eye Protection against Oxidative Stress. Cornea 2015, 34, 693–697. [Google Scholar] [CrossRef]
- Choi, W.; Kim, J.C.; Kim, W.S.; Oh, H.J.; Yang, J.M.; Lee, J.B.; Yoon, K.C. Clinical Effect of Antioxidant Glasses Containing Extracts of Medicinal Plants in Patients with Dry Eye Disease: A Multi-Center, Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. PLoS ONE 2015, 10, e0139761. [Google Scholar] [CrossRef]
- Galbis-Estrada, C.; Pinazo-Durán, M.D.; Martínez-Castillo, S.; Morales, J.M.; Monleón, D.; Zanon-Moreno, V. A Metabolomic Approach to Dry Eye Disorders. The Role of Oral Supplements with Antioxidants and Omega 3 Fatty Acids. Mol. Vis. 2015, 21, 555. [Google Scholar]
- Park, C.Y.; Gu, N.; Lim, C.Y.; Oh, J.H.; Chang, M.; Kim, M.; Rhee, M.Y. The Effect of Vaccinium uliginosum Extract on Tablet Computer-Induced Asthenopia: Randomized Placebo-Controlled Study. BMC Complement. Altern. Med. 2016, 16, 296. [Google Scholar] [CrossRef]
- Yin, L.; Pi, Y.L.; Zhang, M.N. The Effect of Vaccinium uliginosum on Rabbit Retinal Structure and Light-Induced Function Damage. Chin. J. Integr. Med. 2012, 18, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Fan, S.J.; Zhang, M. nian Protective Effects of Anthocyanins Extracted from Vaccinium uliginosum on 661W Cells Against Microwave-Induced Retinal Damage. Chin. J. Integr. Med. 2022, 28, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.I.; Kim, J.; Choung, S.Y. Polyphenol-Enriched Fraction of Vaccinium uliginosum L. Protects Selenite-Induced Cataract Formation in the Lens of Sprague-Dawley Rat Pups. Mol. Vis. 2019, 25, 118. [Google Scholar] [PubMed]
- Yoon, S.M.; Lee, B.L.; Guo, Y.R.; Choung, S.Y. Preventive Effect of Vaccinium uliginosum L. Extract and Its Fractions on Age-Related Macular Degeneration and Its Action Mechanisms. Arch. Pharm. Res. 2016, 39, 21–32. [Google Scholar] [CrossRef]
- Lee, B.L.; Kang, J.H.; Kim, H.M.; Jeong, S.H.; Jang, D.S.; Jang, Y.P.; Choung, S.Y. Polyphenol-Enriched Vaccinium uliginosum L. Fractions Reduce Retinal Damage Induced by Blue Light in A2E-Laden ARPE19 Cell Cultures and Mice. Nutr. Res. 2016, 36, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.L.; Choung, S.Y.; Jeong, K.W. Protective Mechanisms of Polyphenol-Enriched Fraction of Vaccinium uliginosum L. Against Blue Light-Induced Cell Death of Human Retinal Pigmented Epithelial Cells. J. Funct. Foods 2017, 39, 28–36. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Quality of Life and Visual Acuity of Visglyc Eye Drops on Dry Eye Patients. Available online: https://www.clinicaltrials.gov/study/NCT04063644 (accessed on 9 August 2023).
- ClinicalTrials.gov. The Effect of DA9301 on Tablet Computer-Induced Asthenopia. Available online: https://www.clinicaltrials.gov/study/NCT02641470 (accessed on 9 August 2023).
