The Impact of Exposure to Iodine and Fluorine in Drinking Water on Thyroid Health and Intelligence in School-Age Children: A Cross-Sectional Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sampling
2.2. Collection of Drinking Water, Urine and Blood Samples, and Anthropometry
2.3. Thyroid Volume Measurement and Thyroid Nodule Examination
2.4. Biochemical Examination of Thyroid Function
2.5. IQ Evaluation
2.6. Investigation of Other Related Factors
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Effect of Different Water Iodine and Fluorine Areas on Thyroid Health of School-Age Children
3.3. Influence of Different Water Iodine and Water Fluoride Areas on the Intelligence of School-Age Children
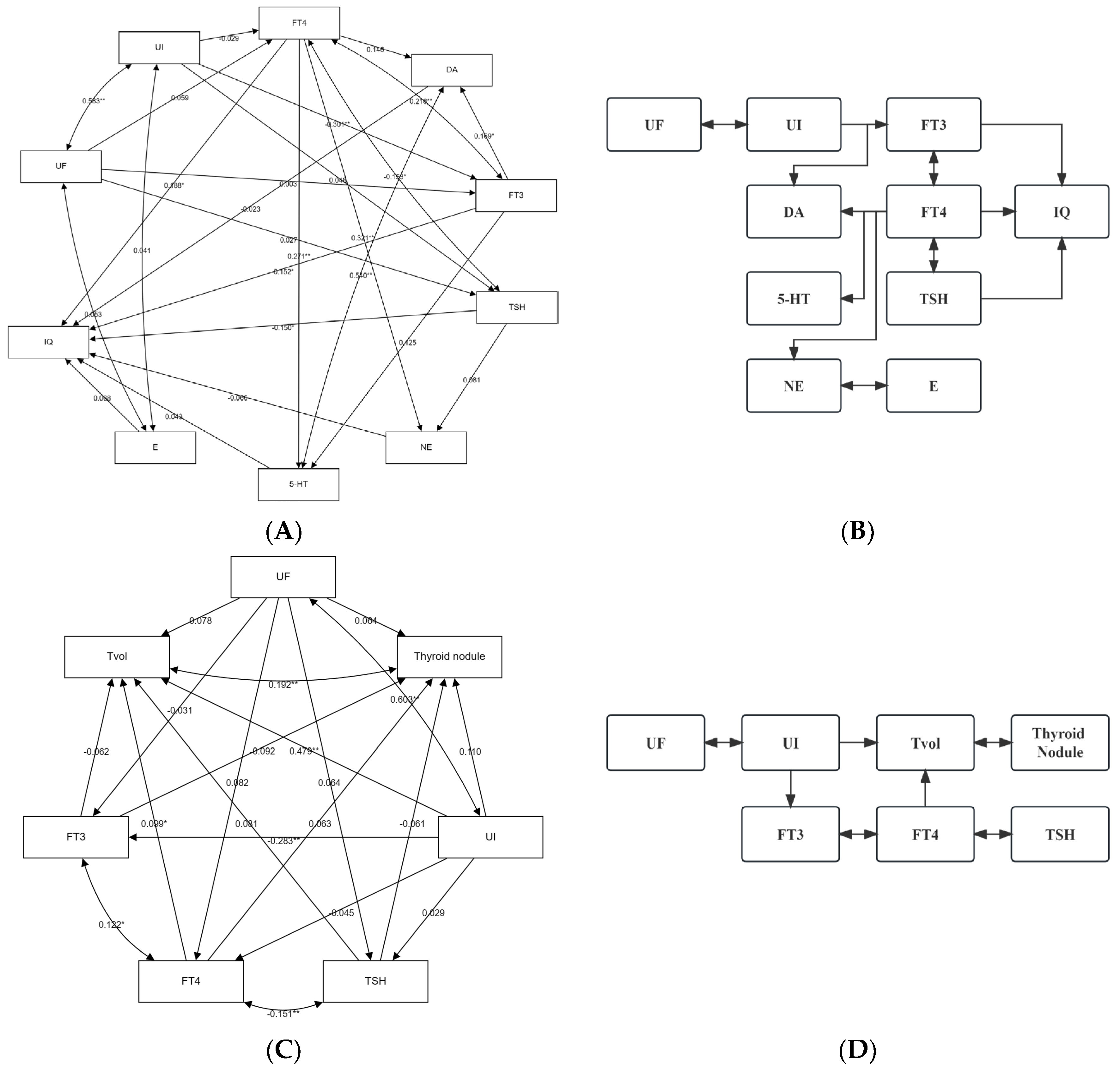
3.4. Relationship between Urinary Iodine, Urinary Fluorine, and Thyroid Health of School-Age Children
3.5. Relationship between Urinary Iodine, Urinary Fluoride, and Intelligence of School-Age Children
3.6. The Role of Thyroid Hormones and/or Monoamine Neurotransmitters with the Influence of Iodine and Fluorine on the Intelligence and Thyroid Health of School-Age Children
3.7. Interaction between Iodine and Fluorine on Thyroid and Intelligence of School-Age Children
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, R.; Yan, M.; Han, P.; Wang, T.; Li, B.; Zhou, S.; Zheng, T.; Hu, Y.; Borthwick, A.G.L.; Zheng, C.; et al. Deficiency and excess of groundwater iodine and their health associations. Nat. Commun. 2022, 13, 7354. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Barberio, M.D.; Banzato, F.; Billi, A.; Boschetti, T.; Franchini, S.; Gori, F.; Petitta, M. Climate change and its effect on groundwater quality. Environ. Geochem. Health 2023, 45, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Guo, J.; Xie, Y.; Bian, R.; Wang, N.; Qi, W.; Liu, H. Distribution, sources, and potential health risks of fluoride, total iodine, and nitrate in rural drinking water sources of North and East China. Sci. Total Environ. 2023, 898, 165561. [Google Scholar] [CrossRef] [PubMed]
- Velasco, I.; Bath, S.C.; Rayman, M.P. Iodine as Essential Nutrient during the First 1000 Days of Life. Nutrients 2018, 10, 290. [Google Scholar] [CrossRef]
- Jha, S.K.; Singh, R.K.; Damodaran, T.; Mishra, V.K.; Sharma, D.K.; Rai, D. Fluoride in groundwater: Toxicological exposure and remedies. J. Toxicol. Environ. Health Part B 2013, 16, 52–66. [Google Scholar] [CrossRef]
- Griebel-Thompson, A.K.; Sands, S.; Chollet-Hinton, L.; Christifano, D.; Sullivan, D.K.; Hull, H.; Carlson, S.E. A Scoping Review of Iodine and Fluoride in Pregnancy in Relation to Maternal Thyroid Function and Offspring Neurodevelopment. Adv. Nutr. 2023, 14, 317–338. [Google Scholar] [CrossRef]
- Farebrother, J.; Zimmermann, M.B.; Andersson, M. Excess iodine intake: Sources, assessment, and effects on thyroid function. Ann. N. Y. Acad. Sci. 2019, 1446, 44–65. [Google Scholar] [CrossRef]
- Mou, Y.; Qu, T.; Wang, R.; Zhang, Y.; He, Z.; Gu, S. The association of high-fluoride and high-iodine combined exposure with dental fluorosis and goiter: A meta-analysis. Environ. Geochem. Health 2023, 45, 3143–3153. [Google Scholar] [CrossRef]
- Du, Y.; Zhou, G.; Gong, B.; Ma, J.; An, N.; Gao, M.; Yang, M.; Ma, Q.; Huang, H.; Zuo, Q.; et al. Iodine Modifies the Susceptibility of Thyroid to Fluoride Exposure in School-age Children: A Cross-sectional Study in Yellow River Basin, Henan, China. Biol. Trace Elem. Res. 2021, 199, 3658–3666. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, W.; Jiang, P.; Li, X.; Liu, C.; Chai, C. Role of nitric oxide and vascular endothelial growth factor in fluoride-induced goitrogenesis in rats. Environ. Toxicol. Pharmacol. 2012, 34, 209–217. [Google Scholar] [CrossRef]
- Peckham, S.; Lowery, D.; Spencer, S. Are fluoride levels in drinking water associated with hypothyroidism prevalence in England? A large observational study of GP practice data and fluoride levels in drinking water. J. Epidemiol. Commun. Health 2015, 69, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xing, R.; Liu, S.; Yu, H.; Li, P. gamma-Aminobutyric acid ameliorates fluoride-induced hypothyroidism in male Kunming mice. Life Sci. 2016, 146, 1–7. [Google Scholar] [CrossRef]
- Kumar, V.; Chahar, P.; Kajjari, S.; Rahman, F.; Bansal, D.K.; Kapadia, J.M. Fluoride, Thyroid Hormone Derangements and its Correlation with Tooth Eruption Pattern Among the Pediatric Population from Endemic and Non-endemic Fluorosis Areas. J. Contemp. Dent. Pract. 2018, 19, 1512–1516. [Google Scholar]
