Effects of 12-Week Supplementation with Coffee Diterpene Cafestol in Healthy Subjects with Increased Waist Circumference: A Randomized, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Visits
2.3. Tests
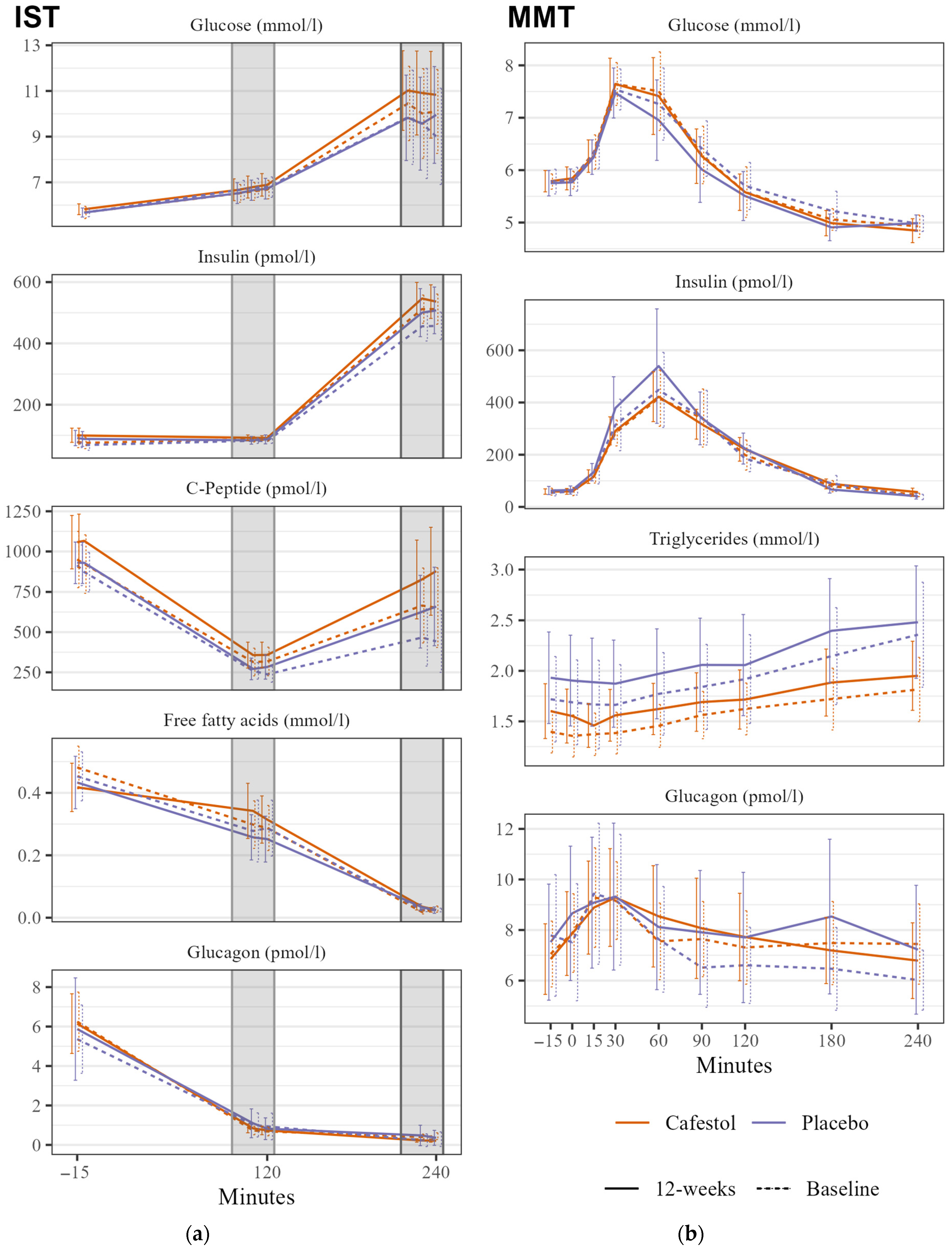
2.3.1. MMT
2.3.2. IST
2.3.3. MRI Scan
2.3.4. ABPM
2.3.5. CGM
2.4. Participants
2.5. Interventions
2.6. Outcomes
2.7. Ancillary Outcomes
2.8. Sample Size
2.9. Randomization
2.10. Blinding
2.11. Statistical Analysis
2.12. Registration
3. Results
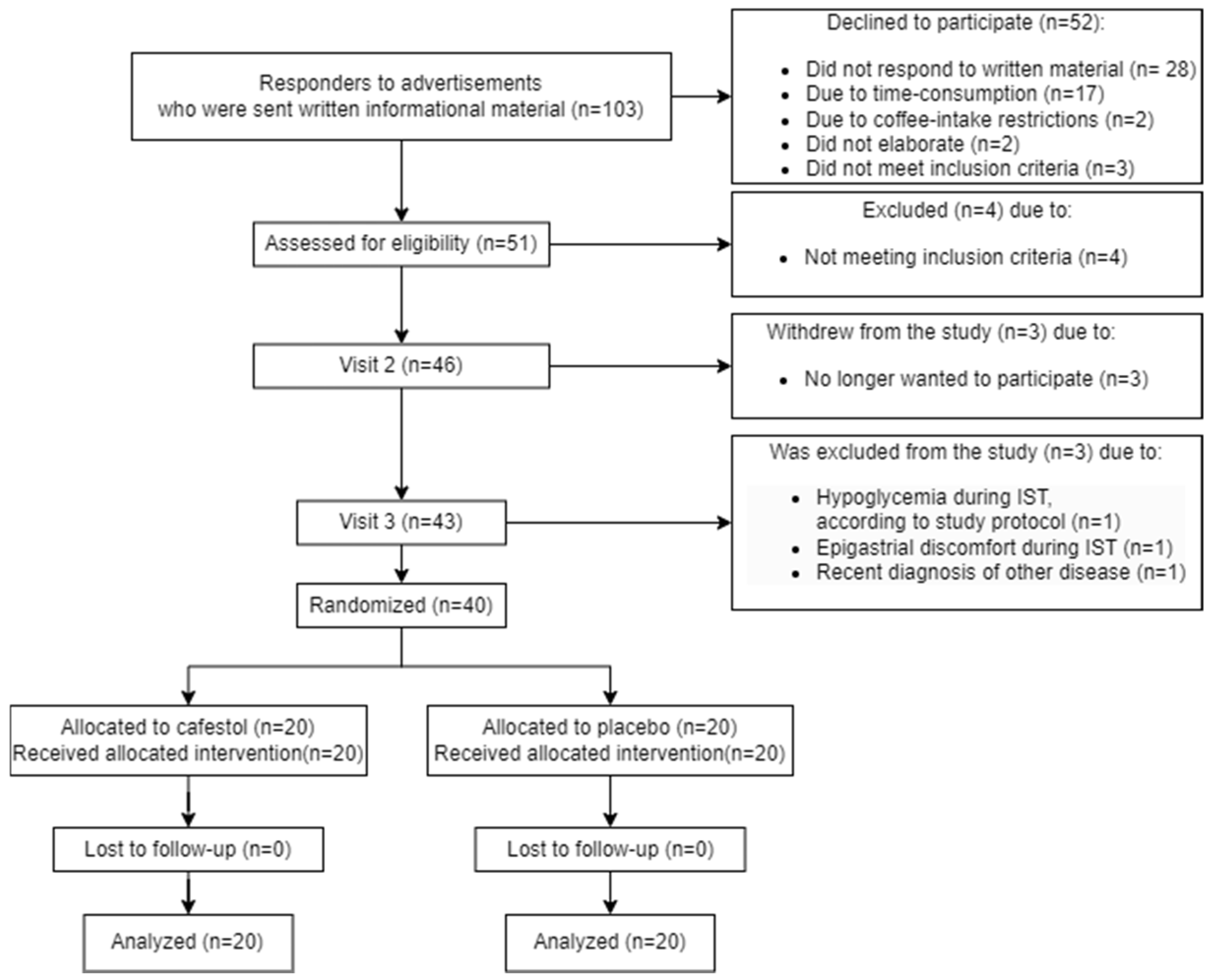
3.1. Participant Flow
3.2. Recruitment
3.3. Baseline Data
3.4. Outcomes
3.4.1. Primary Outcomes
3.4.2. Secondary Outcomes
3.4.3. Ancillary Analyses
3.5. Harms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Statistical Analysis
References
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Uusitupa, M.; Khan, T.A.; Viguiliouk, E.; Kahleova, H.; Rivellese, A.A.; Hermansen, K.; Pfeiffer, A.; Thanopoulou, A.; Salas-Salvado, J.; Schwab, U.; et al. Prevention of Type 2 Diabetes by Lifestyle Changes: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2611. [Google Scholar] [CrossRef] [PubMed]
- Carlstrom, M.; Larsson, S.C. Coffee consumption and reduced risk of developing type 2 diabetes: A systematic review with meta-analysis. Nutr. Rev. 2018, 76, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Bhupathiraju, S.N.; Chen, M.; van Dam, R.M.; Hu, F.B. Caffeinated and Decaffeinated Coffee Consumption and Risk of Type 2 Diabetes: A Systematic Review and a Dose-Response Meta-analysis. Diabetes Care 2014, 37, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef]
- Alperet, D.J.; Rebello, S.A.; Khoo, E.Y.; Tay, Z.; Seah, S.S.; Tai, B.C.; Tai, E.S.; Emady-Azar, S.; Chou, C.J.; Darimont, C.; et al. The effect of coffee consumption on insulin sensitivity and other biological risk factors for type 2 diabetes: A randomized placebo-controlled trial. Am. J. Clin. Nutr. 2020, 111, 448–458. [Google Scholar] [CrossRef]
- Wedick, N.M.; Brennan, A.M.; Sun, Q.; Hu, F.B.; Mantzoros, C.S.; van Dam, R.M. Effects of caffeinated and decaffeinated coffee on biological risk factors for type 2 diabetes: A randomized controlled trial. Nutr. J. 2011, 10, 93. [Google Scholar] [CrossRef]
