Tackling Cravings in Medical Weight Management: An Update on Pathophysiology and an Integrated Approach to Treatment
Abstract
:1. Introduction
2. Method
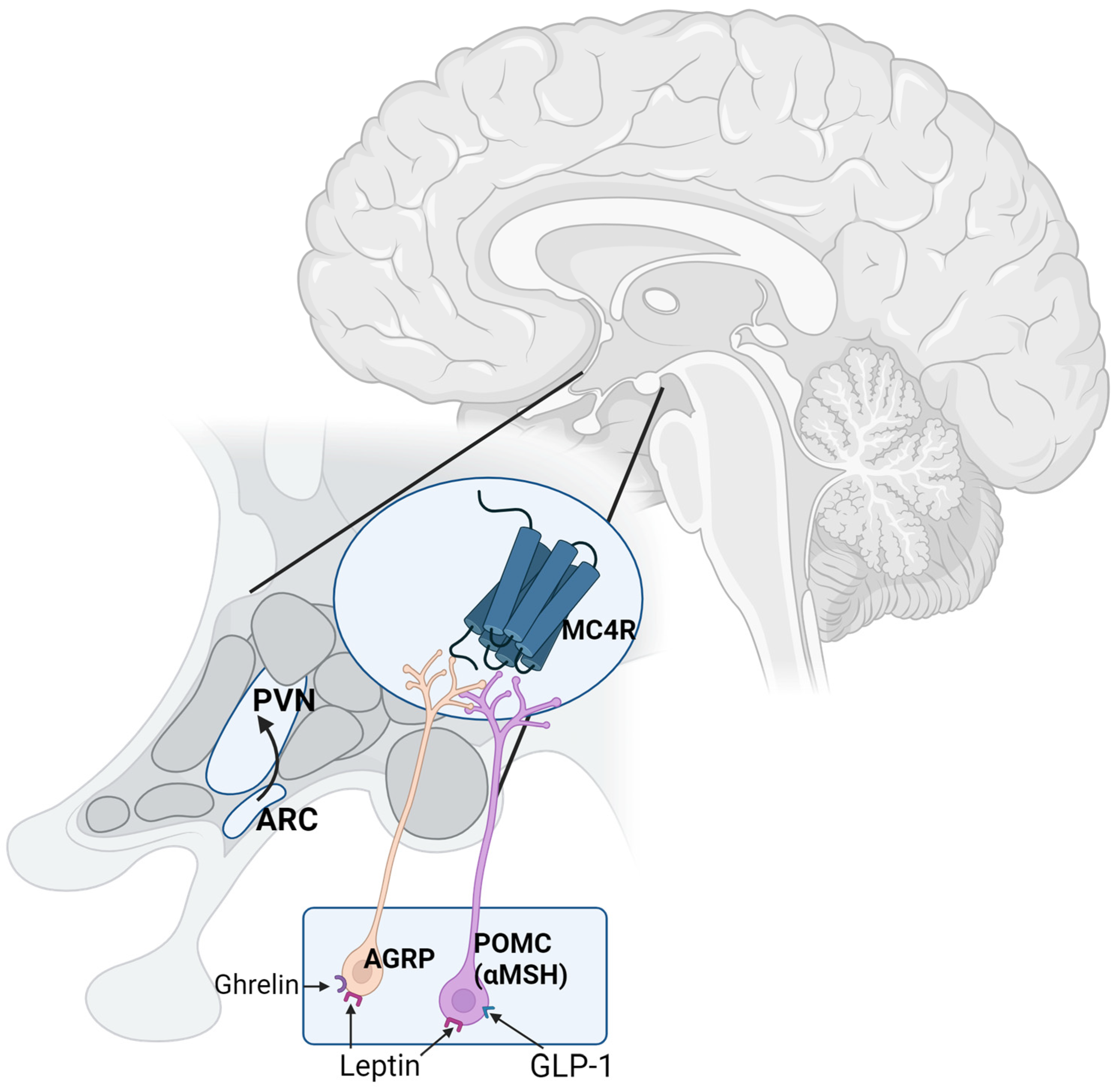
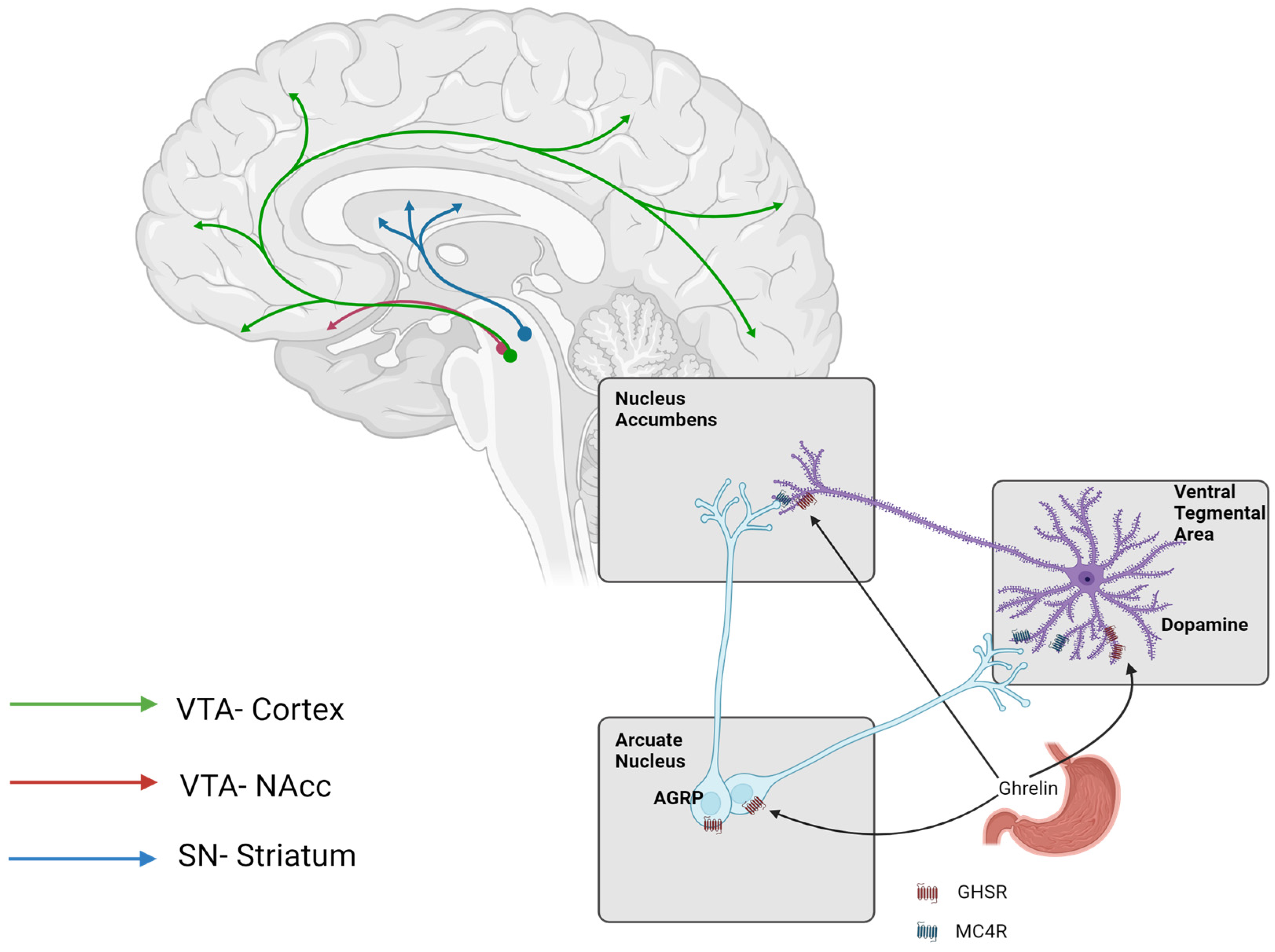
3. Pathophysiology of Cravings
4. Identifying Cravings and Their Spectrum of Manifestations
New Advances in Craving Measurement
5. Craving Management Options
5.1. Lifestyle Factors
5.2. Psychological Interventions
5.3. Medications
5.4. Integrating Medications with Lifestyle and Psychological Strategies
5.5. New Advances in Craving Management
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McVay, M.A.; Copeland, A.L.; Geiselman, P.J. Eating disorder pathology and menstrual cycle fluctuations in eating variables in oral contraceptive users and non-users. Eat. Behav. 2011, 12, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Hallam, J.; Boswell, R.G.; DeVito, E.E.; Kober, H. Gender-related Differences in Food Craving and Obesity. Yale J. Biol. Med. 2016, 89, 161–173. [Google Scholar] [PubMed]
- Dang, N.; Khalil, D.; Sun, J.; Naveed, A.; Soumare, F.; Nusslock, R.; Hamidovic, A. Behavioral Symptomatology in the Premenstruum. Brain Sci. 2022, 12, 814. [Google Scholar] [CrossRef] [PubMed]
- Hormes, J.M. Perimenstrual chocolate craving: From pharmacology and physiology to cognition and culture. In Human Health Handbooks; Brill: Leiden, The Netherlands, 2014; pp. 137–153. [Google Scholar] [CrossRef]
- Hill, D.; Conner, M.; Clancy, F.; Moss, R.; Wilding, S.; Bristow, M.; O’Connor, D.B. Stress and eating behaviours in healthy adults: A systematic review and meta-analysis. Health Psychol. Rev. 2022, 16, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Hewagalamulage, S.D.; Lee, T.K.; Clarke, I.J.; Henry, B.A. Stress, cortisol, and obesity: A role for cortisol responsiveness in identifying individuals prone to obesity. Domest. Anim. Endocrinol. 2016, 56, S112–S120. [Google Scholar] [CrossRef]
- Degroote, C.; Renner, B.; Wickl, J.; Leven, A.; Wirtz, P.H. Eating After Acute Psychosocial Stress in Healthy Men and Women: Sex Differences and Endocrine Mechanisms. J. Clin. Endocrinol. Metab. 2024, 109, e543–e551. [Google Scholar] [CrossRef]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar]
- Mitchell, K.S.; Wolf, E.J. PTSD, food addiction, and disordered eating in a sample of primarily older veterans: The mediating role of emotion regulation. Psychiatry Res. 2016, 243, 23–29. [Google Scholar] [CrossRef]
- Whatnall, M.; Skinner, J.A.; Leary, M.; Burrows, T.L. Food Addiction: A Deep Dive into ‘Loss of Control’ and ‘Craving’. Curr. Addict. Rep. 2022, 9, 318–325. [Google Scholar] [CrossRef]
- Rebello, C.J.; Greenway, F.L. Reward-Induced Eating: Therapeutic Approaches to Addressing Food Cravings. Adv. Ther. 2016, 33, 1853–1866. [Google Scholar] [CrossRef]
- Vainik, U.; Garcia-Garcia, I.; Dagher, A. Uncontrolled eating: A unifying heritable trait linked with obesity, overeating, personality and the brain. Eur. J. Neurosci. 2019, 50, 2430–2445. [Google Scholar] [CrossRef] [PubMed]
- Hormes, J.M.; Orloff, N.C.; Timko, C.A. Chocolate craving and disordered eating. Beyond the gender divide? Appetite 2014, 83, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Orloff, N.C.; Hormes, J.M. Pickles and ice cream! Food cravings in pregnancy: Hypotheses, preliminary evidence, and directions for future research. Front. Psychol. 2014, 5, 1076. [Google Scholar] [CrossRef] [PubMed]
- Bayley, T.M.; Dye, L.; Jones, S.; DeBono, M.; Hill, A.J. Food cravings and aversions during pregnancy: Relationships with nausea and vomiting. Appetite 2002, 38, 45–51. [Google Scholar] [CrossRef]
- Potenza, M.N.; Grilo, C.M. How Relevant is Food Craving to Obesity and Its Treatment? Front. Psychiatry 2014, 5, 164. [Google Scholar] [CrossRef]
