Extra Virgin Olive Oil Reduces the Risk of Non-Alcoholic Fatty Liver Disease in Females but Not in Males: Results from the NUTRIHEP Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Assessments
2.3. Statistical Analysis
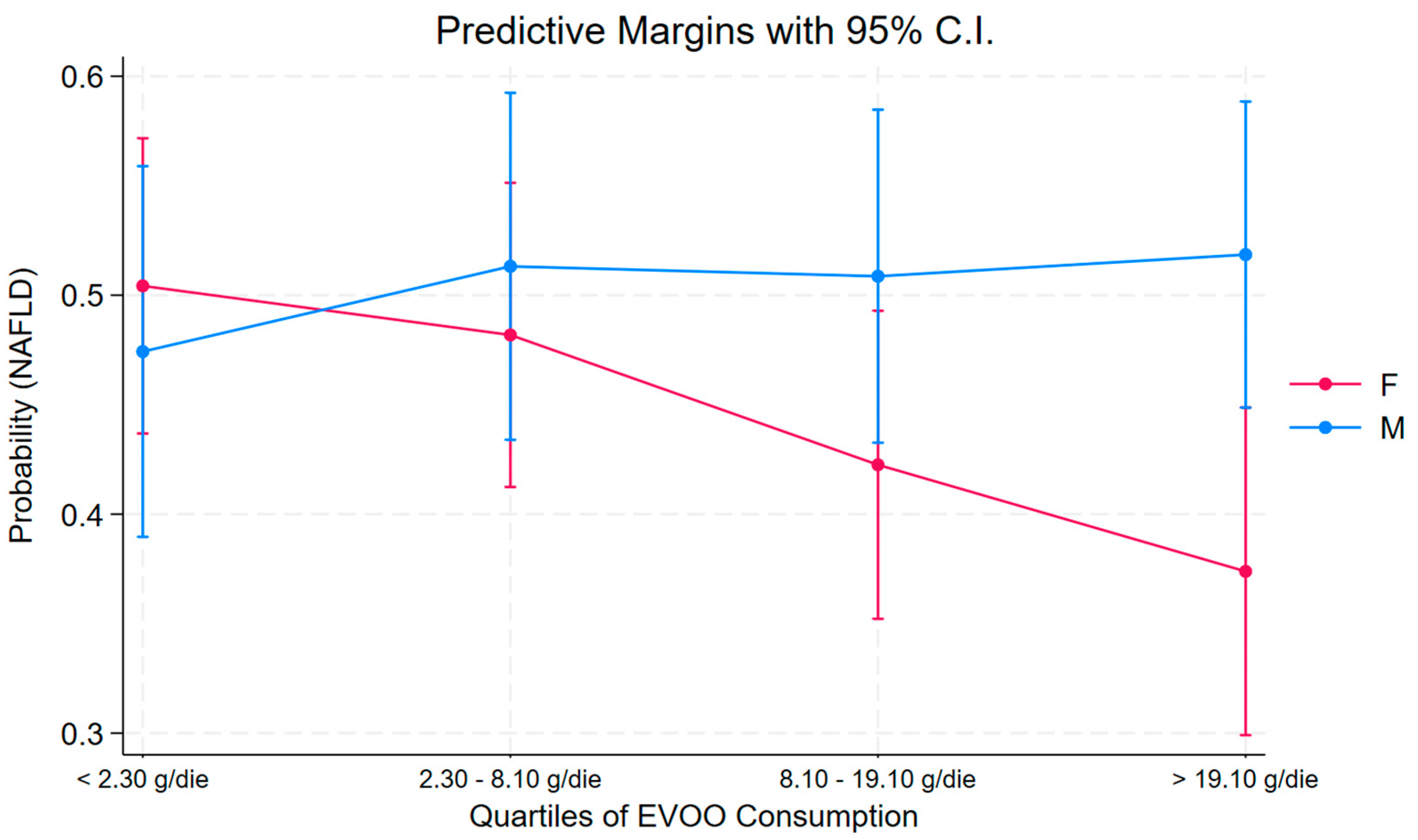
3. Results
4. Discussion
5. Conclusions
Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Sayiner, M.; Koenig, A.; Henry, L.; Younossi, Z.M. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin. Liver Dis. 2016, 20, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Abbate, M.; Montemayor, S.; Mascaró, C.M.; Casares, M.; Tejada, S.; Abete, I.; Zulet, A.M.; Tur, J.A.; et al. Oxidative Stress and Pro-Inflammatory Status in Patients with Non-Alcoholic Fatty Liver Disease. Antioxidants 2020, 9, 759. [Google Scholar] [CrossRef]
- Kazankov, K.; Jørgensen, S.M.D.; Thomsen, K.L.; Møller, H.L.; Vilstrup, H.; George, J.; Schuppan, D.; Grønbæk, H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef]
- Luci, C.; Bourinet, M.; Leclère, P.S.; Anty, R.; Gual, P. Chronic Inflammation in Non-Alcoholic Steatohepatitis: Molecular Mechanisms and Therapeutic Strategies. Front. Endocrinol. 2020, 11, 597648. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Scorletti, E.; Mosca, A.; Alisi, A.; Byrne, C.D.; Targher, G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism 2020, 111S, 154170. [Google Scholar] [CrossRef]
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—Mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD as a driver of chronic kidney disease. J. Hepatol. 2020, 72, 785–801. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Newsome, P.N. Non-alcoholic fatty liver disease and the interface between primary and secondary care. Lancet Gastroenterol. Hepatol. 2018, 3, 509–517. [Google Scholar] [CrossRef]
- Carter, J.; Wang, S.; Friedman, S.L. Ten Thousand Points of Light: Heterogeneity Among the Stars of NASH Fibrosis. Hepatology 2021, 74, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Jonas, W.; Schürmann, A. Genetic and epigenetic factors determining NAFLD risk. Mol. Metab. 2021, 50, 101111. [Google Scholar] [CrossRef]
- Choudhary, N.S.; Duseja, A. Genetic and epigenetic disease modifiers: Non-alcoholic fatty liver disease (NAFLD) and alcoholic liver disease (ALD). Transl. Gastroenterol. Hepatol. 2021, 6, 2. [Google Scholar] [CrossRef]
- Berná, G.; Romero-Gomez, M. The role of nutrition in non-alcoholic fatty liver disease: Pathophysiology and management. Liver Int. 2020, 40, 102–108. [Google Scholar] [CrossRef]