- Ohnaka, K.; Ikeda, M.; Maki, T.; Okada, T.; Shimazoe, T.; Adachi, M.; Nomura, M.; Takayanagi, R.; Kono, S. Effects of 16-week consumption of caffeinated and decaffeinated instant coffee on glucose metabolism in a randomized controlled trial. J. Nutr. Metab. 2012, 2012, 207426. [Google Scholar] [CrossRef]
- Gross, G.; Jaccaud, E.; Huggett, A.C. Analysis of the content of the diterpenes cafestol and kahweol in coffee brews. Food Chem. Toxicol. 1997, 35, 547–554. [Google Scholar] [CrossRef]
- Urgert, R.; van der Weg, G.; Kosmeijer-Schuil, T.G.; van de Bovenkamp, P.; Hovenier, R.; Katan, M.B. Levels of the Cholesterol-Elevating Diterpenes Cafestol and Kahweol in Various Coffee Brews. J. Agric. Food Chem. 1995, 43, 2167–2172. [Google Scholar] [CrossRef]
- Mellbye, F.B.; Jeppesen, P.B.; Hermansen, K.; Gregersen, S. Cafestol, a Bioactive Substance in Coffee, Stimulates Insulin Secretion and Increases Glucose Uptake in Muscle Cells: Studies in Vitro. J. Nat. Prod. 2015, 78, 2447–2451. [Google Scholar] [CrossRef]
- Mellbye, F.B.; Jeppesen, P.B.; Shokouh, P.; Laustsen, C.; Hermansen, K.; Gregersen, S. Cafestol, a Bioactive Substance in Coffee, Has Antidiabetic Properties in KKAy Mice. J. Nat. Prod. 2017, 80, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuka, H.; Shino, A.; Suzuoki, Z. General survey of diabetic features of yellow KK mice. Endocrinol. Jpn. 1970, 17, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Mellbye, F.D.; Hermansen, K.; Jeppesen, P.B.; Gregersen, S. Acute effects of the coffee diterpene cafestol on glucose metabolism in non-diabetic subjects with abdominal obesity. Rev. Diabet. Stud. 2023, 19, 34–42. [Google Scholar]
- Mellbye, F.D.; Hermansen, K.; Jeppesen, P.B.; Gregersen, S. Cafestol and Kahweol acutely reduce glucose levels in subjects with Type 2 Diabetes. Rev. Diabet. Stud. Press. 2024. [Google Scholar]