- Boswell, R.G.; Kober, H. Food cue reactivity and craving predict eating and weight gain: A meta-analytic review. Obes. Rev. 2016, 17, 159–177. [Google Scholar] [CrossRef]
- Dingemans, A.; Danner, U.; Parks, M. Emotion Regulation in Binge Eating Disorder: A Review. Nutrients 2017, 9, 1274. [Google Scholar] [CrossRef]
- Hormes, J.M. The Clinical Significance of Craving Across the Addictive Behaviors: A Review. Curr. Addict. Rep. 2017, 4, 132–141. [Google Scholar] [CrossRef]
- Kavanagh, D.J.; Andrade, J.; May, J. Imaginary relish and exquisite torture: The elaborated intrusion theory of desire. Psychol. Rev. 2005, 112, 446–467. [Google Scholar] [CrossRef]
- Karlsson, J.; Persson, L.O.; Sjostrom, L.; Sullivan, M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2000, 24, 1715–1725. [Google Scholar] [CrossRef]
- French, S.A.; Epstein, L.H.; Jeffery, R.W.; Blundell, J.E.; Wardle, J. Eating behavior dimensions. Associations with energy intake and body weight. A review. Appetite 2012, 59, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Vainik, U.; Dagher, A.; Dube, L.; Fellows, L.K. Neurobehavioural correlates of body mass index and eating behaviours in adults: A systematic review. Neurosci. Biobehav. Rev. 2013, 37, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Port, J.D.; Acosta, A. Integrative Hedonic and Homeostatic Food Intake Regulation by the Central Nervous System: Insights from Neuroimaging. Brain Sci. 2022, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.H.; Kim, M.; Kim, S.Y. Brain circuits for promoting homeostatic and non-homeostatic appetites. Exp. Mol. Med. 2022, 54, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Jais, A.; Bruning, J.C. Arcuate Nucleus-Dependent Regulation of Metabolism-Pathways to Obesity and Diabetes Mellitus. Endocr. Rev. 2022, 43, 314–328. [Google Scholar] [CrossRef]
- Barbosa, D.A.N.; Gattas, S.; Salgado, J.S.; Kuijper, F.M.; Wang, A.R.; Huang, Y.; Kakusa, B.; Leuze, C.; Luczak, A.; Rapp, P.; et al. An orexigenic subnetwork within the human hippocampus. Nature 2023, 621, 381–388. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Hayes, M.R.; Skibicka, K.P. GLP-1 and weight loss: Unraveling the diverse neural circuitry. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R885–R895. [Google Scholar] [CrossRef]
- Rossi, M.A.; Stuber, G.D. Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell Metab. 2018, 27, 42–56. [Google Scholar] [CrossRef]
- Nicola, S.M. Reassessing wanting and liking in the study of mesolimbic influence on food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R811–R840. [Google Scholar] [CrossRef]
- van Zessen, R.; van der Plasse, G.; Adan, R.A. Contribution of the mesolimbic dopamine system in mediating the effects of leptin and ghrelin on feeding. Proc. Nutr. Soc. 2012, 71, 435–445. [Google Scholar] [CrossRef]
- Cone, J.J.; McCutcheon, J.E.; Roitman, M.F. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J. Neurosci. 2014, 34, 4905–4913. [Google Scholar] [CrossRef]
- Alhadeff, A.L.; Betley, J.N. Pass the salt: The central control of sodium intake. Nat. Neurosci. 2017, 20, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.P.; Acosta, A.A.; Ghidewon, M.Y.; McKnight, A.D.; Almeida, M.S.; Nyema, N.T.; Hanchak, N.D.; Patel, N.; Gbenou, Y.S.K.; Adriaenssens, A.E.; et al. Dissociable hindbrain GLP1R circuits for satiety and aversion. Nature 2024, 632, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Merchenthaler, I.; Lane, M.; Shughrue, P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol. 1999, 403, 261–280. [Google Scholar] [CrossRef]
- Dallman, M.F.; Pecoraro, N.; Akana, S.F.; La Fleur, S.E.; Gomez, F.; Houshyar, H.; Bell, M.; Bhatnagar, S.; Laugero, K.D.; Manalo, S. Chronic stress and obesity: A new view of “comfort food”. Proc. Natl. Acad. Sci. USA 2003, 100, 11696–11701. [Google Scholar] [CrossRef]