- Ozlem, K.; Birkan, Y.; Mustafa, K.; Emin, K. Protective Effect of Vaccinium myrtillus on Ischemia- Reperfusion Injury in Rat Ovary. Taiwan. J. Obstet. Gynecol. 2018, 57, 836–841. [Google Scholar] [CrossRef]
- Aqil, F.; Jeyabalan, J.; Agrawal, A.K.; Kyakulaga, A.H.; Munagala, R.; Parker, L.; Gupta, R.C. Exosomal Delivery of Berry Anthocyanidins for the Management of Ovarian Cancer. Food Funct. 2017, 8, 4100–4107. [Google Scholar] [CrossRef]
- Anhê, F.F.; Varin, T.V.; Le Barz, M.; Pilon, G.; Dudonné, S.; Trottier, J.; St-Pierre, P.; Harris, C.S.; Lucas, M.; Lemire, M.; et al. Arctic Berry Extracts Target the Gut-Liver Axis to Alleviate Metabolic Endotoxaemia, Insulin Resistance and Hepatic Steatosis in Diet-Induced Obese Mice. Diabetologia 2018, 61, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Desjardins, Y.; Pilon, G.; Dudonné, S.; Genovese, M.I.; Lajolo, F.M.; Marette, A. Polyphenols and Type 2 Diabetes: A Prospective Review. PharmaNutrition 2013, 1, 105–114. [Google Scholar] [CrossRef]
- Nguyen, B.; Bauman, A.; Gale, J.; Banks, E.; Kritharides, L.; Ding, D. Fruit and Vegetable Consumption and All-Cause Mortality: Evidence from a Large Australian Cohort Study. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Hoggard, N.; Cruickshank, M.; Moar, K.M.; Bestwick, C.; Holst, J.J.; Russell, W.; Horgan, G. A Single Supplement of a Standardised Bilberry (Vaccinium myrtillus L.) Extract (36% Wet Weight Anthocyanins) Modifies Glycaemic Response in Individuals with Type 2 Diabetes Controlled by Diet and Lifestyle. J. Nutr. Sci. 2013, 2, e22. [Google Scholar] [CrossRef]
- Xiao, T.; Guo, Z.; Bi, X.; Zhao, Y. Polyphenolic Profile as Well as Anti-Oxidant and Anti-Diabetes Effects of Extracts from Freeze-Dried Black Raspberries. J. Funct. Foods 2017, 31, 179–187. [Google Scholar] [CrossRef]
- Chan, S.W.; Chu, T.T.W.; Choi, S.W.; Benzie, I.F.F.; Tomlinson, B. Impact of Short-Term Bilberry Supplementation on Glycemic Control, Cardiovascular Disease Risk Factors, and Antioxidant Status in Chinese Patients with Type 2 Diabetes. Phytother. Res. 2021, 35, 3236–3245. [Google Scholar] [CrossRef]
- Karcheva-Bahchevanska, D.P.; Lukova, P.K.; Nikolova, M.M.; Mladenov, R.D.; Iliev, I.N. Effect of Extracts of Bilberries (Vaccinium myrtillus L.) on Amyloglucosidase and α-Glucosidase Activity. Folia Med. 2017, 59, 197–202. [Google Scholar] [CrossRef]
- Sun, X.H.; Zhou, T.T.; Wei, C.H.; Lan, W.Q.; Zhao, Y.; Pan, Y.J.; Wu, V.C.H. Antibacterial Effect and Mechanism of Anthocyanin Rich Chinese Wild Blueberry Extract on Various Foodborne Pathogens. Food Control 2018, 94, 155–161. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.S.; Lee, Y.M.; Sohn, E.; Jo, K.; Kim, J.S. Vaccinium myrtillus Extract Prevents or Delays the Onset of Diabetes--Induced Blood-Retinal Barrier Breakdown. Int. J. Food Sci. Nutr. 2015, 66, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Pemmari, T.; Hämäläinen, M.; Ryyti, R.; Peltola, R.; Moilanen, E. Dried Bilberry (Vaccinium myrtillus L.) Alleviates the Inflammation and Adverse Metabolic Effects Caused by a High-Fat Diet in a Mouse Model of Obesity. Int. J. Mol. Sci. 2022, 23, 11021. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Effects of Berries and Berry Fractions on Metabolic Diseases. Available online: https://www.clinicaltrials.gov/study/NCT01860547 (accessed on 9 August 2023).