- van Rooij, J.; van der Stegen, G.H.; Schoemaker, R.C.; Kroon, C.; Burggraaf, J.; Hollaar, L.; Vroon, T.F.; Smelt, A.H.; Cohen, A.F. A placebo-controlled parallel study of the effect of two types of coffee oil on serum lipids and transaminases: Identification of chemical substances involved in the cholesterol-raising effect of coffee. Am. J. Clin. Nutr. 1995, 61, 1277–1283. [Google Scholar] [CrossRef]
- De Roos, B.; Meyboom, S.; Kosmeijer-Schuil, T.G.; Katan, M.B. Absorption and urinary excretion of the coffee diterpenes cafestol and kahweol in healthy ileostomy volunteers. J. Intern. Med. 1998, 244, 451–460. [Google Scholar] [CrossRef]
- De Roos, B.; Van Tol, A.; Urgert, R.; Scheek, L.M.; Van Gent, T.; Buytenhek, R.; Princen, H.M.; Katan, M.B. Consumption of French-press coffee raises cholesteryl ester transfer protein activity levels before LDL cholesterol in normolipidaemic subjects. J. Intern. Med. 2000, 248, 211–216. [Google Scholar] [CrossRef]
- Urgert, R.; Essed, N.; van der Weg, G.; Kosmeijer-Schuil, T.G.; Katan, M.B. Separate effects of the coffee diterpenes cafestol and kahweol on serum lipids and liver aminotransferases. Am. J. Clin. Nutr. 1997, 65, 519–524. [Google Scholar] [CrossRef]
- Urgert, R.; Meyboom, S.; Kuilman, M.; Rexwinkel, H.; Vissers, M.N.; Klerk, M.; Katan, M.B. Comparison of effect of cafetiere and filtered coffee on serum concentrations of liver aminotransferases and lipids: Six month randomised controlled trial. BMJ 1996, 313, 1362–1366. [Google Scholar] [CrossRef]
- Urgert, R.; Schulz, A.G.; Katan, M.B. Effects of cafestol and kahweol from coffee grounds on serum lipids and serum liver enzymes in humans. Am. J. Clin. Nutr. 1995, 61, 149–154. [Google Scholar] [CrossRef]
- McLaughlin, T.; Yee, G.; Glassford, A.; Lamendola, C.; Reaven, G. Use of a two-stage insulin infusion study to assess the relationship between insulin suppression of lipolysis and insulin-mediated glucose uptake in overweight/obese, nondiabetic women. Metabolism 2011, 60, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.W.; Assimes, T.L.; Tsao, P.S.; Natali, A.; Mari, A.; Quertermous, T.; Reaven, G.M.; Abbasi, F. Measurement of insulin-mediated glucose uptake: Direct comparison of the modified insulin suppression test and the euglycemic, hyperinsulinemic clamp. Metabolism 2013, 62, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008; pp. 1–39. ISBN 9789241501491. [Google Scholar]