- Sadler, J.R.; Thapaliya, G.; Jansen, E.; Aghababian, A.H.; Smith, K.R.; Carnell, S. COVID-19 stress and food intake: Protective and risk factors for stress-related palatable food intake in US adults. Nutrients 2021, 13, 901. [Google Scholar] [CrossRef]
- Bouillon-Minois, J.B.; Trousselard, M.; Thivel, D.; Gordon, B.A.; Schmidt, J.; Moustafa, F.; Oris, C.; Dutheil, F. Ghrelin as a Biomarker of Stress: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 784. [Google Scholar] [CrossRef]
- Douma, E.H.; de Kloet, E.R. Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci. Biobehav. Rev. 2020, 108, 48–77. [Google Scholar] [CrossRef]
- Martin, C.K.; McClernon, F.J.; Chellino, A.; Correa, J.B. Food cravings: A central construct in food intake behavior, weight loss, and the neurobiology of appetitive behavior. In Handbook of Behavior, Food and Nutrition; Springer: Berlin/Heidelberg, Germany, 2011; pp. 741–755. [Google Scholar]
- Meule, A. Assessment of food cravings. In Processed Food Addiction; CRC Press: Boca Raton, FL, USA, 2017; pp. 137–146. [Google Scholar]
- Taylor, M. A review of food craving measures. Eat. Behav. 2019, 32, 101–110. [Google Scholar] [CrossRef]
- Cepeda-Benito, A.; Gleaves, D.H.; Fernandez, M.C.; Vila, J.; Williams, T.L.; Reynoso, J. The development and validation of Spanish versions of the State and Trait Food Cravings Questionnaires. Behav. Res. Ther. 2000, 38, 1125–1138. [Google Scholar] [CrossRef]
- Meule, A.; Hermann, T.; Kübler, A. A short version of the Food Cravings Questionnaire—Trait: The FCQ-T-reduced. Front. Psychol. 2014, 5, 190. [Google Scholar] [CrossRef] [PubMed]
- Nijs, I.M.; Franken, I.H.; Muris, P. The modified Trait and State Food-Cravings Questionnaires: Development and validation of a general index of food craving. Appetite 2007, 49, 38–46. [Google Scholar] [CrossRef]
- Kemps, E.; Tiggemann, M.; Woods, D.; Soekov, B. Reduction of food cravings through concurrent visuospatial processing. Int. J. Eat. Disord. 2004, 36, 31–40. [Google Scholar] [CrossRef] [PubMed]
- May, J.; Andrade, J.; Kavanagh, D.J.; Feeney, G.F.; Gullo, M.J.; Statham, D.J.; Skorka-Brown, J.; Connolly, J.M.; Cassimatis, M.; Young, R.M. The Craving Experience Questionnaire: A brief, theory-based measure of consummatory desire and craving. Addiction 2014, 109, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Meule, A. Twenty Years of the Food Cravings Questionnaires: A Comprehensive Review. Curr. Addict. Rep. 2020, 7, 30–43. [Google Scholar] [CrossRef]
- Gormally, J.; Black, S.; Daston, S.; Rardin, D. The assessment of binge eating severity among obese persons. Addict. Behav. 1982, 7, 47–55. [Google Scholar] [CrossRef]
- Bolger, N.; Laurenceau, J.-P. Intensive Longitudinal Methods: An Introduction to Diary and Experience Sampling Research; Guilford Press: New York, NY, USA, 2013. [Google Scholar]
- Richard, A.; Meule, A.; Reichenberger, J.; Blechert, J. Food cravings in everyday life: An EMA study on snack-related thoughts, cravings, and consumption. Appetite 2017, 113, 215–223. [Google Scholar] [CrossRef]
- Forman, E.M.; Hoffman, K.L.; Juarascio, A.S.; Butryn, M.L.; Herbert, J.D. Comparison of acceptance-based and standard cognitive-based coping strategies for craving sweets in overweight and obese women. Eat. Behav. 2013, 14, 64–68. [Google Scholar] [CrossRef]
- Rosenberg, H. Clinical and laboratory assessment of the subjective experience of drug craving. Clin. Psychol. Rev. 2009, 29, 519–534. [Google Scholar] [CrossRef]
- Ko, C.H.; Liu, G.C.; Yen, J.Y.; Chen, C.Y.; Yen, C.F.; Chen, C.S. Brain correlates of craving for online gaming under cue exposure in subjects with Internet gaming addiction and in remitted subjects. Addict. Biol. 2013, 18, 559–569. [Google Scholar] [CrossRef]