- Waugh, D.T. Fluoride Exposure Induces Inhibition of Sodium/Iodide Symporter (NIS) Contributing to Impaired Iodine Absorption and Iodine Deficiency: Molecular Mechanisms of Inhibition and Implications for Public Health. Int. J. Environ. Res. Public Health 2019, 16, 1086. [Google Scholar] [CrossRef] [PubMed]
- Kutlucan, A.; Kale Koroglu, B.; Numan Tamer, M.; Aydin, Y.; Baltaci, D.; Akdogan, M.; Ozturk, M.; Vural, H.; Ermis, F. The investigation of effects of fluorosis on thyroid volume in school-age children. Med. Glas. 2013, 10, 93–98. [Google Scholar]
- Savchenkov, M.F.; Efimova, N.V.; Manueva, R.S.; Nikolaeva, L.A.; Shin, N.S. Thyroid gland pathology in children population exposed to the combination of iodine deficiency and fluoride pollution of environment. Gig. Sanit. 2016, 95, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.V.; Hall, M.; Green, R.; Chevrier, J.; Ayotte, P.; Martinez-Mier, E.A.; McGuckin, T.; Krzeczkowski, J.; Flora, D.; Hornung, R.; et al. Iodine Status Modifies the Association between Fluoride Exposure in Pregnancy and Preschool Boys’ Intelligence. Nutrients 2022, 14, 2920. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, Q.; Cui, Y.; Yu, L.; Zhao, L.; Hou, C.; Zhang, S.; Zhang, L.; Fu, G.; Liu, Y.; et al. The effects and underlying mechanism of excessive iodide on excessive fluoride-induced thyroid cytotoxicity. Environ. Toxicol. Pharmacol. 2014, 38, 332–340. [Google Scholar] [CrossRef]
- Cui, Y.; Yu, J.; Zhang, B.; Guo, B.; Gao, T.; Liu, H. The relationships between thyroid-stimulating hormone and/or dopamine levels in peripheral blood and IQ in children with different urinary iodine concentrations. Neurosci. Lett. 2020, 729, 134981. [Google Scholar] [CrossRef]
- Malin, A.J.; Riddell, J.; McCague, H.; Till, C. Fluoride exposure and thyroid function among adults living in Canada: Effect modification by iodine status. Environ. Int. 2018, 121, 667–674. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, H.; Yu, Z.; Aoki, K.; Misumi, J.; Zhang, X. Long-term Effects of Various Iodine and Fluorine Doses on the Thyroid and Fluorosis in Mice. Endocr. Regul. 1998, 32, 63–70. [Google Scholar] [PubMed]
- Wirth, E.K.; Meyer, F. Neuronal effects of thyroid hormone metabolites. Mol. Cell Endocrinol. 2017, 458, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, W.; Wang, Q.; Li, Q.; Wang, H.; Wang, J.; Teng, T.; Chen, M.; Ji, A.; Li, Y. New Drug Candidate Targeting the 4A1 Orphan Nuclear Receptor for Medullary Thyroid Cancer Therapy. Molecules 2018, 23, 565. [Google Scholar] [CrossRef] [PubMed]
- Previc, F.H. Dopamine and the origins of human intelligence. Brain Cogn. 1999, 41, 299–350. [Google Scholar] [CrossRef]
- Cooper, D.S.; Klibanski, A.; Ridgway, E.C. Dopaminergic modulation of TSH and its subunits: In vivo and in vitro studies. Clin. Endocrinol. 1983, 18, 265–275. [Google Scholar] [CrossRef]
- Yu, X.; Chen, J.; Li, Y.; Liu, H.; Hou, C.; Zeng, Q.; Cui, Y.; Zhao, L.; Li, P.; Zhou, Z.; et al. Threshold effects of moderately excessive fluoride exposure on children’s health: A potential association between dental fluorosis and loss of excellent intelligence. Environ. Int. 2018, 118, 116–124. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Tang, D.; Zhao, J.; Yang, X.; Liu, Y.; Peng, F.; Shu, L.; Wang, J.; He, Z.; et al. Effects of water improvement and defluoridation on fluorosis-endemic areas in China: A meta-analysis. Environ. Pollut. 2021, 270, 116227. [Google Scholar] [CrossRef]
- Veneri, F.; Vinceti, M.; Generali, L.; Giannone, M.E.; Mazzoleni, E.; Birnbaum, L.S.; Consolo, U.; Filippini, T. Fluoride exposure and cognitive neurodevelopment: Systematic review and dose-response meta-analysis. Environ. Res. 2023, 221, 115239. [Google Scholar] [CrossRef]
- WS/T 107.1-2016; Determination of iodine in urine Part 1: AS3+-Ce4+ Catalytic Spectrophotometry. National Disease Control and Prevention Administration: Beijing, China, 2016; p. 8.
- WS/T 89-2015; Determination of Fluoride in Urine Ion-Selective Electrode Method. National Disease Control and Prevention Administration: Beijing, China, 2015; p. 6.
- World Health Organization (WHO). Physical Status: The Use of and Interpretation of Anthropometry, Report of a WHO Expert Committee; WHO Technical Report Series; WHO: Geneva, Switzerland, 1995; Volume 854, pp. 1–452. [Google Scholar]
- Chen, W.; Li, X.; Wu, Y.; Bian, J.; Shen, J.; Jiang, W.; Tan, L.; Wang, X.; Wang, W.; Pearce, E.N.; et al. Associations between iodine intake, thyroid volume, and goiter rate in school-aged Chinese children from areas with high iodine drinking water concentrations. Am. J. Clin. Nutr. 2017, 105, 228–233. [Google Scholar] [CrossRef]
- WS 276-2007; Diagnostic Criteria for Endemic Goitre. National Disease Control and Prevention Administration: Beijing, China, 2007; p. 7.