- McLaughlin, T.; Carter, S.; Lamendola, C.; Abbasi, F.; Yee, G.; Schaaf, P.; Basina, M.; Reaven, G. Effects of moderate variations in macronutrient composition on weight loss and reduction in cardiovascular disease risk in obese, insulin-resistant adults. Am. J. Clin. Nutr. 2006, 84, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Targher, G. Elevated serum gamma-glutamyltransferase activity is associated with increased risk of mortality, incident type 2 diabetes, cardiovascular events, chronic kidney disease and cancer—A narrative review. Clin. Chem. Lab. Med. 2010, 48, 147–157. [Google Scholar] [CrossRef]
- Fraser, A.; Harris, R.; Sattar, N.; Ebrahim, S.; Davey Smith, G.; Lawlor, D.A. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: The British Women’s Heart and Health Study and meta-analysis. Diabetes Care 2009, 32, 741–750. [Google Scholar] [CrossRef]
- Lee, D.H.; Silventoinen, K.; Jacobs, D.R., Jr.; Jousilahti, P.; Tuomileto, J. gamma-Glutamyltransferase, obesity, and the risk of type 2 diabetes: Observational cohort study among 20,158 middle-aged men and women. J. Clin. Endocrinol. Metab. 2004, 89, 5410–5414. [Google Scholar] [CrossRef]
- Weusten-Van der Wouw, M.P.; Katan, M.B.; Viani, R.; Huggett, A.C.; Liardon, R.; Liardon, R.; Lund-Larsen, P.G.; Thelle, D.S.; Ahola, I.; Aro, A.; et al. Identity of the cholesterol-raising factor from boiled coffee and its effects on liver function enzymes. J. Lipid Res. 1994, 35, 721–733. [Google Scholar] [CrossRef]
- Su, H.Y.; Sheu, W.H.; Chin, H.M.; Jeng, C.Y.; Chen, Y.D.; Reaven, G.M. Effect of weight loss on blood pressure and insulin resistance in normotensive and hypertensive obese individuals. Am. J. Hypertens. 1995, 8, 1067–1071. [Google Scholar] [CrossRef]