- Yki-Järvinen, H.; Luukkonen, P.K.; Hodson, L.; Moore, J.B. Dietary carbohydrates and fats in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 770–786. [Google Scholar] [CrossRef]
- Della Torre, S. Non-alcoholic Fatty Liver Disease as a Canonical Example of Metabolic Inflammatory-Based Liver Disease Showing a Sex-Specific Prevalence: Relevance of Estrogen Signaling. Front. Endocrinol. 2020, 11, 572490. [Google Scholar] [CrossRef]
- Della Torre, S. Beyond the X Factor: Relevance of Sex Hormones in NAFLD Pathophysiology. Cells 2021, 10, 2502. [Google Scholar] [CrossRef] [PubMed]
- Bilson, J.; Sethi, J.K.; Byrne, C.D. Non-alcoholic fatty liver disease: A multi-system disease influenced by ageing and sex, and affected by adipose tissue and intestinal function. Proc. Nutr. Soc. 2022, 81, 146–161. [Google Scholar] [CrossRef]
- Bonfiglio, C.; Cuccaro, F.; Campanella, A.; Rosso, N.; Tatoli, R.; Giannelli, G.; Donghia, R. Effect of Intake of Extra Virgin Olive Oil on Mortality in a South Italian Cohort with and without NAFLD. Nutrients 2023, 15, 4593. [Google Scholar] [CrossRef]
- Grzymisławska, M.; Puch, E.A.; Zawada, A.; Grzymisławski, M. Do nutritional behaviors depend on biological sex and cultural gender? Adv. Clin. Exp. Med. 2020, 29, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Donghia, R.; Campanella, A.; Bonfiglio, C.; Cuccaro, F.; Tatoli, R.; Giannelli, G. Protective Role of Lycopene in Subjects with Liver Disease: NUTRIHEP Study. Nutrients 2024, 16, 562. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Baswiskar, N.; Acharya, S.; Kumar, S. Fatty liver disease—Non alcoholic to metabolic—A transition of concepts!! J. Family Med. Prim. Care 2024, 13, 2857–2862. [Google Scholar] [CrossRef]
- Zou, H.; Ma, X.; Pan, W.; Xie, Y. Comparing similarities and differences between NAFLD, MAFLD, and MASLD in the general U.S. population. Front. Nutr. 2024, 11, 1411802. [Google Scholar] [CrossRef]
- De, A.; Bhagat, N.; Mehta, M.; Taneja, S.; Duseja, A. Metabolic dysfunction-associated steatotic liver disease (MASLD) definition is better than MAFLD criteria for lean patients with NAFLD. J. Hepatol. 2024, 80, e61–e62. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Amor, L.; Sierra, A.L.; Cárdeans, A.; López-Bermudo, L.; López-Beas, J.; Andújar, E.; Pérez-Alegre, M.; Gallego-Durán, R.; Varela, L.M.; Martin-Montalvo, A.; et al. Extra virgin olive oil improved body weight and insulin sensitivity in high fat diet-induced obese LDLr-/-.Leiden mice without attenuation of steatohepatitis. Sci. Rep. 2021, 11, 8250. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Shidfar, F.; Bahrololumi, S.S.; Doaei, S.; Mohammadzadeh, A.; Gholamalizadeh, M.; Mohammadimanesh, A. The Effects of Extra Virgin Olive Oil on Alanine Aminotransferase, Aspartate Aminotransferase, and Ultrasonographic Indices of Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Patients Undergoing Low Calorie Diet. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1053710. [Google Scholar] [CrossRef] [PubMed]