- Zhai, X.; Zhang, L.; Chen, L.; Lian, X.; Liu, C.; Shi, B.; Shi, L.; Tong, N.; Wang, S.; Weng, J.; et al. An Age-Specific Serum Thyrotropin Reference Range for the Diagnosis of Thyroid Diseases in Older Adults: A Cross-Sectional Survey in China. Thyroid. 2018, 28, 1571–1579. [Google Scholar] [CrossRef]
- Liu, H.-L.; Lam, L.T.; Zeng, Q.; Han, S.-Q.; Fu, G.; Hou, C.-C. Effects of drinking water with high iodine concentration on the intelligence of children in Tianjin, China. J. Public. Health 2009, 31, 32–38. [Google Scholar] [CrossRef]
- Sun, H.; Chen, W.; Wang, D.; Jin, Y.; Chen, X.; Xu, Y.; Huang, L. Inverse association between intelligence quotient and urinary retinol binding protein in Chinese school-age children with low blood lead levels: Results from a cross-sectional investigation. Chemosphere 2015, 128, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Nunez, E.; Steyerberg, E.W.; Nunez, J. Regression modeling strategies. Rev. Esp. Cardiol. 2011, 64, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Padyukov, L.; Silva, C.; Stolt, P.; Alfredsson, L.; Klareskog, L. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. 2004, 50, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Knol, M.J.; van der Tweel, I.; Grobbee, D.E.; Numans, M.E.; Geerlings, M.I. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int. J. Epidemiol. 2007, 36, 1111–1118. [Google Scholar] [CrossRef]
- Wang, M.; Liu, L.; Li, H.; Li, Y.; Liu, H.; Hou, C.; Zeng, Q.; Li, P.; Zhao, Q.; Dong, L.; et al. Thyroid function, intelligence, and low-moderate fluoride exposure among Chinese school-age children. Environ. Int. 2020, 134, 105229. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Zhang, D.; Duan, Y.; Li, F.; Li, W.; Chen, L.; Jia, G. Reference Intervals for Thyroid Hormones and Thyroid Volume in Children Aged 7 ~ 13 Years from Iodine-Sufficient Areas in Tianjin, China. Biol. Trace Elem. Res. 2023, 201, 5575–5584. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, Y.H.; Lim, Y.-H.; Kim, B.-N.; Kim, J.I.; Hong, Y.-C.; Park, Y.J.; Shin, C.H.; Cho, S.W.; Lee, Y.A. Effects of iodine status on thyroid volume and goiter in children living in an iodine-replete area. Eur. Thyroid. J. 2023, 12, e230219. [Google Scholar] [CrossRef]
- An, S.; Fan, L.; Li, M.; Wang, Z.; Liu, S.; Ji, Y.; Liu, X.L.; Du, Y.; Meng, F.; Liu, P. Relationship between water iodine and children’s goiters. Brit. J. Nutr. 2022, 128, 1798–1805. [Google Scholar] [CrossRef]
- Leung, A.M.; Braverman, L.E. Consequences of excess iodine. Nat. Rev. Endocrinol. 2014, 10, 136–142. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, X.; Sun, Q.; Shan, Z.; Teng, W. Effects of Excess Fluoride and Iodide on Thyroid Function and Morphology. Biol. Trace Elem. Res. 2016, 170, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Jenq, S.F.; Jap, T.S.; Hsieh, M.S.; Chiang, H. The characterization of adenyl cyclase activity in FRTL-5 cell line. Zhonghua Yi Xue Za Zhi 1993, 51, 159–165. [Google Scholar] [PubMed]
- Niedziela, M. Thyroid nodules. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 245–277. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.J. Thyroid nodules in children and adolescents. Curr. Opin. Endocrinol. 2019, 26, 266–274. [Google Scholar] [CrossRef]