- Gorji, Z.; Varkaneh, H.K.; Talaei, S.; Nazary-Vannani, A.; Clark, C.C.T.; Fatahi, S.; Rahmani, J.; Salamat, S.; Zhang, Y. The effect of green-coffee extract supplementation on obesity: A systematic review and dose-response meta-analysis of randomized controlled trials. Phytomedicine 2019, 63, 153018. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Kolesarova, A. The anti-obesity and health-promoting effects of tea and coffee. Physiol. Res. 2021, 70, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Tung, Y.C.; Yang, G.; Li, S.; Ho, C.T. Molecular mechanisms of the anti-obesity effect of bioactive compounds in tea and coffee. Food Funct. 2016, 7, 4481–4491. [Google Scholar] [CrossRef] [PubMed]
- Urgert, R.; Katan, M.B. The cholesterol-raising factor from coffee beans. Annu. Rev. Nutr. 1997, 17, 305–324. [Google Scholar] [CrossRef] [PubMed]
| Data—Mean (SD) | Cafestol (N = 20) | Placebo (N = 20) | Total (N = 40) | |
|---|---|---|---|---|
| Age (years) | 60.2 (15.7) | 57.2 (10.2) | 58.7 (13.2) | |
| Weight (kg) | 96.7 (11.7) | 94.1 (15.3) | 95.4 (13.5) | |
| BMI (kg/m2) | 32.7 (3.5) | 31.6 (3.1) | 32.1 (3.3) | |
| Coffee intake (cups/day) | Total | 3.5 (2.8) | 5.3 (3.6) | 4.4 (3.3) |
| With cafestol | 1.3 (2.3) | 2.4 (3.2) | 1.9 (2.8) | |
| Waist circumference (cm) | Male | 114.4 (6.1) | 112.1 (6.1) | 113.5 (6.0) |
| Female | 104.6 (8.8) | 102.3 (8.3) | 103.2 (8.3) | |
| All participants | 110.6 (8.5) | 106.1 (8.8) | 108.3 (8.9) | |
| IST Glucose (mmol/L) | Start | 8.1 (1.9) | 7.8 (2.3) | 7.9 (2.1) |
| Stage 1 SSPG | 6.6 (0.9) | 6.7 (0.9) | 6.7 (0.9) | |
| Stage 2 SSPG | 10.4 (3.9) | 9.7 (4.8) | 10.0 (4.3) | |
| Mixed Meal | Glucose tAUC (mmol/L × 240 min) | 1427 (134) | 1438 (168) | 1432 (150) |
| CGM | Mean glucose (mmol/L) | 5.8 (0.5) | 5.9 (0.5) | 5.9 (0.5) |
| Time in Range (%) | 96 (8) | 98 (2) | 97 | |
| Glucose variability (24 h-standard deviation) | 1.0 (0.3) | 1.0 (0.2) | 1.0 (0.2) | |
| MRI | Liver fat % | 8.2 (8.2) | 8.6 (8.8) | 8.4 (8.4) |
| Visceral fat (L) | 7.9 (2.8) | 7.0 (2.6) | 7.4 (2.7) | |
| Subcutaneous fat (L) | 13.9 (4.7) | 13.8 (4.0) | 13.8 (4.3) | |
| 24-h blood pressure (mmHg) | Systolic BP (awake) | 129 (12) | 133 (15) | 131 (14) |
| Diastolic BP (awake) | 76 (6) | 82 (7) | 79 (7) | |
| Systolic BP (asleep) | 114 (15) | 115 (20) | 114 (18) | |
| Diastolic BP (asleep) | 64 (5) | 67 (12) | 66 (9) | |