- Soto-Alarcon, S.; Valenzuela, R.; Valenzuela, A.; Videla, L.A. Liver Protective Effects of Extra Virgin Olive Oil: Interaction between Its Chemical Composition and the Cell-signaling Pathways Involved in Protection. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Comitato, R.; Saba, A.; Turrini, A.; Arganini, C.; Virgili, F. Sex hormones and macronutrient metabolism. Crit. Rev. Fodd. Sci. Nutr. 2015, 55, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.J.; Gardner, A.W.; Arciero, P.J.; Calles-Escandon, J.; Poehlman, E.T. Gender differences in fat oxidation and sympathetic nervous system activity at rest and during submaximal exercise in older individuals. Clin. Sci. 1998, 95, 59–66. [Google Scholar] [CrossRef]
- Poehlman, E.T.; Tchernof, A. Traversing the menopause: Changes in energy expenditure and body composition. Coron. Artery Dis. 1998, 9, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Clegg, D.J. Sex differences in the regulation of body weight. Physiol. Behav. 2009, 97, 199–204. [Google Scholar] [CrossRef]
- Killinger, D.W.; Perel, E.; Daniilescu, D.; Kharlip, L.; Lindsay, W.R. The relationship between aromatase activity and body fat distribution. Steroids 1987, 50, 61–72. [Google Scholar] [CrossRef]
- McTernan, P.G.; Anderson, L.A.; Anwar, A.J.; Eggo, M.C.; Crocker, J.; Barnett, A.H.; Stewart, P.M.; Kumar, S. Glucocorticoid regulation of p450 aromatase activity in human adipose tissue: Gender and site differences. J. Clin. Endocrinol. Metab. 2002, 87, 1327–1336. [Google Scholar] [CrossRef]
- Liu, M.; Shen, L.; Yang, Q.; Nauli, A.M.; Bingamon, M.; Wang, D.Q.H.; Ulrich-Lai, Y.M.; Tso, P. Sexual dimorphism in intestinal absorption and lymphatic transport of dietary lipids. J. Physiol. 2021, 599, 5015–5030. [Google Scholar] [CrossRef]
- Karlstadt, R.G.; Hogan, D.L.; Foxx-Orenstein, A. Normal Physiology of the Gastrointestinal Tract and Gender Differences. Princ. Gender-Spec. Med. 2004, 1, 377–396. [Google Scholar]
- Narayanan, S.P.; Anderson, B.; Bharucha, A.E. Sex- and Gender-Related Differences in Common Functional Gastroenterologic Disorders. Mayo Clin. Proc. 2021, 96, 1071–1089. [Google Scholar] [CrossRef] [PubMed]