- Cherella, C.E.; Angell, T.E.; Richman, D.M.; Frates, M.C.; Benson, C.B.; Moore, F.D.; Barletta, J.A.; Hollowell, M.; Smith, J.R.; Alexander, E.K.; et al. Differences in Thyroid Nodule Cytology and Malignancy Risk Between Children and Adults. Thyroid 2019, 29, 1097–1104. [Google Scholar] [CrossRef]
- Spachmo, B.; Arukwe, A. Endocrine and developmental effects in Atlantic salmon (Salmo salar) exposed to perfluorooctane sulfonic or perfluorooctane carboxylic acids. Aquat. Toxicol. 2012, 108, 112–124. [Google Scholar] [CrossRef]
- Serrano-Nascimento, C.; Salgueiro, R.B.; Pantaleão, T.; da Costa, V.M.C.; Nunes, M.T. Maternal Exposure to Iodine Excess Throughout Pregnancy and Lactation Induces Hypothyroidism in Adult Male Rat Offspring. Sci. Rep. 2017, 7, 15591. [Google Scholar] [CrossRef]
- Gupta, A.; Ly, S.; Castroneves, L.A.; Frates, M.C.; Benson, C.B.; Feldman, H.A.; Wassner, A.J.; Smith, J.R.; Marqusee, E.; Alexander, E.K.; et al. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. J. Clin. Endocr. Metab. 2013, 98, 3238–3245. [Google Scholar] [CrossRef]
- Suzuki, S.; Suzuki, S.; Iwadate, M.; Matsuzuka, T.; Shimura, H.; Ohira, T.; Furuya, F.; Suzuki, S.; Yasumura, S.; Yokoya, S.; et al. Possible Association Between Thyroid Nodule Formation and Developmental Alterations in the Pituitary-Thyroid Hormone Axis in Children and Adolescents: The Fukushima Health Management Survey. Thyroid 2022, 32, 1316–1327. [Google Scholar] [CrossRef]
- Uyghurturk, D.A.; Goin, D.E.; Martinez-Mier, E.A.; Woodruff, T.J.; DenBesten, P.K. Maternal and fetal exposures to fluoride during mid-gestation among pregnant women in northern California. Environ. Health 2020, 19, 38. [Google Scholar] [CrossRef]
- Duan, Q.; Jiao, J.; Chen, X.; Wang, X. Association between water fluoride and the level of children’s intelligence: A dose-response meta-analysis. Public. Health 2018, 154, 87–97. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, C.; Lv, J.; Cui, Y.; Wang, Y.; Hou, C.; Yu, J.; Guo, B.; Liu, H.; Li, L. Fluoride exposure, dopamine relative gene polymorphism and intelligence: A cross-sectional study in China. Ecotox. Environ. Safe 2021, 209, 111826. [Google Scholar] [CrossRef] [PubMed]
- Sabour, S.; Ghorbani, Z. Developmental fluoride neurotoxicity: Clinical importance versus statistical significance. Environ. Health Persp. 2013, 121, A70. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, J.; Zhang, Z. Effects of fluoride on synaptic membrane fluidity and PSD-95 expression level in rat hippocampus. Biol. Trace Elem. Res. 2011, 139, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhang, S.; Liu, H.; Guan, Z.; Zeng, Q.; Zhang, C.; Lei, R.; Xia, T.; Wang, Z.; Yang, L.; et al. Low glucose utilization and neurodegenerative changes caused by sodium fluoride exposure in rat’s developmental brain. Neuromol Med. 2014, 16, 94–105. [Google Scholar] [CrossRef]
- Söderqvist, S.; Nutley, S.B.; Peyrard-Janvid, M.; Matsson, H.; Humphreys, K.; Kere, J.; Klingberg, T. Dopamine, working memory, and training induced plasticity: Implications for developmental research. Dev. Psychol. 2012, 48, 836–843. [Google Scholar] [CrossRef]
- Schlagenhauf, F.; Rapp, M.A.; Huys, Q.J.M.; Beck, A.; Wüstenberg, T.; Deserno, L.; Buchholz, H.; Kalbitzer, J.; Buchert, R.; Bauer, M.; et al. Ventral striatal prediction error signaling is associated with dopamine synthesis capacity and fluid intelligence. Hum. Brain Mapp. 2013, 34, 1490–1499. [Google Scholar] [CrossRef]