- Yang, C.L.; Tucker, R.M. Beneficial effects of a high protein breakfast on fullness disappear after a night of short sleep in nonobese, premenopausal women. Physiol. Behav. 2021, 229, 113269. [Google Scholar] [CrossRef] [PubMed]
- Hoertel, H.A.; Will, M.J.; Leidy, H.J. A randomized crossover, pilot study examining the effects of a normal protein vs. high protein breakfast on food cravings and reward signals in overweight/obese “breakfast skipping”, late-adolescent girls. Nutr. J. 2014, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Vidafar, P.; Cain, S.W.; Shechter, A. Relationship between Sleep and Hedonic Appetite in Shift Workers. Nutrients 2020, 12, 2835. [Google Scholar] [CrossRef] [PubMed]
- Tasali, E.; Wroblewski, K.; Kahn, E.; Kilkus, J.; Schoeller, D.A. Effect of Sleep Extension on Objectively Assessed Energy Intake Among Adults With Overweight in Real-life Settings: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 365–374. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Hooper, S.; Knight, A.; Hooper, D. Effectiveness of Natural Frequency Technology((R)) on cognition, sleep, and mood of adults with high perceived stress: A randomized, double-blind, placebo-controlled crossover study. Brain Behav. 2020, 10, e01712. [Google Scholar] [CrossRef]
- Li, A.; Li, X.; Zhou, T.; Ma, H.; Heianza, Y.; Williamson, D.A.; Smith, S.R.; Bray, G.A.; Sacks, F.M.; Qi, L. Sleep Disturbance and Changes in Energy Intake and Body Composition During Weight Loss in the POUNDS Lost Trial. Diabetes 2022, 71, 934–944. [Google Scholar] [CrossRef]
- Drenowatz, C.; Evensen, L.H.; Ernstsen, L.; Blundell, J.E.; Hand, G.A.; Shook, R.P.; Hébert, J.R.; Burgess, S.; Blair, S.N. Cross-sectional and longitudinal associations between different exercise types and food cravings in free-living healthy young adults. Appetite 2017, 118, 82–89. [Google Scholar] [CrossRef]
- Mason, C.; de Dieu Tapsoba, J.; Duggan, C.; Wang, C.Y.; Alfano, C.M.; McTiernan, A. Eating behaviors and weight loss outcomes in a 12-month randomized trial of diet and/or exercise intervention in postmenopausal women. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 113. [Google Scholar] [CrossRef]
- Martinez-Avila, W.D.; Sanchez-Delgado, G.; Acosta, F.M.; Jurado-Fasoli, L.; Oustric, P.; Labayen, I.; Blundell, J.E.; Ruiz, J.R. Eating Behavior, Physical Activity and Exercise Training: A Randomized Controlled Trial in Young Healthy Adults. Nutrients 2020, 12, 3685. [Google Scholar] [CrossRef]
- Annesi, J.J. Exercise Predicts Long-Term Weight Loss in Women With Class 1 and Class 2 Obesity Through Effects on Emotional Eating and its Correlates. J. Phys. Act. Health 2018, 15, 57–63. [Google Scholar] [CrossRef]
- Annesi, J.J.; Mareno, N. Indirect effects of exercise on emotional eating through psychological predictors of weight loss in women. Appetite 2015, 95, 219–227. [Google Scholar] [CrossRef]
- Codella, R.; Terruzzi, I.; Luzi, L. Sugars, exercise and health. J. Affect. Disord. 2017, 224, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Herring, M.P.; O’Connor, P.J.; Dishman, R.K. The effect of exercise training on anxiety symptoms among patients: A systematic review. Arch. Intern. Med. 2010, 170, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, T.; Nozaki, T.; Sawamoto, R.; Komaki, G.; Miyata, N.; Hosoi, M.; Sudo, N. Effects of Weight Loss on Sweet Taste Preference and Palatability following Cognitive Behavioral Therapy for Women with Obesity. Obes. Facts 2019, 12, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Ang, X.Q.; Giles, E.L.; Traviss-Turner, G. Emotional eating interventions for adults living with overweight or obesity: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2023, 20, 2722. [Google Scholar] [CrossRef] [PubMed]
- Katterman, S.N.; Kleinman, B.M.; Hood, M.M.; Nackers, L.M.; Corsica, J.A. Mindfulness meditation as an intervention for binge eating, emotional eating, and weight loss: A systematic review. Eat. Behav. 2014, 15, 197–204. [Google Scholar] [CrossRef]