- Homma, H.; Kurachi, H.; Nischio, Y.; Takeda, T.; Yamamoto, T.; Adachi, K.; Morishige, K.; Ohmichi, M.; Matsuzawa, Y.; Murata, Y. Estrogen suppresses transcription of lipoprotein lipase gene. Existence of a unique estrogen response element on the lipoprotein lipase promoter. J. Biol. Chem. 2000, 275, 11404–11411. [Google Scholar] [CrossRef] [PubMed]
- Price, T.M.; O’Brien, S.N.; Welter, B.H.; George, R.; Anandjiwala, J.; Kilgore, N. Estrogen regulation of adipose tissue lipoprotein lipase--possible mechanism of body fat distribution. Am. J. Obstet. Gynecol. 1998, 178, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.B.; Kristensen, K.; Hermann, P.A.; Katzenellenbogen, J.A.; Richelsen, B. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J. Clin. Endocrinol. Metab. 2004, 89, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Pascot, A.; Lemieux, I.; Bergeron, J.; Tremblay, A.; Nadeau, A.; Prud’homme, D.; Couillard, C.; Lamarche, B.; Després, J.P. HDL particle size: A marker of the gender difference in the metabolic risk profile. Atherosclerosis 2002, 160, 399–406. [Google Scholar] [CrossRef]
- Donghia, R.; Schiano Di Cola, R.; Cesaro, F.; Vitale, A.; Lippolis, G.; Lisco, T.; Isernia, R.; De Pergola, G.; De Nucci, S.; Rinaldi, R.; et al. Gender and Liver Steatosis Discriminate Different Physiological Patterns in Obese Patients Undergoing Bariatric Surgery: Obesity Center Cohort. Nutrients 2023, 15, 2381. [Google Scholar] [CrossRef]

| Parameters * | Total Cohort (n = 1426) | Gender | p ^ | |
|---|---|---|---|---|
| Female (n = 808) | Male (n = 618) | |||
| Age (years) | 54.87 ± 14.34 | 54.48 ± 13.80 | 55.37 ± 15.00 | 0.20 |
| Civil Status (%) | <0.001 ψ | |||
| Single | 164 (12.53) | 88 (11.91) | 76 (13.33) | |
| Married or Cohabiting | 1054 (80.52) | 580 (78.48) | 474 (83.16) | |
| Divorced or Separated | 30 (2.29) | 23 (3.11) | 7 (1.23) | |
| Widower | 61 (4.66) | 48 (6.50) | 13 (2.28) | |
| Education (%) | 0.04 ψ | |||
| None | 17 (1.30) | 11 (1.49) | 6 (1.05) | |
| Elementary School | 272 (20.80) | 172 (23.27) | 100 (17.57) | |
| Secondary School | 389 (29.74) | 214 (28.96) | 175 (30.76) | |
| High School | 459 (35.09) | 238 (32.21) | 221 (38.84) | |
| Degree | 58 (4.43) | 33 (4.47) | 25 (4.39) | |
| Post-Degree | 113 (8.64) | 71 (9.61) | 42 (7.38) | |
| Job (%) | <0.001 ψ | |||
| Managers and Professionals | 110 (7.75) | 43 (5.34) | 67 (10.89) | |
| Crafts, Agricultural, and Sales Workers | 514 (36.20) | 275 (34.16) | 239 (38.86) | |
| Elementary Occupations | 193 (13.59) | 98 (12.17) | 95 (15.45) | |
| Housewife | 153 (10.77) | 152 (18.88) | 1 (0.16) | |
| Pensioner | 374 (26.34) | 183 (22.73) | 191 (31.06) | |
| Unemployed | 76 (5.35) | 54 (6.71) | 22 (3.58) | |