- Kupnicka, P.; Listos, J.; Tarnowski, M.; Kolasa-Wołosiuk, A.; Wąsik, A.; Łukomska, A.; Barczak, K.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Fluoride Affects Dopamine Metabolism and Causes Changes in the Expression of Dopamine Receptors (D1R and D2R) in Chosen Brain Structures of Morphine-Dependent Rats. Int. J. Mol. Sci. 2020, 21, 2361. [Google Scholar] [CrossRef]
- Fisher, D.A. Hypothyroxinemia in premature infants: Is thyroxine treatment necessary? Thyroid 1999, 9, 715–720. [Google Scholar] [CrossRef]
- Ghassabian, A.; El Marroun, H.; Peeters, R.P.; Jaddoe, V.W.; Hofman, A.; Verhulst, F.C.; Tiemeier, H.; White, T. Downstream effects of maternal hypothyroxinemia in early pregnancy: Nonverbal IQ and brain morphology in school-age children. J. Clin. Endocr. Metab. 2014, 99, 2383–2390. [Google Scholar] [CrossRef]
- Levie, D.; Korevaar, T.I.M.; Bath, S.C.; Dalmau-Bueno, A.; Murcia, M.; Espada, M.; Dineva, M.; Ibarluzea, J.M.; Sunyer, J.; Tiemeier, H.; et al. Thyroid Function in Early Pregnancy, Child IQ, and Autistic Traits: A Meta-Analysis of Individual Participant Data. J. Clin. Endocr. Metab. 2018, 103, 2967–2979. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, T.; Sienaert, P. The use of triiodothyronine (T3) in the treatment of bipolar depression: A review of the literature. J. Affect. Disord. 2018, 229, 410–414. [Google Scholar] [CrossRef]
- Sokolov, S.T.; Kutcher, S.P.; Joffe, R.T. Basal thyroid indices in adolescent depression and bipolar disorder. J. Am. Acad. Child. Psychiatry 1994, 33, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Trotman, H.D.; Holtzman, C.W.; Ryan, A.T.; Shapiro, D.I.; MacDonald, A.N.; Goulding, S.M.; Brasfield, J.L.; Walker, E.F. The development of psychotic disorders in adolescence: A potential role for hormones. Horm. Behav. 2013, 64, 411–419. [Google Scholar] [CrossRef]
- Cleare, A.J.; McGregor, A.; O’Keane, V. Neuroendocrine evidence for an association between hypothyroidism, reduced central 5-HT activity and depression. Clin. Endocrinol. 1995, 43, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Heinz, A.; Whybrow, P.C. Thyroid hormones, serotonin and mood: Of synergy and significance in the adult brain. Mol. Psychiatr. 2002, 7, 140–156. [Google Scholar] [CrossRef]
- Bauer, M.; Goetz, T.; Glenn, T.; Whybrow, P.C. The thyroid-brain interaction in thyroid disorders and mood disorders. J. Neuroendocrinol. 2008, 20, 1101–1114. [Google Scholar] [CrossRef]
- Whybrow, P.C.; Prange, A.J. A hypothesis of thyroid-catecholamine-receptor interaction. Its relevance to affective illness. Arch. Gen. Psychiatry 1981, 38, 106–113. [Google Scholar] [CrossRef]
- Kelly, T.; Lieberman, D.Z. The use of triiodothyronine as an augmentation agent in treatment-resistant bipolar II and bipolar disorder NOS. J. Affect. Disord. 2009, 116, 222–226. [Google Scholar] [CrossRef]


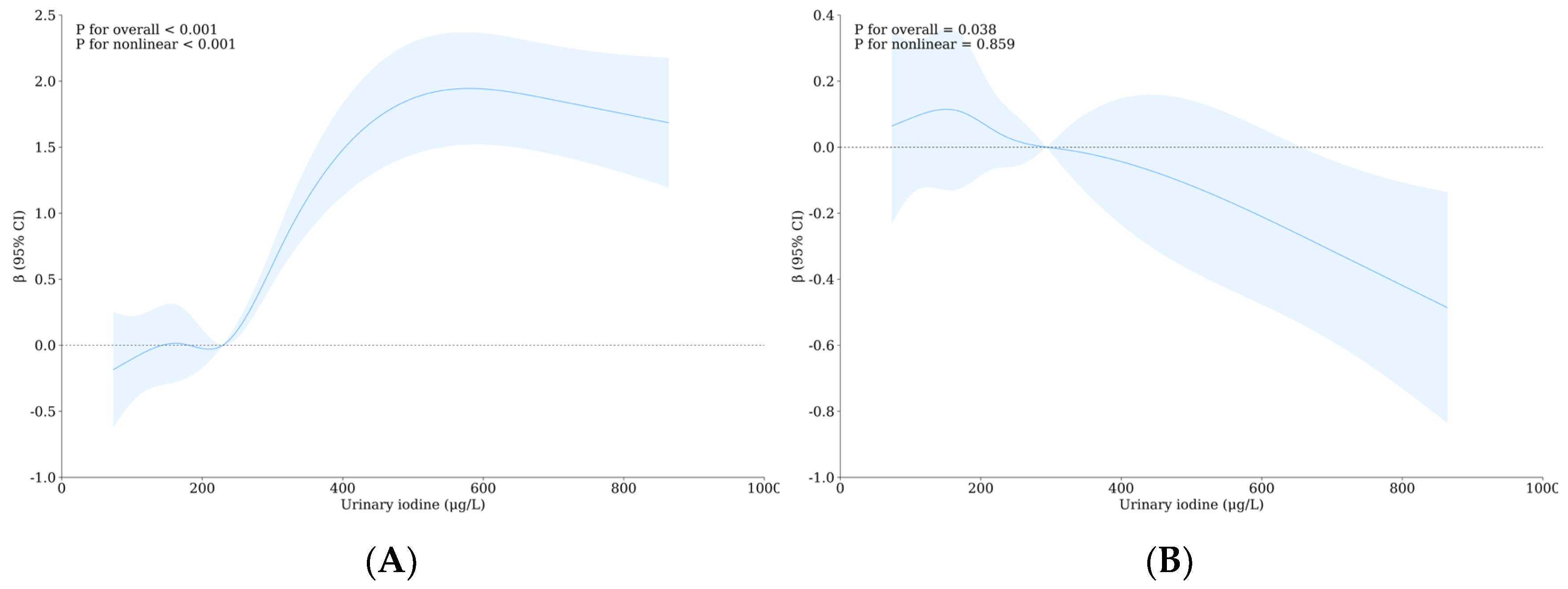
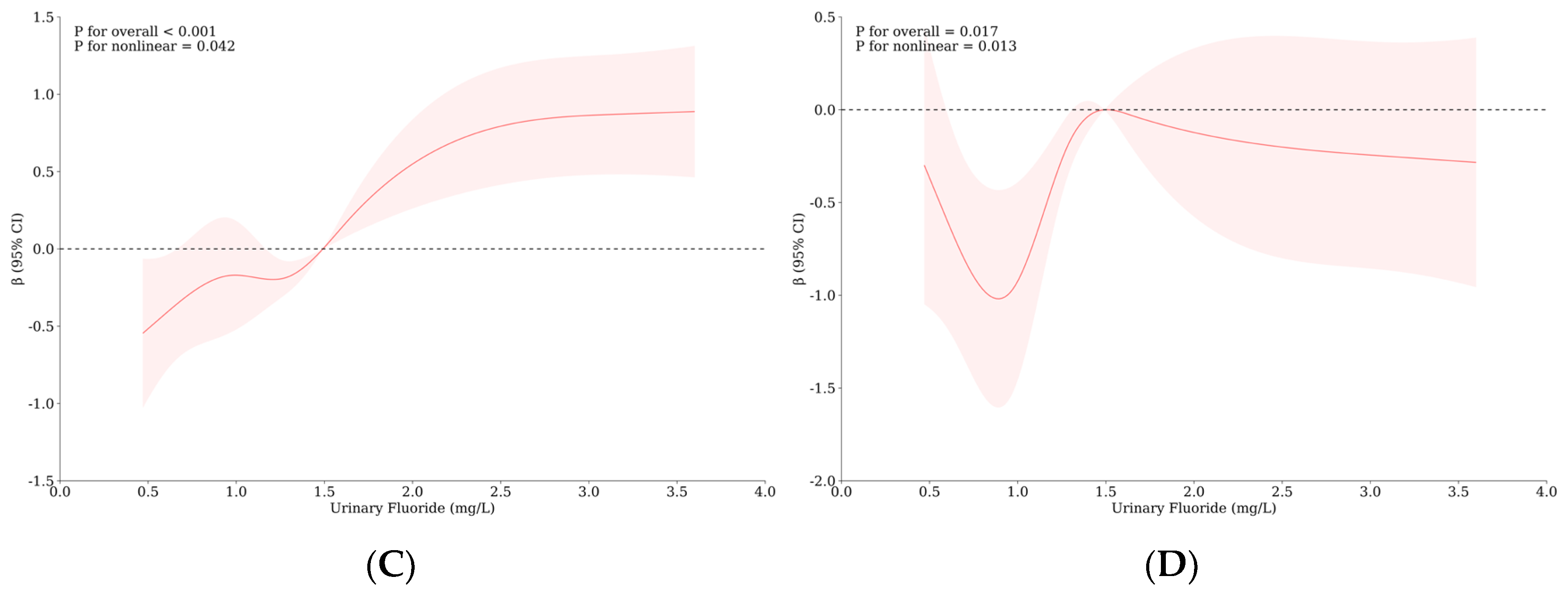
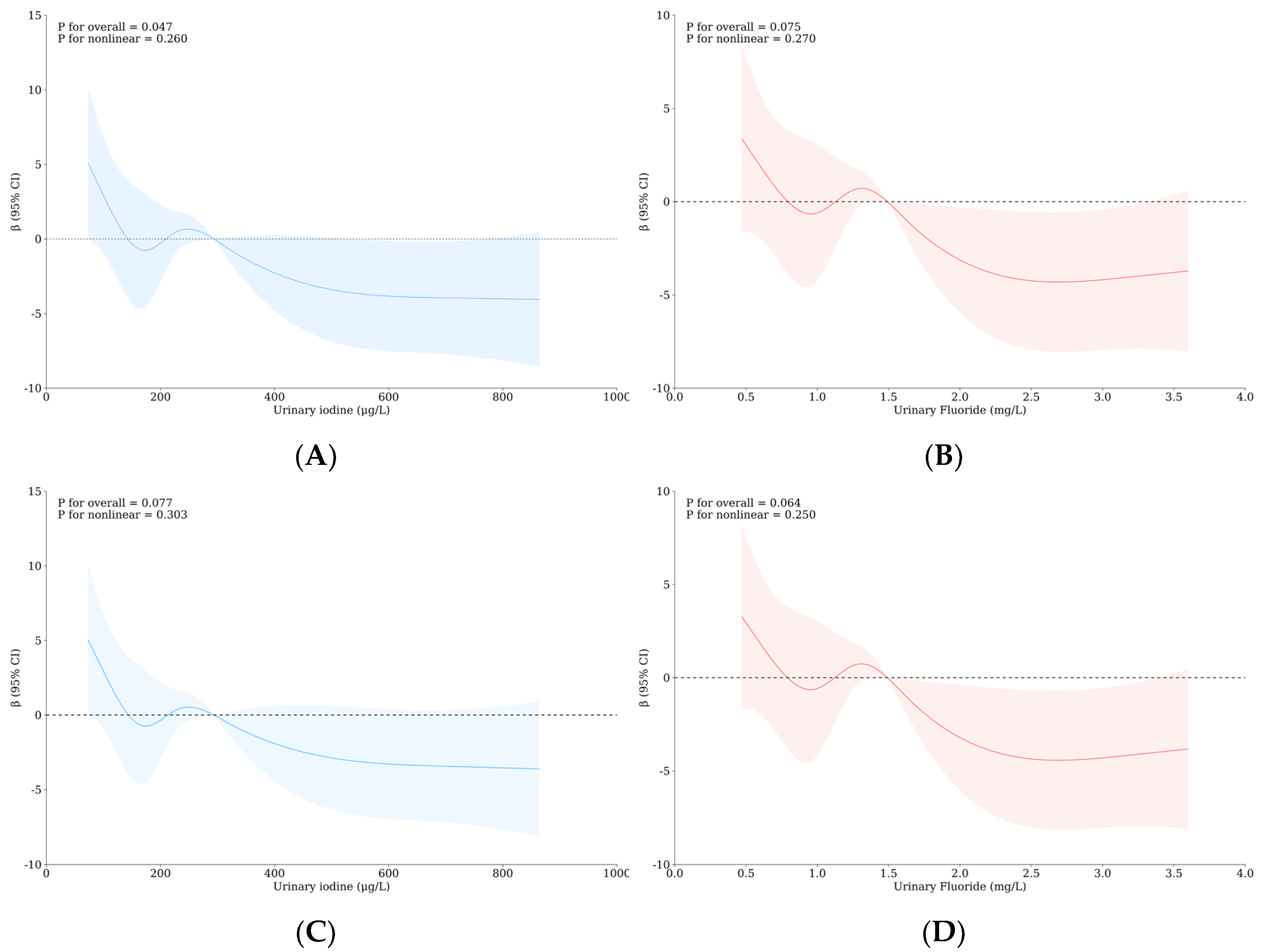
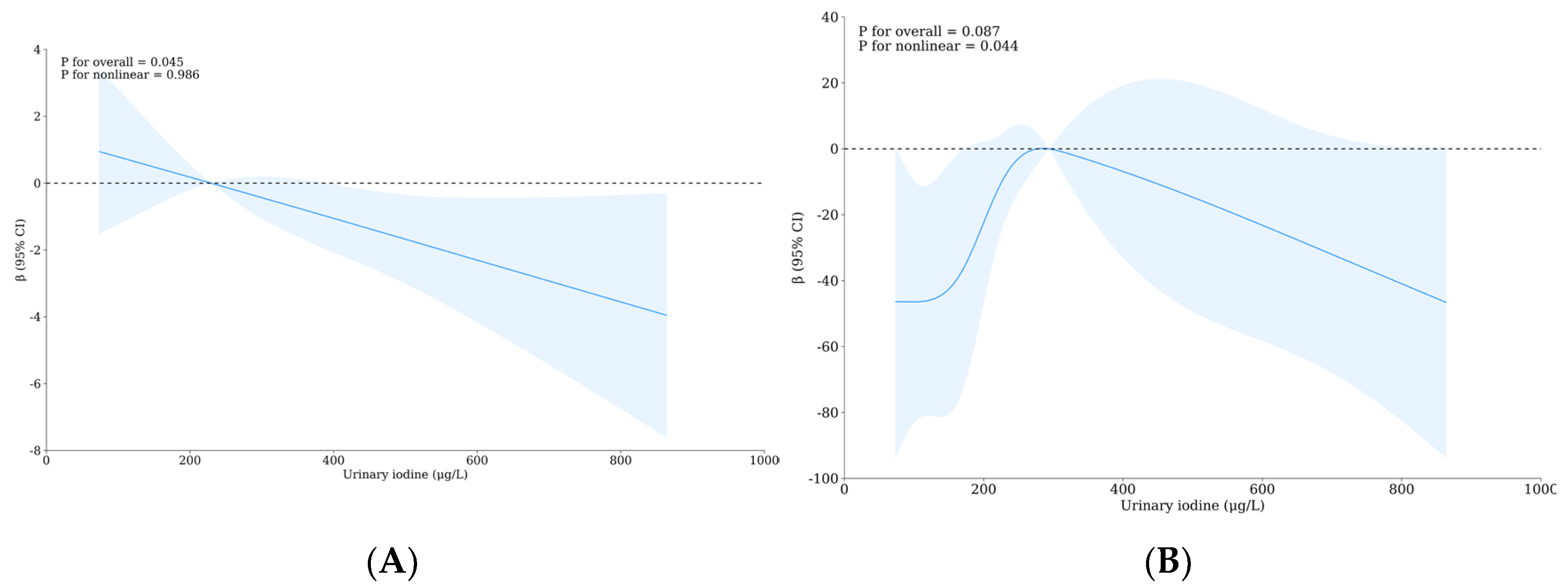

| Variable | Level | WI-WF Group a, b | p | Overall | UI-UF Group a, b | p | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-H | N-L | N-M | H-H | L-L | L-H | H-L | H-H | ||||||
| Sample size | 100 | 99 | 100 | 100 | 399 | 115 | 22 | 93 | 135 | 365 | |||
| Age (mean ± SD) | years old | 9.35 ± 1.21 | 9.24 ± 1.26 | 9.58 ± 1.22 | 9.72 ± 0.79 | 0.013 * | 9.47 ± 1.15 | 9.40 ± 1.31 | 9.82 ± 1.15 | 9.26 ± 1.17 | 9.64 ± 0.97 | 0.036 * | 9.48 ± 1.16 |