- Morillo-Sarto, H.; Lopez-Del-Hoyo, Y.; Perez-Aranda, A.; Modrego-Alarcon, M.; Barcelo-Soler, A.; Borao, L.; Puebla-Guedea, M.; Demarzo, M.; Garcia-Campayo, J.; Montero-Marin, J. ‘Mindful eating’ for reducing emotional eating in patients with overweight or obesity in primary care settings: A randomized controlled trial. Eur. Eat. Disord. Rev. 2023, 31, 303–319. [Google Scholar] [CrossRef]
- Kabat-Zinn, J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. Gen. Hosp. Psychiatry 1982, 4, 33–47. [Google Scholar] [CrossRef]
- Hayes, S.C.; Strosahl, K.D.; Wilson, K.G. Acceptance and Commitment Therapy: The Process and Practice of Mindful Change; Guilford Press: New York, NY, USA, 2011. [Google Scholar]
- Tapper, K. Mindfulness and craving: Effects and mechanisms. Clin. Psychol. Rev. 2018, 59, 101–117. [Google Scholar] [CrossRef]
- Cooper, Z.; Fairburn, C.G. A new cognitive behavioural approach to the treatment of obesity. Behav. Res. Ther. 2001, 39, 499–511. [Google Scholar] [CrossRef]
- Abilés, V.; Abilés, J.; Rodriguez-Ruiz, S.; Luna, V.; Martín, F.; Gándara, N.; Fernandez-Santaella, M. Effectiveness of cognitive behavioral therapy on weight loss after two years of bariatric surgery in morbidly obese patients. Nutr. Hosp. 2013, 28, 1109–1114. [Google Scholar] [PubMed]
- Masheb, R.M.; Grilo, C.M.; White, M.A. An examination of eating patterns in community women with bulimia nervosa and binge eating disorder. Int. J. Eat. Disord. 2011, 44, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Palavras, M.A.; Hay, P.; Filho, C.A.; Claudino, A. The Efficacy of Psychological Therapies in Reducing Weight and Binge Eating in People with Bulimia Nervosa and Binge Eating Disorder Who Are Overweight or Obese-A Critical Synthesis and Meta-Analyses. Nutrients 2017, 9, 299. [Google Scholar] [CrossRef]
- Hilbert, A. Psychological and medical treatments for binge-eating disorder: A research update. Physiol. Behav. 2023, 269, 114267. [Google Scholar] [CrossRef] [PubMed]
- Tapper, K. Can mindfulness influence weight management related eating behaviors? If so, how? Clin. Psychol. Rev. 2017, 53, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.E.; Epel, E.S.; Kristeller, J.; Moran, P.J.; Dallman, M.; Lustig, R.H.; Acree, M.; Bacchetti, P.; Laraia, B.A.; Hecht, F.M.; et al. Effects of a mindfulness-based intervention on mindful eating, sweets consumption, and fasting glucose levels in obese adults: Data from the SHINE randomized controlled trial. J. Behav. Med. 2016, 39, 201–213. [Google Scholar] [CrossRef]
- Alberts, H.J.; Mulkens, S.; Smeets, M.; Thewissen, R. Coping with food cravings. Investigating the potential of a mindfulness-based intervention. Appetite 2010, 55, 160–163. [Google Scholar] [CrossRef]
- Alberts, H.J.E.M.; Thewissen, R.; Raes, L. Dealing with problematic eating behaviour. The effects of a mindfulness-based intervention on eating behaviour, food cravings, dichotomous thinking and body image concern. Appetite 2012, 58, 847–851. [Google Scholar] [CrossRef]
- Mason, A.E.; Jhaveri, K.; Cohn, M.; Brewer, J.A. Testing a mobile mindful eating intervention targeting craving-related eating: Feasibility and proof of concept. J. Behav. Med. 2018, 41, 160–173. [Google Scholar] [CrossRef]
- Wardzinski, E.K.; Richter, J.; Moenikes, S.; Duysen, K.U.; Oltmanns, K.M. Nondietary psychological app program leads to sustained weight loss due to trained physiological satiety perception. Appl. Psychol. Health Well Being 2024. [Google Scholar] [CrossRef]
- Forman, E.M.; Hoffman, K.L.; McGrath, K.B.; Herbert, J.D.; Brandsma, L.L.; Lowe, M.R. A comparison of acceptance- and control-based strategies for coping with food cravings: An analog study. Behav. Res. Ther. 2007, 45, 2372–2386. [Google Scholar] [CrossRef] [PubMed]