| Smoker (Yes) (%) | 175 (12.29) | 75 (9.29) | 100 (16.21) | <0.001 ψ |
| BMI (kg/m2) | 27.69 ± 5.04 | 27.50 ± 5.47 | 27.95 ± 4.41 | 0.009 |
| Systolic Pressure (mmHg) | 121.32 ± 15.85 | 118.86 ± 16.31 | 124.54 ± 14.61 | <0.0001 |
| Diastolic Pressure (mmHg) | 77.85 ± 7.99 | 76.32 ± 7.89 | 79.86 ± 7.68 | <0.0001 |
| Diabetes (Yes) (%) | 92 (6.87) | 48 (6.37) | 44 (7.50) | 0.42 ψ |
| Heart Attack (Yes) (%) | 18 (1.34) | 5 (0.66) | 13 (2.21) | 0.01 ψ |
| NAFLD (Yes) (%) | 707 (49.58) | 363 (44.93) | 344 (55.66) | <0.001 ψ |
| Blood Variables | ||||
| RBC (M/mcL) | 4.94 ± 0.55 | 4.75 ± 0.46 | 5.19 ± 0.55 | <0.0001 |
| Hemoglobin (g/dL) | 13.96 ± 1.49 | 13.27 ± 1.21 | 14.86 ± 1.33 | <0.0001 |
| Hematocrit (L/L) | 41.74 ± 3.77 | 40.15 ± 3.26 | 43.80 ± 3.38 | <0.0001 |
| MCV (μm3) | 85.03 ± 8.20 | 84.98 ± 8.10 | 85.10 ± 8.34 | 0.56 |
| MCH (pg) | 28.44 ± 2.95 | 28.11 ± 2.81 | 28.87 ± 3.07 | <0.0001 |
| MCHC (g/dL) | 33.40 ± 1.12 | 33.02 ± 1.02 | 33.90 ± 1.06 | <0.0001 |
| RDW-CV (%) | 13.81 ± 1.50 | 13.83 ± 1.48 | 13.78 ± 1.53 | 0.17 |
| Platelets (K/mcL) | 233.32 ± 60.96 | 241.10 ± 58.61 | 223.15 ± 62.51 | <0.0001 |
| WBC (K/mcL) | 5.84 ± 1.70 | 5.62 ± 1.69 | 6.13 ± 1.66 | <0.0001 |
| Neutrophils (%) | 57.69 ± 7.80 | 57.73 ± 7.78 | 57.64 ± 7.84 | 0.76 |
| Lymphocytes (%) | 31.70 ± 7.25 | 32.04 ± 7.16 | 31.26 ± 7.35 | 0.04 |
| Monocytes (%) | 7.31 ± 1.71 | 7.04 ± 1.58 | 7.66 ± 1.80 | <0.0001 |
| Eosinophils (%) | 2.75 ± 1.96 | 2.64 ± 2.09 | 2.89 ± 1.77 | 0.0001 |
| Basophils (%) | 0.53 ± 0.31 | 0.54 ± 0.33 | 0.51 ± 0.29 | 0.04 |
| Neutrophils (109/L) | 3.41 ± 1.31 | 3.29 ± 1.31 | 3.58 ± 1.28 | <0.0001 |
| Lymphocytes (109/L) | 1.81 ± 0.55 | 1.76 ± 0.54 | 1.87 ± 0.55 | 0.0002 |
| Monocytes (109/L) | 0.42 ± 0.14 | 0.39 ± 0.12 | 0.47 ± 0.16 | <0.0001 |
| Eosinophils (109/L) | 0.16 ± 0.13 | 0.15 ± 0.13 | 0.18 ± 0.13 | <0.0001 |
| Basophils (109/L) | 0.03 ± 0.04 | 0.03 ± 0.05 | 0.03 ± 0.02 | 0.48 |
| Insulin (mmol/L) | 7.70 ± 5.86 | 7.22 ± 5.03 | 8.32 ± 6.75 | 0.009 |
| HOMA | 1.92 ± 2.01 | 1.75 ± 1.57 | 2.16 ± 2.46 | 0.0001 |
| Glycemia (mg/dL) | 95.56 ± 17.55 | 93.30 ± 16.73 | 98.52 ± 18.16 | <0.0001 |
| HbA1c (mmol/mol) | 38.10 ± 6.97 | 37.91 ± 6.55 | 38.34 ± 7.47 | 0.88 |
| Total Bilirubin (mg/dL) | 0.72 ± 0.38 | 0.65 ± 0.34 | 0.81 ± 0.41 | <0.0001 |
| GOT (U/L) | 22.07 ± 11.62 | 20.84 ± 13.14 | 23.68 ± 9.03 | <0.0001 |
| SGPT (IU/L) | 22.49 ± 16.49 | 19.91 ± 18.33 | 25.89 ± 12.95 | <0.0001 |
| GGT (U/L) | 17.93 ± 14.28 | 14.86 ± 12.74 | 21.96 ± 15.18 | <0.0001 |
| Alkaline Phosphatase (U/L) | 53.13 ± 16.07 | 53.52 ± 16.80 | 52.62 ± 15.05 | 0.51 |
| Albumin (U/L) | 4.08 ± 0.27 | 4.04 ± 0.24 | 4.15 ± 0.29 | <0.0001 |