| Fasting blood samples | HbA1c (%, mmol/mol) | 5.5%, 36.6 (3.1) | 5.4%, 35.5 (3.1) | 5.5%, 36.1 (3.1) |
| HOMA-IR (C-peptide) | 43.1 (16.1) | 40.1 (10.8) | 41.6 (13.6) | |
| HOMA-IR (Insulin) | 4.2 (2.4) | 4.0 (2.2) | 4.1 (2.3) | |
| Cholesterol (mmol/L) | 4.6 (1.0) | 5.1 (1.3) | 4.8 (1.1) | |
| HDL-cholesterol (mmol/L) | 1.1 (0.3) | 1.3 (0.4) | 1.2 (0.4) | |
| LDL-cholesterol (mmol/L) | 2.9 (0.7) | 2.9 (0.8) | 2.9 (0.8) | |
| Triglycerides (mmol/L) | 1.3 (0.5) | 1.6 (1.0) | 1.4 (0.8) | |
| Data—N (%) | ||||
| Sex | Male | 11 (55%) | 7 (35%) | 18 (45%) |
| Female | 9 (45%) | 13 (65%) | 22 (55%) | |
| Cholesterol-Lowering drug use | 6 (30%) | 4 (20%) | 10 (25%) | |
| Anti-Hypertensive drug use | 4 (20%) | 4 (20%) | 8 (20%) | |
| Family history of type 2 diabetes | 7 (35%) | 7 (35%) | 14 (35%) | |
| Group difference | ||||
|---|---|---|---|---|
| Absolute βcafestol | Relative βcafestol | 95%CI | p | |
| Insulin Suppression Test | ||||
| Stage 1 SSPG (mmol/L) | 0.18 | −0.21–0.56 | 0.4 | |
| Stage 2 SSPG (mmol/L) | 0.44 | −1.14–2.02 | 0.6 | |
| Mixed Meal Test | ||||
| Glucose tAUC (mmol/L × 240 min) | 36.6 | −22.1–95.2 | 0.22 | |
| Insulin tAUC (pmol/L × 240 min) | −4923 | −12,154–2307 | 0.18 | |
| Glucagon tAUC (pmol/L × 240 min) | −319 | −747–108 | 0.14 | |
| Triglyceride tAUC (mmol/L × 240 min) | 0.98 | 0.84–1.14 | 0.8 | |
| MRI | ||||
| Liver fat % | −0.14 | −2.2–2.0 | 0.9 | |
| Visceral fat (L) | −0.39 | −0.7–−0.1 | 0.014 * | |
| Subcutaneous fat (L) | 0.08 | −0.7–0.9 | 0.8 | |
| 24 h Blood Pressure (mmHg) | ||||
| Daytime systolic † | −5.1 | −13–3 | 0.20 | |
| Daytime diastolic † | −2.2 | −7–3 | 0.4 | |
| Nighttime systolic ‡ | −1.4 | −12–9 | 0.8 | |
| Nighttime diastolic ‡ | −2.0 | −9–5 | 0.6 | |
| Continuous Glucose Monitor | ||||
| Mean Glucose (mmol/L) § | 1.01 | 0.96–1.07 | 0.6 | |
| Time in Range (%) § | 0.6 | −0.8–2.1 | 0.4 | |
| Glucose Variability § | 0.03 | −0.08–0.15 | 0.6 | |
| Fasting Blood samples | ||||
| HbA1c (mmol/mol) | 0.96 | 0.95–1.02 | 0.5 | |
| Group Difference | ||||
|---|---|---|---|---|
| Absolute βcafestol | Relative βcafestol | 95%CI | p | |
| Fasting Blood samples | ||||
| LDL Cholesterol (mmol/L) † | −0.06 | −0.30–0.18 | 0.6 | |