- Hooper, N.; Sandoz, E.K.; Ashton, J.; Clarke, A.; McHugh, L. Comparing thought suppression and acceptance as coping techniques for food cravings. Eat. Behav. 2012, 13, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Wegner, D.M. Ironic processes of mental control. Psychol. Rev. 1994, 101, 34. [Google Scholar] [CrossRef] [PubMed]
- Erskine, J.A.; Georgiou, G.J.; Kvavilashvili, L. I suppress, therefore I smoke: Effects of thought suppression on smoking behavior. Psychol. Sci. 2010, 21, 1225–1230. [Google Scholar] [CrossRef]
- Hayes, S.C.; Luoma, J.B.; Bond, F.W.; Masuda, A.; Lillis, J. Acceptance and commitment therapy: Model, processes and outcomes. Behav. Res. Ther. 2006, 44, 1–25. [Google Scholar] [CrossRef]
- Kavanagh, D.J.; May, J.; Andrade, J. Tests of the elaborated intrusion theory of craving and desire: Features of alcohol craving during treatment for an alcohol disorder. Br. J. Clin. Psychol. 2009, 48, 241–254. [Google Scholar] [CrossRef]
- Hamilton, J.; Fawson, S.; May, J.; Andrade, J.; Kavanagh, D.J. Brief guided imagery and body scanning interventions reduce food cravings. Appetite 2013, 71, 158–162. [Google Scholar] [CrossRef]
- Bishop, S.R.; Lau, M.; Shapiro, S.; Carlson, L.; Anderson, N.D.; Carmody, J.; Segal, Z.V.; Abbey, S.; Speca, M.; Velting, D. Mindfulness: A proposed operational definition. Clin. Psychol. Sci. Pract. 2004, 11, 230. [Google Scholar] [CrossRef]
- Tapper, K.; Turner, A. The effect of a mindfulness-based decentering strategy on chocolate craving. Appetite 2018, 130, 157–162. [Google Scholar] [CrossRef]
- Wilson, E.; Senior, V.; Tapper, K. The effect of visualisation and mindfulness-based decentering on chocolate craving. Appetite 2021, 164, 105278. [Google Scholar] [CrossRef]
- Schumacher, S.; Kemps, E.; Tiggemann, M. Cognitive defusion and guided imagery tasks reduce naturalistic food cravings and consumption: A field study. Appetite 2018, 127, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.; Kemps, E.; Tiggemann, M. Acceptance- and imagery-based strategies can reduce chocolate cravings: A test of the elaborated-intrusion theory of desire. Appetite 2017, 113, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Tiggemann, M.; Kemps, E. The phenomenology of food cravings: The role of mental imagery. Appetite 2005, 45, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Daniel, T.O.; Stanton, C.M.; Epstein, L.H. The future is now: Reducing impulsivity and energy intake using episodic future thinking. Psychol. Sci. 2013, 24, 2339–2342. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; Khalil, M.; Dickson, J.; May, J.; Kavanagh, D.J. Functional Imagery Training to reduce snacking: Testing a novel motivational intervention based on Elaborated Intrusion theory. Appetite 2016, 100, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Werthmann, J.; Tuschen-Caffier, B.; Ströbele, L.; Kübel, S.L.; Renner, F. Healthy cravings? Impact of imagined healthy food consumption on craving for healthy foods and motivation to eat healthily—Results of an initial experimental study. Appetite 2023, 183, 106458. [Google Scholar] [CrossRef]
- Wolz, I.; Nannt, J.; Svaldi, J. Laboratory-based interventions targeting food craving: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e12996. [Google Scholar] [CrossRef]
- Wharton, S.; Batterham, R.L.; Bhatta, M.; Buscemi, S.; Christensen, L.N.; Frias, J.P.; Jodar, E.; Kandler, K.; Rigas, G.; Wadden, T.A.; et al. Two-year effect of semaglutide 2.4 mg on control of eating in adults with overweight/obesity: STEP 5. Obesity 2023, 31, 703–715. [Google Scholar] [CrossRef]
- Martin, C.K.; Ravussin, E.; Sanchez-Delgado, G.; Nishiyama, H.; Li, J.; Urva, S.; Pratt, E.J.; Milicevic, Z.; Haupt, A.; Coskun, T. 128-OR: The effect of tirzepatide during weight loss on food intake, appetite, food preference, and food craving in people with obesity. Diabetes 2023, 72, 128-OR. [Google Scholar] [CrossRef]