| FT3 (mean ± SD) | pmol/L | 6.86 ± 0.62 | 6.70 ± 0.85 | 6.87 ± 0.80 | 6.34 ± 0.65 | <0.001 ** | 6.72 ± 0.76 | 6.83 ± 0.65 | 6.95 ± 0.42 | 6.82 ± 0.63 | 6.47 ± 0.80 | <0.001 ** | 6.71 ± 0.71 |
| FT4 (mean ± SD) | pmol/L | 16.28 ± 1.53 | 17.36 ± 2.00 | 16.86 ± 1.92 | 17.07 ± 1.92 | <0.001 ** | 16.88 ± 1.88 | 16.79 ± 1.78 | 17.41 ± 1.71 | 16.48 ± 1.96 | 17.16 ± 2.00 | 0.047 * | 16.88 ± 1.91 |
| IQ (mean ± SD) | scores | 112.07 ± 15.50 | 114.04 ± 11.05 | 114.22 ± 11.95 | 108.33 ± 14.51 | 0.006 ** | 112.16 ± 13.55 | 114.59 ± 13.73 | 107.86 ± 13.78 | 112.18 ± 12.76 | 110.90 ± 13.65 | 0.070 | 112.20 ± 13.54 |
| BMI (median [IQR]) | kg/m2 | 17.12 (15.75, 20.5) | 16.89 (15.44, 18.31) | 16.22 (14.54, 18.90) | 16.35 (14.83, 18.56) | 0.020 * | 16.74 (15.23, 18.90) | 16.96 (15.46, 19.14) | 15.76 (14.37, 19.38) | 16.73 (15.33, 18.65) | 16.35 (15.02, 19.08) | 0.515 | 16.74 (15.22, 18.90) |
| Tvol (median [IQR]) | mL | 0.99 (0.88, 1.13) | 1.29 (1.20, 1.46) | 1.14 (0.93, 1.30) | 4.08 (3.38, 4.95) | <0.001 ** | 1.26 (1.02, 2.24) | 1.16 (1.02, 1.36) | 1.01 (0.94, 1.47) | 1.23 (1.08, 1.69) | 2.67 (1.22, 4.26) | <0.001 ** | 1.27 (1.02, 2.40) |
| TSH (median [IQR]) | μIU/mL | 2.73 (1.98, 3.28) | 2.97 (2.20, 4.16) | 2.64 (2.19, 3.69) | 2.71 (1.75, 4.32) | 0.167 | 2.75 (2.06, 3.77) | 2.64 (2.16, 3.64) | 2.91 (2.26, 3.53) | 2.84 (2.18, 3.96) | 2.80 (2.05, 4.08) | 0.740 | 2.77 (2.09, 3.75) |
| UI (median [IQR]) | μg/L | 187.65 (133.40, 234.15) | 217.20 (158.38, 289.73) | 209.15 (137.68, 255.12) | 476.30 (333.45, 629.45) | <0.001 ** | 229.30 (160.65, 337.20) | 139.50 (110.10, 162.65) | 167.15 (135.90, 183.43) | 253.50 (222.50, 308.90) | 376.00 (270.80, 525.45) | <0.001 ** | 227.50 (159.70, 336.30) |
| UF (median [IQR]) | mg/L | 1.01 (0.75, 1.40) | 1.05 (0.76, 1.52) | 1.24 (0.89, 1.47) | 2.18 (1.52, 2.79) | <0.001 ** | 1.29 (0.89, 1.84) | 0.89 (0.71, 1.07) | 1.62 (1.46, 1.89) | 1.01 (0.83, 1.24) | 2.10 (1.64, 2.70) | <0.001 ** | 1.29 (0.89, 1.86) |
| 5-HT (median [IQR]) | ng/mL | 125.43 (78.84, 253.54) | 139.39 (97.00, 334.83) | 134.10 (83.37, 232.35) | 104.45 (70.79, 202.08) | 0.036 * | 127.44 (79.08, 245.91) | 114.81 (74.45, 241.98) | 123.17 (70.33, 218.88) | 158.76 (87.49, 300.60) | 124.04 (79.93, 230.67) | 0.200 | 128.16 (79.77, 245.91) |
| NE (median [IQR]) | ng/L | 1169.83 (854.96, 1369.12) | 1184.24 (885.91, 1588.78) | 1008.21 (808.65, 1281.21) | 1078.17 (924.48, 1311.28) | 0.124 | 1094.07 (853.77, 1366.29) | 1107.81 (842.60, 1305.38) | 1249.23 (943.59, 1483.40) | 1006.82 (7714.1, 1329.36) | 1119.66 (923.62, 1413.85) | 0.244 | 1094.19 (854.78, 1367.70) |
| DA (median [IQR]) | mg/mL | 7.34 (4.80, 13.28) | 4.21 (2.54, 8.82) | 6.06 (2.88, 12.64) | 4.57 (1.95, 9.48) | 0.004 ** | 5.77 (2.86, 11.54) | 5.95 (3.74, 11.68) | 6.99 (3.99, 12.26) | 6.84 (2.93, 14.37) | 5.32 (2.65, 9.79) | 0.299 | 5.98 (3.04, 11.44) |
| E (median [IQR]) | ng/L | 334.15 (286.60, 488.81) | 347.47 (259.41, 487.11) | 356.00 (255.79, 496.39) | 386.13 (284.82, 624.17) | 0.240 | 350.30 (268.68, 517.98) | 318.08 (258.29, 502.60) | 402.09 (245.86, 585.47) | 334.98 (284.33, 467.08) | 391.74 (265.15, 561.89) | 0.475 | 345.55 (266.33, 522.50) |
| Gender (%) | Boys | 50 (50.00) | 48 (48.48) | 50 (50.00) | 53 (53.00) | 0.934 | 201 (50.38) | 64 (55.65) | 14 (63.64) | 45 (48.39) | 70 (51.85) | 0.535 | 193 (52.88) |
| Girls | 50 (50.00) | 51 (51.52) | 50 (50.00) | 47 (47.00) | 198 (49.62) | 51 (44.35) | 8 (36.36) | 48 (51.61) | 65 (48.15) | 172 (47.12) | |||
