Oral Anti-Inflammatory and Symbiotic Effects of Fermented Lingonberry Juice—Potential Benefits in IBD
Abstract
:1. Introduction
2. Lingonberries
3. Inflammatory Bowel Disease
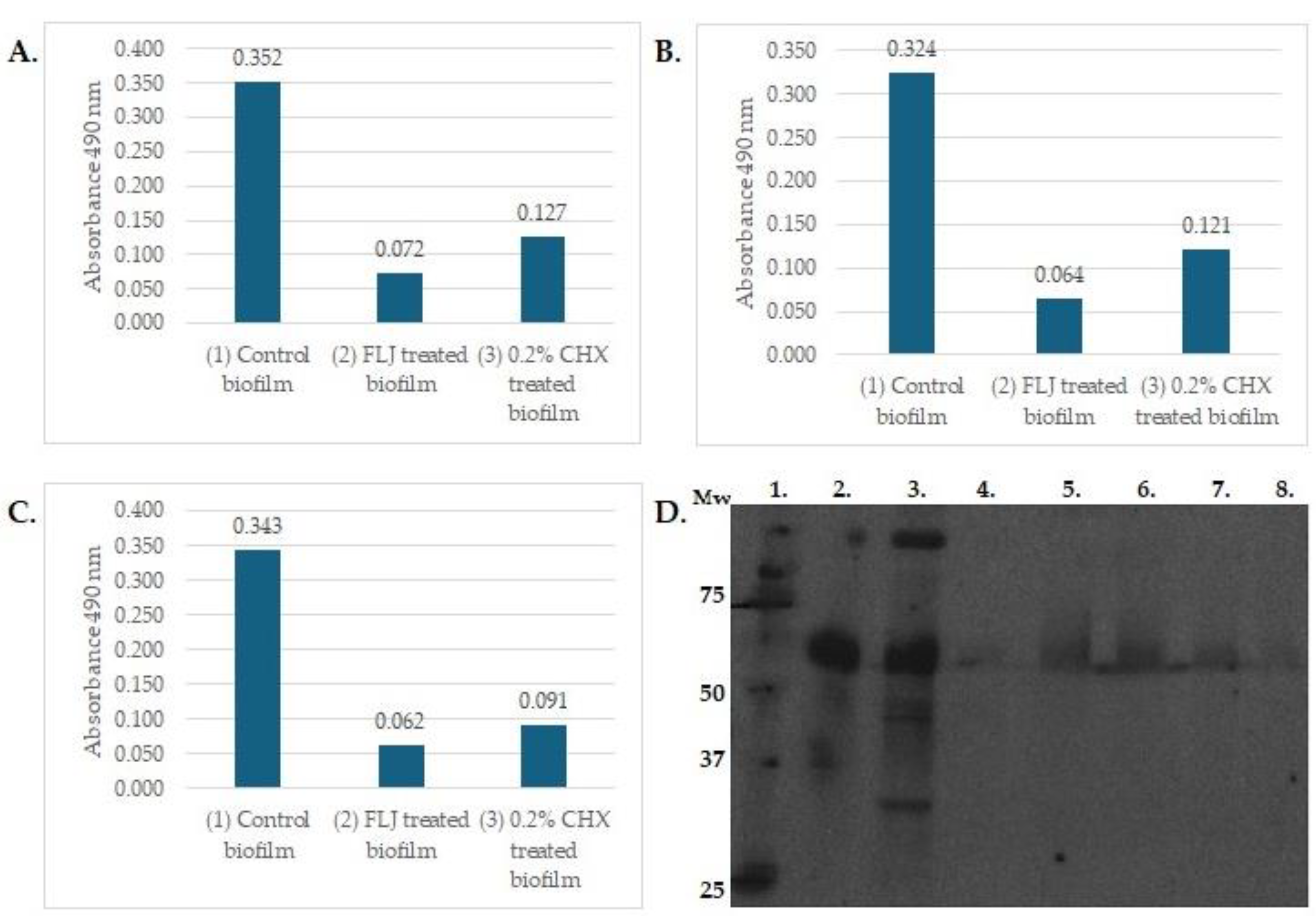
4. Oral–Gut Interactions
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elzayat, H.; Mesto, G.; Al-Marzooq, F. Unraveling the Impact of Gut and Oral Microbiome on Gut Health in Inflammatory Bowel Diseases. Nutrients 2023, 15, 3377. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zang, S.Q.; Wei, J.; Yu, H.C.; Yang, Z.; Wu, H.M.; Kang, Y.; Tao, H.; Yang, M.F.; Jin, L.; et al. High-throughput sequencing provides insights into oral microbiota dysbiosis in association with inflammatory bowel disease. Genomics 2021, 113 Pt 2, 664–676. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- García-Gamboa, R.; Kirchmayr, M.R.; Gradilla-Hernández, M.S.; Pérez-Brocal, V.; Moya, A.; González-Avila, M. The intestinal mycobiota and its relationship with overweight, obesity and nutritional aspects. J. Hum. Nutr. Diet. 2021, 34, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 167–174. [Google Scholar] [CrossRef]
- Haș, I.M.; Tit, D.M.; Bungau, S.G.; Pavel, F.M.; Teleky, B.E.; Vodnar, D.C.; Vesa, C.M. Cardiometabolic Risk: Characteristics of the Intestinal Microbiome and the Role of Polyphenols. Int. J. Mol. Sci. 2023, 24, 13757. [Google Scholar] [CrossRef]
- Shao, T.; Hsu, R.; Rafizadeh, D.L.; Wang, L.; Bowlus, C.L.; Kumar, N.; Mishra, J.; Timilsina, S.; Ridgway, W.M.; Gershwin, M.E.; et al. The gut ecosystem and immune tolerance. J. Autoimmun. 2023, 141, 103114. [Google Scholar] [CrossRef]
- Grau-Del Valle, C.; Fernández, J.; Solá, E.; Montoya-Castilla, I.; Morillas, C.; Bañuls, C. Association between gut microbiota and psychiatric disorders: A systematic review. Front. Psychol. 2023, 14, 1215674. [Google Scholar] [CrossRef]
- Moszak, M.; Pelczyńska, M.; Wesołek, A.; Stenclik, D.; Bogdański, P. Does gut microbiota affect the success of weight loss? Evidence and speculation. Nutrition 2023, 116, 112111. [Google Scholar] [CrossRef]
- Beheshti-Maal, A.; Shahrokh, S.; Ansari, S.; Mirsamadi, E.S.; Yadegar, A.; Mirjalali, H.; Zali, M.R. Gut mycobiome: The probable determinative role of fungi in IBD patients. Mycoses 2021, 64, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Nishida, A. Alteration of the Gut Microbiome in Inflammatory Bowel Disease. Digestion 2023, 104, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Pärnänen, P. A Preparation for Balancing the Composition of the Oral Microbial Flora. EP2585087B1, 13 December 2017. [Google Scholar]
- Pärnänen, P.; Nikula-Ijäs, P.; Sorsa, T. Antimicrobial and anti-inflammatory lingonberry mouthwash—A clinical pilot study in the oral cavity. Microorganisms 2019, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Pärnänen, P.; Lomu, S.; Räisänen, I.T.; Tervahartiala, T.; Sorsa, T. Antimicrobial and Anti-Inflammatory Oral Effects of Fermented Lingonberry Juice—A One-Year Prospective Human Intervention Study. Eur. J. Dent. 2023, 17, 1235–1240. [Google Scholar] [CrossRef]