| Iron (mg/dL) | 90.29 ± 31.45 | 85.04 ± 31.29 | 97.17 ± 30.33 | <0.0001 |
| Cholesterol (mg/dL) | 191.57 ± 35.51 | 194.67 ± 34.26 | 187.49 ± 36.72 | <0.0001 |
| HDL (mg/dL) | 50.83 ± 12.71 | 55.28 ± 12.51 | 45.00 ± 10.41 | <0.0001 |
| Triglycerides (mg/dL) | 99.12 ± 69.31 | 86.56 ± 54.28 | 115.63 ± 82.30 | <0.0001 |
| Ceruloplasmin (mg/dL) | 30.94 ± 7.26 | 33.19 ± 7.66 | 28.00 ± 5.44 | <0.0001 |
| α1AT (mg/dL) | 157.73 ± 31.16 | 161.76 ± 31.69 | 152.44 ± 29.65 | <0.0001 |
| CRP (mg/L) | 0.26 ± 0.56 | 0.27 ± 0.62 | 0.24 ± 0.46 | 0.16 |
| Ferritin (ng/mL) | 92.16 ± 91.36 | 56.47 ± 56.74 | 139.05 ± 105.92 | <0.0001 |
| Diet Habits | ||||
| Alcohol (g/die) | 9.91 ± 15.17 | 4.22 ± 7.30 | 17.41 ± 19.11 | <0.0001 |
| EVOO (g/die) | 15.33 ± 23.21 | 13.59 ± 19.29 | 17.63 ± 27.39 | 0.002 |
| EVOO Quartiles (%) | ||||
| <2.30 g | 230 (25.30) | 145 (28.05) | 85 (21.68) | |
| 2.30–8.10 g | 227 (24.97) | 135 (26.11) | 92 (23.47) | |
| 8.10–19.10 g | 226 (24.86) | 125 (24.18) | 101 (25.77) | |
| >19.10 g | 226 (24.86) | 112 (21.66) | 114 (29.08) | |
| Parameter | Female | Male | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | Se (OR) | p | 95% C.I. | OR | Se (OR) | p | 95% C.I. | |
| Quartile | ||||||||
| <2.30 g/die [Ref.] | -- | -- | -- | -- | -- | -- | -- | -- |
| 2.30–8.10 g/die | 0.90 | 0.29 | 0.73 | 0.48 to 1.68 | 1.26 | 0.49 | 0.54 | 0.59 to 2.69 |
| 8.10–19.10 g/die | 0.59 | 0.19 | 0.11 | 0.31 to 1.12 | 1.27 | 0.48 | 0.53 | 0.61 to 2.65 |
| >19.10 g/die | 0.43 | 0.15 | 0.02 | 0.21 to 0.85 | 1.34 | 0.49 | 0.42 | 0.65 to 2.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donghia, R.; Tatoli, R.; Campanella, A.; Losurdo, G.; Di Leo, A.; De Pergola, G.; Bonfiglio, C.; Giannelli, G. Extra Virgin Olive Oil Reduces the Risk of Non-Alcoholic Fatty Liver Disease in Females but Not in Males: Results from the NUTRIHEP Cohort. Nutrients 2024, 16, 3234. https://doi.org/10.3390/nu16193234
Donghia R, Tatoli R, Campanella A, Losurdo G, Di Leo A, De Pergola G, Bonfiglio C, Giannelli G. Extra Virgin Olive Oil Reduces the Risk of Non-Alcoholic Fatty Liver Disease in Females but Not in Males: Results from the NUTRIHEP Cohort. Nutrients. 2024; 16(19):3234. https://doi.org/10.3390/nu16193234
Chicago/Turabian StyleDonghia, Rossella, Rossella Tatoli, Angelo Campanella, Giuseppe Losurdo, Alfredo Di Leo, Giovanni De Pergola, Caterina Bonfiglio, and Gianluigi Giannelli. 2024. "Extra Virgin Olive Oil Reduces the Risk of Non-Alcoholic Fatty Liver Disease in Females but Not in Males: Results from the NUTRIHEP Cohort" Nutrients 16, no. 19: 3234. https://doi.org/10.3390/nu16193234