- Wang, G.J.; Zhao, J.; Tomasi, D.; Kojori, E.S.; Wang, R.; Wiers, C.E.; Caparelli, E.C.; Volkow, N.D. Effect of combined naltrexone and bupropion therapy on the brain’s functional connectivity. Int. J. Obes. 2018, 42, 1890–1899. [Google Scholar] [CrossRef]
- Greenway, F.L.; Fujioka, K.; Plodkowski, R.A.; Mudaliar, S.; Guttadauria, M.; Erickson, J.; Kim, D.D.; Dunayevich, E.; Group, C.-I.S. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010, 376, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Dalton, M.; Finlayson, G.; Walsh, B.; Halseth, A.E.; Duarte, C.; Blundell, J.E. Early improvement in food cravings are associated with long-term weight loss success in a large clinical sample. Int. J. Obes. 2017, 41, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]
- Maselli, D.B.; Camilleri, M. Effects of GLP-1 and Its Analogs on Gastric Physiology in Diabetes Mellitus and Obesity. Adv. Exp. Med. Biol. 2021, 1307, 171–192. [Google Scholar] [CrossRef]
- Kawatani, M.; Yamada, Y.; Kawatani, M. Glucagon-like peptide-1 (GLP-1) action in the mouse area postrema neurons. Peptides 2018, 107, 68–74. [Google Scholar] [CrossRef]
- Wadden, T.A.; Foreyt, J.P.; Foster, G.D.; Hill, J.O.; Klein, S.; O’Neil, P.M.; Perri, M.G.; Pi-Sunyer, F.X.; Rock, C.L.; Erickson, J.S.; et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: The COR-BMOD trial. Obesity 2011, 19, 110–120. [Google Scholar] [CrossRef]
- Wadden, T.A.; Bailey, T.S.; Billings, L.K.; Davies, M.; Frias, J.P.; Koroleva, A.; Lingvay, I.; O’Neil, P.M.; Rubino, D.M.; Skovgaard, D.; et al. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA 2021, 325, 1403–1413. [Google Scholar] [CrossRef]
- Jeffers, L.; Manner, J.; Jepson, R.; McAteer, J. Healthcare professionals’ perceptions and experiences of obesity and overweight and its management in primary care settings: A qualitative systematic review. Prim. Health Care Res. Dev. 2024, 25, e5. [Google Scholar] [CrossRef]
- Song, S.; Zilverstand, A.; Gui, W.; Pan, X.; Zhou, X. Reducing craving and consumption in individuals with drug addiction, obesity or overeating through neuromodulation intervention: A systematic review and meta-analysis of its follow-up effects. Addiction 2022, 117, 1242–1255. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Kwan, A.T.; Rosenblat, J.D.; Teopiz, K.M.; Mansur, R.B. Psychotropic drug–related weight gain and its treatment. Am. J. Psychiatry 2024, 181, 26–38. [Google Scholar] [CrossRef]

| Questionnaire | Craving Domain | Strengths | Weaknesses |
|---|---|---|---|
| Past/Trait Food Cravings | |||
| Control of Eating Questionnaire (CoEQ) | Tonic experience, frequency, severity, type of craving (sweet, savoury), positive mood | Strong criterion validity, assesses hunger/satiety; four-factor structure replicated | Frequency and severity are not measured separately |
| The Food Craving Inventory (FCI) | Frequency of specific food cravings across four types of food (high fat, sweets, carbohydrates, and fast-food fats) | Four-factor structure replicated in clinical samples; suitable for cross-cultural use; good test–retest reliability and criterion validity; useful for clinicians | Mixed evidence for predictive validity |
| Food Craving Questionnaire—Trait (FCQ-T) [43] and FCQ-T-reduced (FCQ-T-r) [44] | Multi-dimensional (cognitive, emotional, contextual, behavioural, physiological); commonly craved foods | FCQ-T: good test–retest reliability for total score; psychometric evaluation in clinical samples; good predictive, construct, and criterion validity; suitable for cross-cultural use. FCQ-T-r: shorter/less burdensome (15 items; single-factor structure replicated (healthy/clinical samples)) | FCQ-T: lengthy/burdensome for participants to complete (39 items); risk of inaccurate responding or missing date; lower test–retest reliability in overweight and obesity relative to healthy samples; unstable factor structure |