- Heyman-Lindén, L.; Kotowska, D.; Sand, E.; Bjursell, M.; Plaza, M.; Turner, C.; Holm, C.; Fåk, F.; Berger, K. Lingonberries alter the gut microbiota and prevent low-grade inflammation in high-fat diet fed mice. Food Nutr. Res. 2016, 60, 29993. [Google Scholar] [CrossRef]
- Lähteenmäki, H.; Tervahartiala, T.; Räisänen, I.T.; Pärnänen, P.; Sorsa, T. Fermented lingonberry juice’s effects on active MMP-8 (aMMP-8), bleeding on probing (BOP), and visible plaque index (VPI) in dental implants—A clinical pilot mouthwash study. Clin. Exp. Dent. Res. 2022, 8, 1322–1330. [Google Scholar] [CrossRef]
- Ryyti, R.; Hämäläinen, M.; Peltola, R.; Moilanen, E. Beneficial effects of lingonberry (Vaccinium vitis-idaea L.) supplementation on metabolic and inflammatory adverse effects induced by high-fat diet in a mouse model of obesity. PLoS ONE 2020, 15, e0232605. [Google Scholar] [CrossRef]
- Pärnänen, P.; Sorsa, T.; Tervahartiala, T.; Nikula-Ijäs, P. Isolation, characterization and regulation of moonlighting proteases from Candida glabrata cell wall. Microb. Pathog. 2020, 149, 104547. [Google Scholar] [CrossRef]
- Törrönen, R.; Kolehmainen, M.; Sarkkinen, E.; Mykkänen, H.; Niskanen, L. Postprandial glucose, insulin, and free fatty acid responses to sucrose consumed with blackcurrants and lingonberries in healthy women. Am. J. Clin. Nutr. 2012, 96, 527–533. [Google Scholar] [CrossRef]
- Liu, J.; Hefni, M.E.; Witthöft, C.M.; Bergström, M.; Burleigh, S.; Nyman, M.; Hållenius, F. On the effect of flavonoids and dietary fibre in lingonberries on atherosclerotic plaques, lipid profiles and gut microbiota composition in Apoe−/− mice. Int. J. Food Sci. Nutr. 2022, 73, 1080–1090. [Google Scholar] [CrossRef]
- Marungruang, N.; Kovalenko, T.; Osadchenko, I.; Voss, U.; Huang, F.; Burleigh, S.; Ushakova, G.; Skibo, G.; Nyman, M.; Prykhodko, O.; et al. Lingonberries and their two separated fractions differently alter the gut microbiota, improve metabolic functions, reduce gut inflammatory properties, and improve brain function in ApoE-/- mice fed high-fat diet. Nutr. Neurosci. 2020, 23, 600–612. [Google Scholar] [CrossRef]
- Pärnänen, P.; Lomu, S.; Räisänen, I.T.; Tervahartiala, T.; Sorsa, T. Effects of Fermented Lingonberry Juice Mouthwash on Salivary Parameters—A One-Year Prospective Human Intervention Study. Dent. J. 2022, 10, 69. [Google Scholar] [CrossRef]
- Kowalska, K. Lingonberry (Vaccinium vitis-idaea L.) Fruit as a Source of Bioactive Compounds with Health-Promoting Effects—A Review. Int. J. Mol. Sci. 2021, 22, 5126. [Google Scholar] [CrossRef]
- Rytter, H.; Combet, E.; Chassaing, B. Probiotic: Is diet part of the efficacy equation? Gut Microbes 2023, 15, 2222438. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.H. Salivary Factors that Maintain the Normal Oral Commensal Microflora. J. Dent. Res. 2020, 99, 644–649. [Google Scholar] [CrossRef]
- Ramage, G.; Saville, S.P.; Wickes, B.L.; López-Ribot, J.L. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 2002, 68, 5459–5463. [Google Scholar] [CrossRef] [PubMed]