| Thyroid nodule (%) | No | 99 (99.00) | 91 (91.92) | 96 (96.00) | 73 (73.00) | <0.001 ** | 359 (89.97) | 107 (93.04) | 22 (100.00) | 90 (96.77) | 109 (80.74) | <0.010 ** | 328 (89.86) |
| Yes | 1 (1.00) | 8 (8.08) | 4 (4.00) | 27 (27.00) | 40 (10.03) | 8 (6.96) | 0 (0.00) | 3 (3.23) | 26 (19.26) | 37 (10.14) | |||
| Goiter (%) | Yes | 0 (0.00) | 0 (0.00) | 0 (0.00) | 15 (15.00) | <0.001 ** | 15 (3.76) | 0 (0.00) | 0 (0.00) | 1 (1.08) | 13 (9.63) | <0.010 ** | 14 (3.84) |
| No | 100 (100.00) | 99 (100.00) | 100 (100.00) | 85 (85.00) | 384 (96.24) | 115 (100.00) | 22 (100.00) | 92 (98.92) | 122 (90.37) | 351 (96.16) | |||
| Factor | WI-WF Group d | |||||
|---|---|---|---|---|---|---|
| N-M b | N-H b | H-H b | ||||
| Crude OR/ β (95% CI) a | Adjusted OR/ β (95% CI) a,c | Crude OR/ β (95% CI) a | Adjusted OR/ β (95% CI) a,c | Crude OR/ β (95% CI) a | Adjusted OR/ β (95% CI) a,c | |
| Thyroid nodule | 0.47 (0.14~1.63) | 0.50 (0.13~1.92) | 0.12 (0.01~0.94) * | 0.12 (0.01~1.15) | 4.21 (1.80~9.81) * | 5.27 (1.84~15.12) * |
| Thyroid abnormal | 0.56 (0.25~1.25) | 0.55 (0.23~1.33) | 0.29 (0.11~0.76) * | 0.33 (0.12~0.93) * | 4.32 (2.27~8.23) * | 5.68 (2.63~12.25) * |
| Thyroid disease | 0.98 (0.65~1.47) | 1.07 (0.57–2.03) | 1.28 (0.79~2.08) | 1.08 (0.58–2.00) | 0.84 (0.57~1.23) | 1.06 (0.56–2.02) |
| Tvol | −0.16 (−0.35~0.03) | −0.15 (−0.35~0.04) | −0.27 (−0.46~−0.08) * | −0.31 (−0.50~−0.12) * | 2.91 (2.72~3.10) * | 2.96 (2.75~3.16) * |
| FT3 | 0.17 (−0.04~0.39) | 0.18 (−0.04~0.40) | 0.16 (−0.05~0.38) | 0.15 (−0.07~0.37) | −0.36 (−0.58~−0.13) * | −0.38 (−0.62~−0.14) * |
| FT4 | −0.50 (−1.03~0.02) | −0.39 (−0.94~0.17) | −1.09 (−1.62~−0.56) * | −1.08 (−1.64~−0.53) * | −0.30 (−0.86~0.27) | −0.23 (−0.84~0.37) |
| TSH | −0.35 (−0.82~0.12) | −0.32 (−0.81~0.17) | −0.61 (−1.08~−0.14) * | −0.66 (−1.15~−0.17) * | −0.10 (−0.60~0.40) | −0.06 (−0.59~0.47) |
| Factor | WI-WF Group d | |||||
|---|---|---|---|---|---|---|
| N-M b | N-H b | H-H b | ||||
| Crude OR/ β (95% CI) a | Adjusted OR/ β (95% CI) a,c | Crude OR/ β (95% CI) a | Adjusted OR/ β (95% CI) a,c | Crude OR/ β (95% CI) a | Adjusted OR/ β (95% CI) a,c | |
| Degree of Intelligence | 1.03 (0.79~1.32) | 1.22 (0.72–1.89) | 0.93 (0.72~1.20) | 1.08 (0.84–1.77) | 0.69 (0.53~0.90) * | 0.77 (0.49–0.95) * |
| IQ | 0.18 (−3.55~3.91) | 0.33 (−3.71~4.36) | −1.97 (−5.70~1.76) | −1.32 (−5.48~2.84) | −5.71 (−9.44~−1.98) * | −5.85 (−10.13~−1.57) * |
| 5-HT | −36.79 (−77.65~4.07) | −27.41 (−71.36~16.55) | −38.28 (−80.99~4.43) | −17.13 (−63.75~29.49) | −69.24 (−114.85~−23.64) * | −55.61 (−104.67~−6.54) * |
| NE | −238.68 (−424.14~−53.22) * | −182.38 (−374.53~9.77) | −102.67 (−300.37~95.03) | −11.02 (−216.24~194.20) | −127.36 (−333.21~78.49) | −76.57 (−288.67~135.53) |
| DA | 2.84 (0.50~5.17) * | 3.34 (0.78~5.89) * | 2.24 (−0.15~4.64) | 3.08 (0.42~5.75) * | −0.42 (−3.01~2.18) | −0.03 (−2.85~2.80) |
| E | 61.64 (−47.66~170.94) | 75.41 (−41.72~192.54) | 37.56 (−78.92~154.03) | 37.36 (−90.11~164.83) | 94.92 (−21.56~211.39) | 115.00 (−10.50~240.50) |
| Factor | UI-UF Group d | |||||
|---|---|---|---|---|---|---|
| L-H b | H-L b | H-H b | ||||
| Crude OR/ β (95% CI) a | Adjusted OR/ β (95% CI) a,c | Crude OR/ β (95% CI) a | Adjusted OR/ β (95% CI) a,c | Crude OR/ β (95% CI) a | Adjusted OR/ β (95% CI) a,c | |
| Thyroid nodule | 0.00 (0.00-Inf) | 0.00 (0.00-Inf) | 0.45 (0.11–1.73) | 0.47 (0.12–1.86) | 3.19 (1.38–7.36) * | 2.85 (1.22–6.69) * |