- ClinicalTrials.gov. The Health Effect of Diet Rich in Nordic Berries (Berry). Available online: https://www.clinicaltrials.gov/study/NCT01414647 (accessed on 10 August 2023).
- ClinicalTrials.gov. Dietary Anthocyanins Improve Lipid Metabolism in a Dose—Dependent Manner. Available online: https://www.clinicaltrials.gov/study/NCT03415503 (accessed on 9 August 2023).
- ClinicalTrials.gov. Safety and Efficacy of Herbal Tea in Type 2 Diabetics (DIABHerbMix). Available online: https://www.clinicaltrials.gov/study/NCT04054284 (accessed on 10 August 2023).
- Bryl-Górecka, P.; Sathanoori, R.; Arevström, L.; Landberg, R.; Bergh, C.; Evander, M.; Olde, B.; Laurell, T.; Fröbert, O.; Erlinge, D. Bilberry Supplementation after Myocardial Infarction Decreases Microvesicles in Blood and Affects Endothelial Vesiculation. Mol. Nutr. Food Res. 2020, 64, 2000108. [Google Scholar] [CrossRef]
- Ashour, O.M.; Elberry, A.A.; Alahdal, A.M.; Al Mohamadi, A.M.; Nagy, A.A.; Abdel-Naim, A.B.; Abdel-Sattar, E.A.; Mohamadin, A.M. Protective Effect of Bilberry (Vaccinium myrtillus) against Doxorubicin-Induced Oxidative Cardiotoxicity in Rats. Med. Sci. Monit. 2011, 17, 110–115. [Google Scholar] [CrossRef]
- Habanova, M.; Saraiva, J.A.; Haban, M.; Schwarzova, M.; Chlebo, P.; Predna, L.; Gažo, J.; Wyka, J. Intake of Bilberries (Vaccinium myrtillus L.) Reduced Risk Factors for Cardiovascular Disease by Inducing Favorable Changes in Lipoprotein Profiles. Nutr. Res. 2016, 36, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Rafieiankopaei, M.; Sahebkar, A.; Shamsi, F.; Goli-malekabadi, N. Anti-Hyperglycemic and Anti-Hyperlipidemic Effects of Vaccinium myrtillus Fruit in Experimentally Induced Diabetes (Antidiabetic Effect of Vaccinium myrtillus Fruit). J. Sci. Food Agric. 2016, 96, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, R.; Zhao, H.; Chen, G.; Jiang, Y.; Lyu, X.; Wu, T. Reduction of Aging-Induced Oxidative Stress and Activation of Autophagy by Bilberry Anthocyanin Supplementation via the AMPK-MTOR Signaling Pathway in Aged Female Rats. J. Agric. Food Chem. 2019, 67, 7832–7843. [Google Scholar] [CrossRef]
- Benassai, E.; Del Bubba, M.; Ancillotti, C.; Colzi, I.; Gonnelli, C.; Calisi, N.; Salvatici, M.C.; Casalone, E.; Ristori, S. Green and Cost-Effective Synthesis of Copper Nanoparticles by Extracts of Non-Edible and Waste Plant Materials from Vaccinium Species: Characterization and Antimicrobial Activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111453. [Google Scholar] [CrossRef]
- Zhou, T.T.; Wei, C.H.; Lan, W.Q.; Zhao, Y.; Pan, Y.J.; Sun, X.H.; Wu, V.C.H. The Effect of Chinese Wild Blueberry Fractions on the Growth and Membrane Integrity of Various Foodborne Pathogens. J. Food Sci. 2020, 85, 1513–1522. [Google Scholar] [CrossRef]
- Satoh, Y.; Ishihara, K. Investigation of the Antimicrobial Activity of Bilberry (Vaccinium myrtillus L.) Extract against Periodontopathic Bacteria. J. Oral Biosci. 2020, 62, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Bayar, Y.; Onaran, A.; Yilar, M.; Gul, F. Determination of the Essential Oil Composition and the Antifungal Activities of Bilberry (Vaccinium myrtillus L.) and Bay Laurel (Laurus nobilis L.). J. Essent. Oil Bear. Plants 2018, 21, 548–555. [Google Scholar] [CrossRef]