| HDL Cholesterol (mmol/L) | 0.03 | −0.10–0.15 | 0.7 | |
| Plasma Glucose (mmol/L) | 0.08 | −0.11–0.26 | 0.4 | |
| Total Cholesterol (mmol/L) | −0.06 | −0.38–0.26 | 0.7 | |
| Triglycerides (mmol/L) | 0.98 | 0.81–1.19 | 0.9 | |
| Alanine Aminotransferase (U/L) | 0.97 | 0.81–1.16 | 0.7 | |
| Aspartate Aminotransferase (U/L) ‡ | 1.07 | 0.96–1.19 | 0.24 | |
| Gamma-Glutamyltransferase (U/L) | 0.85 | 0.73–0.97 | 0.019 * | |
| Creatinine (μmol/L) | 2.90 | −0.48–6.28 | 0.09 | |
| High-sensitivity CRP (mg/L) | 1.03 | 0.56–1.92 | 0.9 | |
| HOMA-IR (Insulin) | 0.99 | 0.77–1.27 | 0.9 | |
| HOMA-IR (C-peptide) | 0.71 | −4.16–5.58 | 0.8 | |
| Ancillary analyses: Insulin Suppression Test | ||||
| Stage 1 FFA (mmol/L) | 0.07 | 0.00–0.14 | 0.048 * | |
| Ancillary analyses: MRI | ||||
| Gluteus muscle fat % | −0.22 | −2.0–1.6 | 0.8 | |
| Psoas major muscle fat % | 0.09 | −0.9–1.1 | 0.9 | |
| Ancillary analyses: Weight & Height | ||||
| Weight (kg) | −1.79 | −3.45–−0.13 | 0.035 * | |
| BMI (kg/m2) | −0.59 | −1.16–−0.02 | 0.043 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mellbye, F.D.; Nguyen, M.D.; Hermansen, K.; Jeppesen, P.B.; Al-Mashhadi, Z.K.; Ringgaard, S.; Gregersen, S. Effects of 12-Week Supplementation with Coffee Diterpene Cafestol in Healthy Subjects with Increased Waist Circumference: A Randomized, Placebo-Controlled Trial. Nutrients 2024, 16, 3232. https://doi.org/10.3390/nu16193232
Mellbye FD, Nguyen MD, Hermansen K, Jeppesen PB, Al-Mashhadi ZK, Ringgaard S, Gregersen S. Effects of 12-Week Supplementation with Coffee Diterpene Cafestol in Healthy Subjects with Increased Waist Circumference: A Randomized, Placebo-Controlled Trial. Nutrients. 2024; 16(19):3232. https://doi.org/10.3390/nu16193232
Chicago/Turabian StyleMellbye, Fredrik D., Mi D. Nguyen, Kjeld Hermansen, Per B. Jeppesen, Zheer K. Al-Mashhadi, Steffen Ringgaard, and Søren Gregersen. 2024. "Effects of 12-Week Supplementation with Coffee Diterpene Cafestol in Healthy Subjects with Increased Waist Circumference: A Randomized, Placebo-Controlled Trial" Nutrients 16, no. 19: 3232. https://doi.org/10.3390/nu16193232