- Ribaldone, D.G.; Brigo, S.; Mangia, M.; Saracco, G.M.; Astegiano, M.; Pellicano, R. Oral Manifestations of Inflammatory Bowel Disease and the Role of Non-Invasive Surrogate Markers of Disease Activity. Medicines 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Lankarani, K.B.; Sivandzadeh, G.R.; Hassanpour, S. Oral manifestation in inflammatory bowel disease: A review. World J. Gastroenterol. 2013, 19, 8571–8579. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Dhaneshwar, S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, D.; Huang, J.; Liu, K.; Liu, H.; Wu, H.; Bao, C. Probiotics for inflammatory bowel disease: Is there sufficient evidence? Open Life Sci. 2024, 19, 20220821. [Google Scholar] [CrossRef]
- Zurba, Y.; Gros, B.; Shehab, M. Exploring the Pipeline of Novel Therapies for Inflammatory Bowel Disease; State of the Art Review. Biomedicines 2023, 11, 747. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Mohana, T.; Athesh, K.; Hillary, V.E.; Vasconcelos, A.B.S.; Farias de Franca, M.N.; Montalvão, M.M.; Ceasar, S.A.; Jothi, G.; Sridharan, G.; et al. Anti-inflammatory natural products modulate interleukins and their related signaling markers in inflammatory bowel disease: A systematic review. J. Pharm. Anal. 2023, 13, 1408–1428. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Davila, M.M.; Papada, E. The Role of Plant-Derived Natural Products in the Management of Inflammatory Bowel Disease—What Is the Clinical Evidence So Far? Life 2023, 13, 1703. [Google Scholar] [CrossRef] [PubMed]
- Somineni, H.K.; Weitzner, J.H.; Venkateswaran, S.; Dodd, A.; Prince, J.; Karikaran, A.; Sauer, C.G.; Abramowicz, S.; Zwick, M.E.; Cutler, D.J.; et al. Site- and Taxa-Specific Disease-Associated Oral Microbial Structures Distinguish Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2021, 27, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Baima, G.; Massano, A.; Squillace, E.; Caviglia, G.P.; Buduneli, N.; Ribaldone, D.G.; Aimetti, M. Shared microbiological and immunological patterns in periodontitis and IBD: A scoping review. Oral Dis. 2022, 28, 1029–1041. [Google Scholar] [CrossRef]
- Byrd, K.M.; Gulati, A.S. The “Gum-Gut” Axis in Inflammatory Bowel Diseases: A Hypothesis-Driven Review of Associations and Advances. Front. Immunol. 2021, 12, 620124. [Google Scholar] [CrossRef]
- Olsen, I.; Yamazaki, K. Can oral bacteria affect the microbiome of the gut? J. Oral Microbiol. 2019, 11, 1586422. [Google Scholar] [CrossRef]
- Schmidt, T.S.; Hayward, M.R.; Coelho, L.P.; Li, S.S.; Costea, P.I.; Voigt, A.Y.; Wirbel, J.; Maistrenko, O.M.; Alves, R.J.; Bergsten, E.; et al. Extensive transmission of microbes along the gastrointestinal tract. eLife 2019, 8, e42693. [Google Scholar] [CrossRef]