- Lippert, E.; Ruemmele, P.; Obermeier, F.; Goelder, S.; Kunst, C.; Rogler, G.; Dunger, N.; Messmann, H.; Hartmann, A.; Endlicher, E. Anthocyanins Prevent Colorectal Cancer Development in a Mouse Model. Digestion 2017, 95, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Kim, M.; Kim, B. The Effects of Anthocyanin-Rich Bilberry Extract on Transintestinal Cholesterol Excretion. Foods 2021, 10, 2852. [Google Scholar] [CrossRef] [PubMed]
- Mauramo, M.; Onali, T.; Wahbi, W.; Vasara, J.; Lampinen, A.; Mauramo, E.; Kivimäki, A.; Martens, S.; Häggman, H.; Sutinen, M.; et al. Bilberry (Vaccinium myrtillus L.) Powder Has Anticarcinogenic Effects on Oral Carcinoma In Vitro and In Vivo. Antioxidants 2021, 10, 1319. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Agrawal, A.K.; Mudd, A.M.; Kyakulaga, A.H.; Singh, I.P.; Vadhanam, M.V.; Gupta, R.C. Exosomal Formulation of Anthocyanidins against Multiple Cancer Types. Cancer Lett. 2017, 393, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry Anthocyanidins Synergistically Suppress Growth and Invasive Potential of Human Non-Small-Cell Lung Cancer Cells. Cancer Lett. 2012, 325, 54–62. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Botanical Therapy in Treating Mucositis in Patients With Head and Neck Cancer Who Have Undergone Chemoradiation Therapy. Available online: https://www.clinicaltrials.gov/study/NCT01674374 (accessed on 9 August 2023).
- American Herbal Pharmacopoeia. Bilberry Fruit: Vaccinium myrtillus L.: Standards of Analysis, Quality Control, and Therapeutics; American Herbal Pharmacopoeia: Scotts Valley, CA, USA, 2001; p. 25. [Google Scholar]
- Biedermann, L.; Mwinyi, J.; Scharl, M.; Frei, P.; Zeitz, J.; Kullak-Ublick, G.A.; Vavricka, S.R.; Fried, M.; Weber, A.; Humpf, H.U.; et al. Bilberry Ingestion Improves Disease Activity in Mild to Moderate Ulcerative Colitis—An Open Pilot Study. J. Crohns. Colitis 2013, 7, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.; Paur, I.; Bøhn, S.K.; Sakhi, A.K.; Borge, G.I.; Serafini, M.; Erlund, I.; Laake, P.; Tonstad, S.; Blomhoff, R. Bilberry Juice Modulates Plasma Concentration of NF-KappaB Related Inflammatory Markers in Subjects at Increased Risk of CVD. Eur. J. Nutr. 2010, 49, 345–355. [Google Scholar] [CrossRef]
- Arevström, L.; Bergh, C.; Landberg, R.; Wu, H.; Rodriguez-Mateos, A.; Waldenborg, M.; Magnuson, A.; Blanc, S.; Fröbert, O. Freeze-Dried Bilberry (Vaccinium myrtillus) Dietary Supplement Improves Walking Distance and Lipids after Myocardial Infarction: An Open-Label Randomized Clinical Trial. Nutr. Res. 2019, 62, 13–22. [Google Scholar] [CrossRef]











| Polyphenol Compounds | Method of Characterization | References |
|---|---|---|
| delphinidin-3-O-galactoside malvidin-3-O-galactoside malvidin-3-O-arabinoside delphinidin-3-O-arabinoside | CIELAB HPLC-DAD | [46] |
| delphinidin 3-glucoside cyanidin 3-glucoside petunidin 3-glucoside delphinidin 3-glucoside | HPLC-DAD | [47] |