| Thyroid abnormal | 0.00 (0.00-Inf) | 0.00 (0.00-Inf) | 1.10 (0.50–2.38) | 1.07 (0.48–2.41) | 3.30 (1.75–6.24) * | 2.99 (1.56–5.71) * |
| Thyroid disease | 1.13 (0.44–2.92) | 1.08 (0.41–2.81) | 0.91 (0.50–1.64) | 0.97 (0.53–1.78) | 1.01 (0.59–1.72) | 1.10 (0.90–1.34) |
| Tvol | −0.10 (−0.70~0.51) | −0.17 (−0.77~0.44) | 0.38 (0.01~0.74) * | 0.39 (0.02~0.76) * | 1.59 (1.26~1.92) * | 1.55 (1.21~1.89) * |
| FT3 | 0.12 (−0.20~0.44) | 0.10 (−0.22~0.43) | −0.01 (−0.20~0.19) | −0.00 (−0.20~0.20) | −0.36 (−0.54~−0.18) * | −0.35 (−0.53~−0.16) * |
| FT4 | 0.62 (−0.27~1.50) | 0.76 (−0.13~1.65) | −0.31 (−0.85~0.23) | −0.33 (−0.88~0.21) | 0.37 (−0.13~0.87) | 0.43 (−0.08~0.94) |
| TSH | −0.00 (−0.75~0.74) | −0.03 (−0.79~0.72) | 0.34 (−0.13~0.81) | 0.28 (−0.20~0.76) | 0.31 (−0.11~0.74) | 0.27 (−0.16~0.71) |
| Factor | UI-UF Group d | |||||
|---|---|---|---|---|---|---|
| L-H b | H-L b | H-H b | ||||
| Crude OR/β (95% CI) a | Adjusted OR/β (95% CI) a,c | Crude OR/β (95% CI) a | Adjusted OR/β (95% CI) a,c | Crude OR/β (95% CI) a | Adjusted OR/β (95% CI) a,c | |
| Degree of Intelligence | 0.53 (0.11–2.48) | 0.47 (0.10–2.24) | 1.37 (0.67–2.79) | 1.32 (0.64–2.72) | 1.46 (0.76–2.79) | 1.27 (0.65–2.46) |
| IQ | −6.72 (−12.87~−0.58) * | −4.67 (−10.46~1.12) | −2.40 (−6.09~1.28) | −3.14 (−6.66~0.38) | −3.68 (−7.04~−0.33) * | −2.47 (−5.68~0.74) |
| 5-HT | 0.46 (−67.72~68.63) | −16.98 (−83.95~49.99) | 45.24 (2.69~87.78) * | 36.51 (−7.33~80.35) | 9.87 (−28.90~48.63) | 2.98 (−37.95~43.92) |
| NE | 77.92 (−246.77~402.62) | −1.82 (−312.72~309.08) | −10.71 (−208.51~187.10) | −67.51 (−266.17~131.14) | 86.62 (−91.65~264.88) | 37.55 (−145.86~220.96) |
| DA | 1.41 (−2.16~4.97) | 0.64 (−2.96~4.25) | 1.70 (−0.53~3.94) | 1.47 (−0.88~3.82) | −1.03 (−3.05~0.98) | −1.45 (−3.64~0.73) |
| E | 24.84 (−191.14~240.82) | 18.10 (−201.00~237.19) | −60.39 (−173.61~52.83) | −94.76 (−214.31~24.80) | 11.43 (−90.57~113.44) | 0.04 (−110.52~110.60) |
| Index | Thyroid Nodule | IQ | ||||
|---|---|---|---|---|---|---|
| UI | UF | UI & UF | UI | UF | UI & UF | |
| Coefficients (B ± SE) | −14.97 ± 843.46 | −0.81 ± 0.69 | 1.16 ± 0.43 | −0.02 ± 0.01 | −3.02 ± 1.44 | 0.01 ± 0.00 |
| OR (95% CI) | 0.00 (0.00-Inf) | 0.45 (0.11–1.73) | 3.19 (1.38–7.36) | 0.98 (0.97–1.00) | 0.05 (0.00–0.83) | 1.01 (1.00–1.01) |
| p | 0.99 | 0.24 | 0.01 ** | 0.02 ** | 0.04 ** | 0.07 * |
| RERI (95% CI) | 1.37 (0.74~2.01) | 0.97 (0.82~1.13) | ||||
| AP (95% CI) | 3.08 (0.32~5.83) | 0.97 (0.82~1.11) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Yu, X.; Xing, Z.; Ding, P.; Cui, Y.; Liu, H. The Impact of Exposure to Iodine and Fluorine in Drinking Water on Thyroid Health and Intelligence in School-Age Children: A Cross-Sectional Investigation. Nutrients 2024, 16, 2913. https://doi.org/10.3390/nu16172913
Liu S, Yu X, Xing Z, Ding P, Cui Y, Liu H. The Impact of Exposure to Iodine and Fluorine in Drinking Water on Thyroid Health and Intelligence in School-Age Children: A Cross-Sectional Investigation. Nutrients. 2024; 16(17):2913. https://doi.org/10.3390/nu16172913
Chicago/Turabian StyleLiu, Siyu, Xiaomeng Yu, Zhilei Xing, Peisen Ding, Yushan Cui, and Hongliang Liu. 2024. "The Impact of Exposure to Iodine and Fluorine in Drinking Water on Thyroid Health and Intelligence in School-Age Children: A Cross-Sectional Investigation" Nutrients 16, no. 17: 2913. https://doi.org/10.3390/nu16172913