| General Food Craving Questionnaire—Trait (GFCQ-T) 1 [45] | Multi-dimensional (cognitive, emotional, contextual, behavioural, physiological); general desire for food or to eat | Good test–retest reliability for total score; evidence of criterion validity; briefer than FCQ-T; suitable for cross-cultural use | Factor structure not replicated |
| State Food Cravings | |||
| Single-item craving rating scale [46] | Desire or urge to eat | Brief and simple | Single-dimensional (cognitive aspect) |
| The Craving Experience Questionnaire (CEQ) [47] | Strength (state) and frequency (trait) of craving-related thoughts and urges, vividness of imagery, intrusiveness of thoughts | Researcher-defined timeframe (past cravings); specific foods or food generally; brief (10 items); theoretical basis (EI theory); multi-sensory aspect of cravings; strength (state) and frequency (past) of cravings assessed separately | Single-dimensional (cognitive aspect); criterion validity not assessed |
| Food Craving Questionnaire—State (FCQ-S) [43] | Multi-dimensional (cognitive, emotional, contextual, behavioural, physiological); commonly craved foods | Sensitive to acute fluctuations; brief (15 items); specific-food; strong construct validity; tested in clinical samples; suitable for cross-cultural use | Low test–retest reliability; poor predictor of long-term weight change; lack of sensitivity detecting differences between individuals with or without weight-related issues |
| General Food Craving Questionnaire—State (GCFQ-S) 2 [45] | Multi-dimensional (cognitive, emotional, contextual, behavioural, physiological); general desire for food or to eat | Sensitive to acute fluctuations; brief (15 items); tasty food in general; strong construct and criterion validity | Low test–retest reliability |
| Medication | Average Weight Loss | Cravings Reduction | Common Side Effects (>5% and >2 × Placebo) |
|---|---|---|---|
| Liraglutide (Saxenda) | 8–10% in 1 year RCT (SCALE Trials) | Yes (mild effect on Food Cravings Inventory) | Nausea and vomiting Diarrhoea Constipation Dyspepsia |
| Semaglutide (Wegovy) | 15–17% in 1 year RCT (STEP Trials) | Yes (moderate effect on Control of Eating Questionnaire) | Nausea and vomiting Diarrhoea Constipation Dyspepsia |
| Tirzepatide (Mounjaro) | 22–24% in 1 year RCT (Surmount Trials) | Yes (moderate effect on Control of Eating Questionnaire) | Nausea and vomiting Diarrhoea Constipation Dyspepsia |
| Naltrexone-Bupropion (Contrave) | 8–11% in 1 year RCT (COR Trials) | Yes (moderate effect on Control of Eating Questionnaire) | Nausea and vomiting Constipation Dizziness Hot flushes Dry mouth |
| Orlistat (Xenical) | 3% in 6 months RCT | Unlikely | Diarrhoea Faecal urgency Abdominal discomfort |
| Phentermine (Duromine) | 5–7% in 6 months RCT | Yes (Mild effect on Trait and State Food Cravings Questionnaire) | Dry mouth Restlessness |
| Question About Cravings |
|---|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakoschke, N.; Henry, B.A.; Cowley, M.A.; Lee, K. Tackling Cravings in Medical Weight Management: An Update on Pathophysiology and an Integrated Approach to Treatment. Nutrients 2024, 16, 3238. https://doi.org/10.3390/nu16193238
Kakoschke N, Henry BA, Cowley MA, Lee K. Tackling Cravings in Medical Weight Management: An Update on Pathophysiology and an Integrated Approach to Treatment. Nutrients. 2024; 16(19):3238. https://doi.org/10.3390/nu16193238
Chicago/Turabian StyleKakoschke, Naomi, Belinda A. Henry, Michael A. Cowley, and Kevin Lee. 2024. "Tackling Cravings in Medical Weight Management: An Update on Pathophysiology and an Integrated Approach to Treatment" Nutrients 16, no. 19: 3238. https://doi.org/10.3390/nu16193238