| chlorogenic acid quercetin-3-O-galactoside quercetin-3-O-glucuronide delphinidin-3-O-galactoside delphinidin-3-O-glucoside cyanidin-3-O-galactoside petunidin-3-O-glucoside | HPLC-UV/DAD HPLC-ESI-MS MS | [48] |
| delphinidin 3-O-glucoside malvidin 3-O-glucoside myricetin 3-O-hexoside quercetin 3-O-galactoside | HPLC-DAD HPLC-ESI-MS | [49] |
| cyanidin-3-O-glucoside cyanidin-3-O-rutinoside catechin quercetin-3-O-galactoside quercetin-3-O-arabinoside myricetin 3-O-hexose | HPLC-FT-ICR MS/MS | [50] |
| gallic acid vanillic acid ferulic acid caffeic acid p-coumaric acid quercetin | HPLC | [51] |
| (–)-epicatechin kaempferol derivative chlorogenic acid ellagic acid | HPLC | [52] |
| glycosides of quercetin myricetin kaempferol isorhamnetin syringetin laricitrin | HPLC–MS | [53] |
| Dry Samples | Fresh Samples | |||
|---|---|---|---|---|
| Total Anthocyanins Content (mg/g) | Total Phenolics Content (mg/g) | Total Anthocyanins Content (mg/g) | Total Phenolics Content (mg/g) | |
| V. myrtillus | 21.8 ± 0.1 | 26.6 ± 0.1 | 19.4 ± 0.1 | 23.7 ± 0.1 |
| V. uliginosum | 14.3 ± 0.3 | 21.1 ± 0.3 | 12.4 ± 0.2 | 18.2 ± 0.2 |
| NCT Number | Study Title | Clinical Trial Status | Study Design | Condition | References |
|---|---|---|---|---|---|
| NCT01860547 | The Effect of the Bioactives of Sea Buckthorn and Bilberry on the Risk of Metabolic Diseases | Not applicable | Randomized, open-label, crossover assignment |
| [105] |
| NCT01414647 | The Effect of Diet Rich in Nordic Berries on Gut Microbiota, Glucose and Lipid Metabolism and Metabolism on Fenolic Compounds | Not applicable | Randomized, open-label, crossover assignment |
| [106] |
| NCT03415503 | Anthocyanin Supplementation Improves Blood Lipids in a Dose-response Manner in Subjects with Dyslipidemia | Phase 3 | Randomized, double-blind (participant, investigator), parallel assignment |
| [107] |
| NCT04054284 | Safety and Efficacy of a Complex Herbal Tea Mixture in Type 2 Diabetics | Not applicable | Randomized, quadruple -blind (participant, care provider, investigator, outcomes assessor), parallel assignment |
| [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopystecka, A.; Kozioł, I.; Radomska, D.; Bielawski, K.; Bielawska, A.; Wujec, M. Vaccinium uliginosum and Vaccinium myrtillus—Two Species—One Used as a Functional Food. Nutrients 2023, 15, 4119. https://doi.org/10.3390/nu15194119
Kopystecka A, Kozioł I, Radomska D, Bielawski K, Bielawska A, Wujec M. Vaccinium uliginosum and Vaccinium myrtillus—Two Species—One Used as a Functional Food. Nutrients. 2023; 15(19):4119. https://doi.org/10.3390/nu15194119
Chicago/Turabian StyleKopystecka, Agnieszka, Ilona Kozioł, Dominika Radomska, Krzysztof Bielawski, Anna Bielawska, and Monika Wujec. 2023. "Vaccinium uliginosum and Vaccinium myrtillus—Two Species—One Used as a Functional Food" Nutrients 15, no. 19: 4119. https://doi.org/10.3390/nu15194119
APA StyleKopystecka, A., Kozioł, I., Radomska, D., Bielawski, K., Bielawska, A., & Wujec, M. (2023). Vaccinium uliginosum and Vaccinium myrtillus—Two Species—One Used as a Functional Food. Nutrients, 15(19), 4119. https://doi.org/10.3390/nu15194119