- Said, H.S.; Suda, W.; Nakagome, S.; Chinen, H.; Oshima, K.; Kim, S.; Kimura, R.; Iraha, A.; Ishida, H.; Fujita, J.; et al. Dysbiosis of salivary microbiota in inflammatory bowel disease and its association with oral immunological biomarkers. DNA Res. 2014, 21, 15–25. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Drury, B.; Hardisty, G.; Gray, R.D.; Ho, G.T. Neutrophil Extracellular Traps in Inflammatory Bowel Disease: Pathogenic Mechanisms and Clinical Translation. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, H.; Kantarci, A.; Van Dyke, T.E. Oral inflammatory diseases and systemic inflammation: Role of the macrophage. Front. Immunol. 2012, 3, 118. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.A.; Kazmi, N.; Barb, J.J.; Ames, N. The Oral and Gut Bacterial Microbiomes: Similarities, Differences, and Connections. Biol. Res. Nurs. 2021, 23, 7–20. [Google Scholar] [CrossRef]
- Umeizudike, K.; Räisänen, I.; Gupta, S.; Nwhator, S.; Grigoriadis, A.; Sakellari, D.; Sorsa, T. Active matrix metalloproteinase-8: A potential biomarker of oral systemic link. Clin. Exp. Dent. Res. 2022, 8, 359–365. [Google Scholar] [CrossRef]
- Schmidt, J.; Weigert, M.; Leuschner, C.; Hartmann, H.; Raddatz, D.; Haak, R.; Mausberg, R.F.; Kottmann, T.; Schmalz, G.; Ziebolz, D. Active matrix metalloproteinase-8 and periodontal bacteria-interlink between periodontitis and inflammatory bowel disease? J. Periodontal. 2018, 89, 699–707. [Google Scholar] [CrossRef]
- de Mello-Neto, J.M.; Nunes, J.G.R.; Tadakamadla, S.K.; da Silva Figueredo, C.M. Immunological Traits of Patients with Coexistent Inflammatory Bowel Disease and Periodontal Disease: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 8958. [Google Scholar] [CrossRef]
- Förster, T.M.; Mogavero, S.; Dräger, A.; Graf, K.; Polke, M.; Jacobsen, I.D.; Hube, B. Enemies and brothers in arms: Candida albicans and gram-positive bacteria. Cell. Microbiol. 2016, 18, 1709–1715. [Google Scholar] [CrossRef]
- Jamieson, P.E.; Carbonero, F.; Stevens, J.F. Dietary (poly)phenols mitigate inflammatory bowel disease: Therapeutic targets, mechanisms of action, and clinical observations. Curr. Res. Food Sci. 2023, 6, 100521. [Google Scholar] [CrossRef]
| Examples of Bioactivities of Lingonberries | ||
|---|---|---|
| Antimicrobial | In vivo human studies | [13,14,15] |
| Anti-inflammatory and antioxidant | In vivo human studies In vitro mouse study | [13,14,15,16,17] [18] |
| Anti-proteolytic (aMMP-8 ↓) | In vitro and in vivo human study | [14,19] |
| Glucose, insulin, free fatty acids | In vivo human study In vivo mouse study | [20] [21] |
| Gut microbiota | In vitro mouse study | [22] |
| Saliva | In vivo human study | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pärnänen, P.; Räisänen, I.T.; Sorsa, T. Oral Anti-Inflammatory and Symbiotic Effects of Fermented Lingonberry Juice—Potential Benefits in IBD. Nutrients 2024, 16, 2896. https://doi.org/10.3390/nu16172896
Pärnänen P, Räisänen IT, Sorsa T. Oral Anti-Inflammatory and Symbiotic Effects of Fermented Lingonberry Juice—Potential Benefits in IBD. Nutrients. 2024; 16(17):2896. https://doi.org/10.3390/nu16172896
Chicago/Turabian StylePärnänen, Pirjo, Ismo T. Räisänen, and Timo Sorsa. 2024. "Oral Anti-Inflammatory and Symbiotic Effects of Fermented Lingonberry Juice—Potential Benefits in IBD" Nutrients 16, no. 17: 2896. https://doi.org/10.3390/nu16172896






