Styphnolobium japonicum Fruit and Germinated Soybean Embryo Complex Extract for Postmenopausal-Symptom Relief
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. HPLC Analysis
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. NO and DPPH Assay
2.6. MG63 Activation and RANKL Gene Expression Analysis
2.7. qPCR Assay
2.8. Western Blot Analysis
2.9. Immunocytochemical Analysis of ERα in MCF7 Cells
2.10. Animal Housing and Ovariectomy
2.11. Measurement of Vasomotor Symptoms
2.12. Evaluation of Vaginal Epithelial Cell Changes (Vaginal Cornification)
2.13. Assessment of Organ Abnormality and Fat Tissues
2.14. Blood Hormone and Biochemical Analysis
2.15. Microscopic Assessment of Trabecular Bone Loss
2.16. Isolation of Bone Marrow Cells from Femurs
2.17. Assessment of Bone Mineral Contents, Bone Mineral Density, and X-ray Imaging
2.18. Statistical Analysis
3. Results and Discussion
3.1. Optimal Ratio of SJFE and GSEE
3.2. Impacts of SJFE and GSEE Combinations on MCF7 Cells
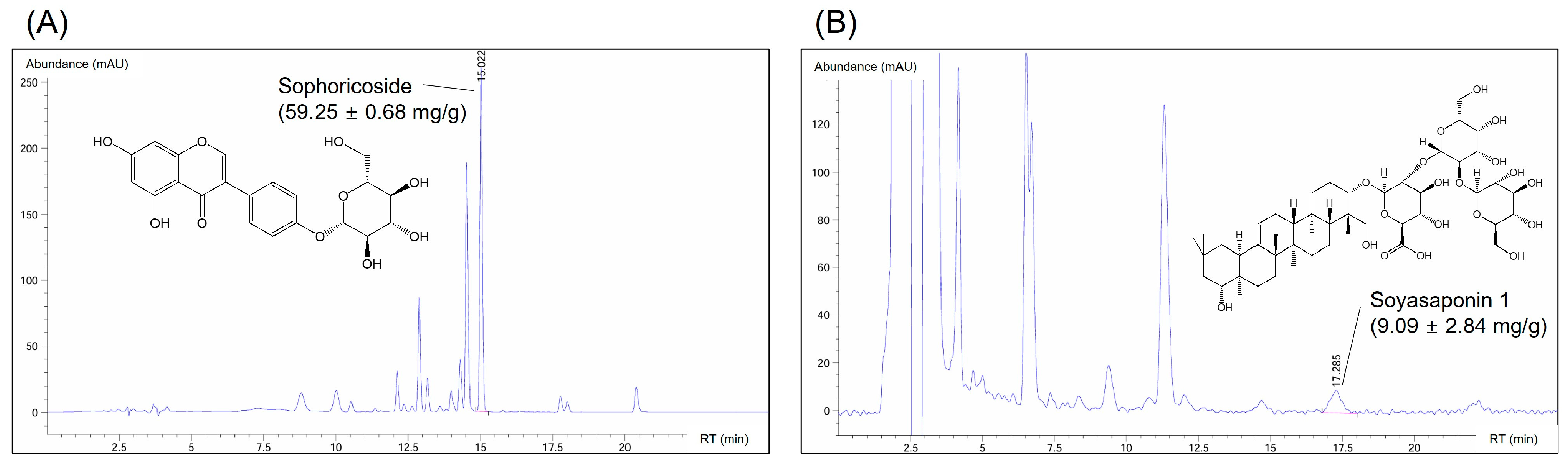
3.3. Composition of Active Compounds in the SJF–GSE Extract
3.4. Body Weight and Vasomotor Symptom Improvement
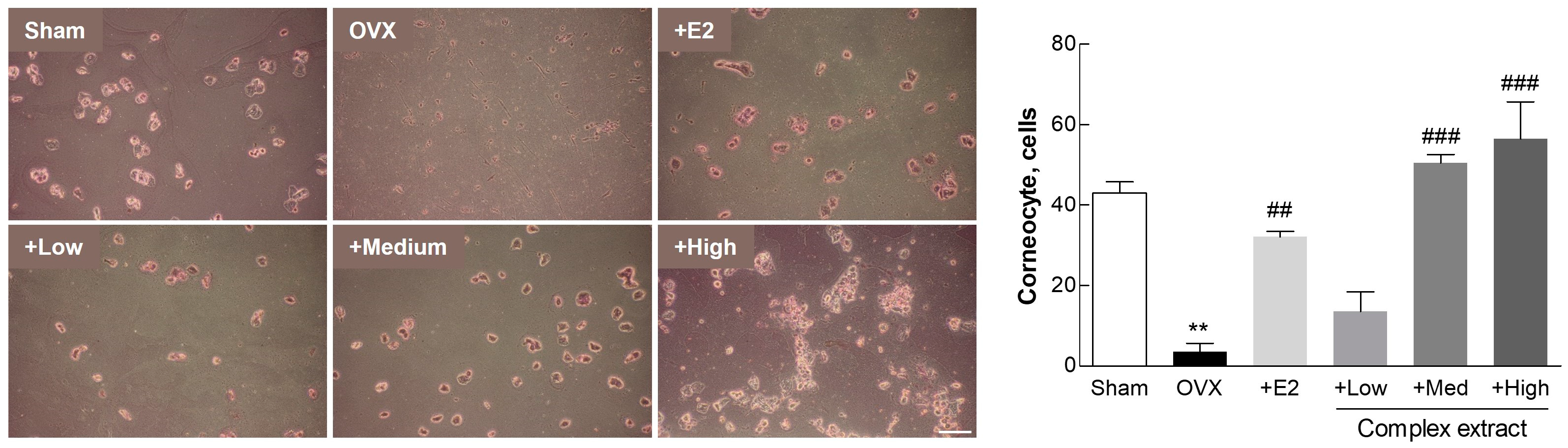
3.5. Postmenopausal Vaginal Symptoms (Vaginal Keratinization)
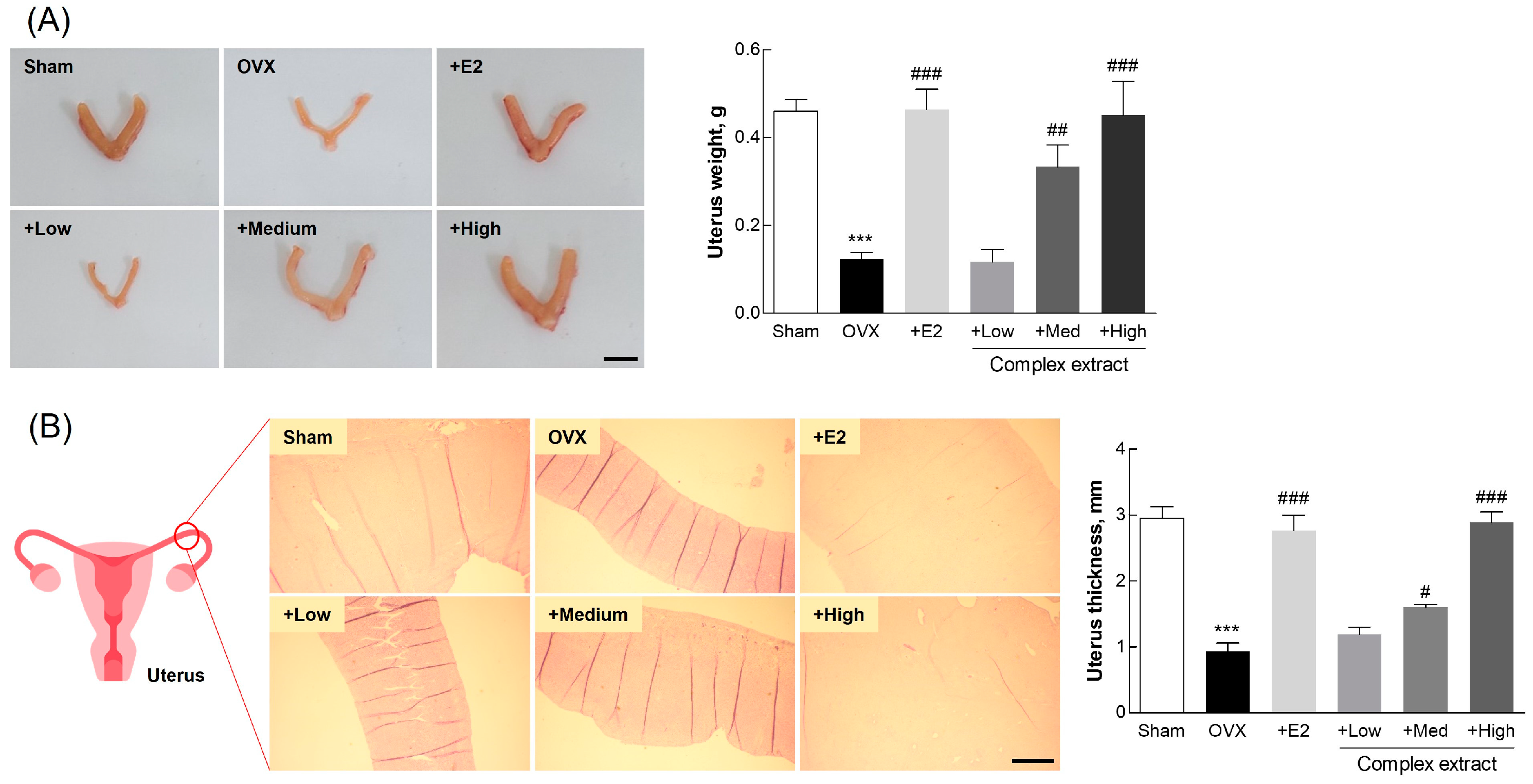
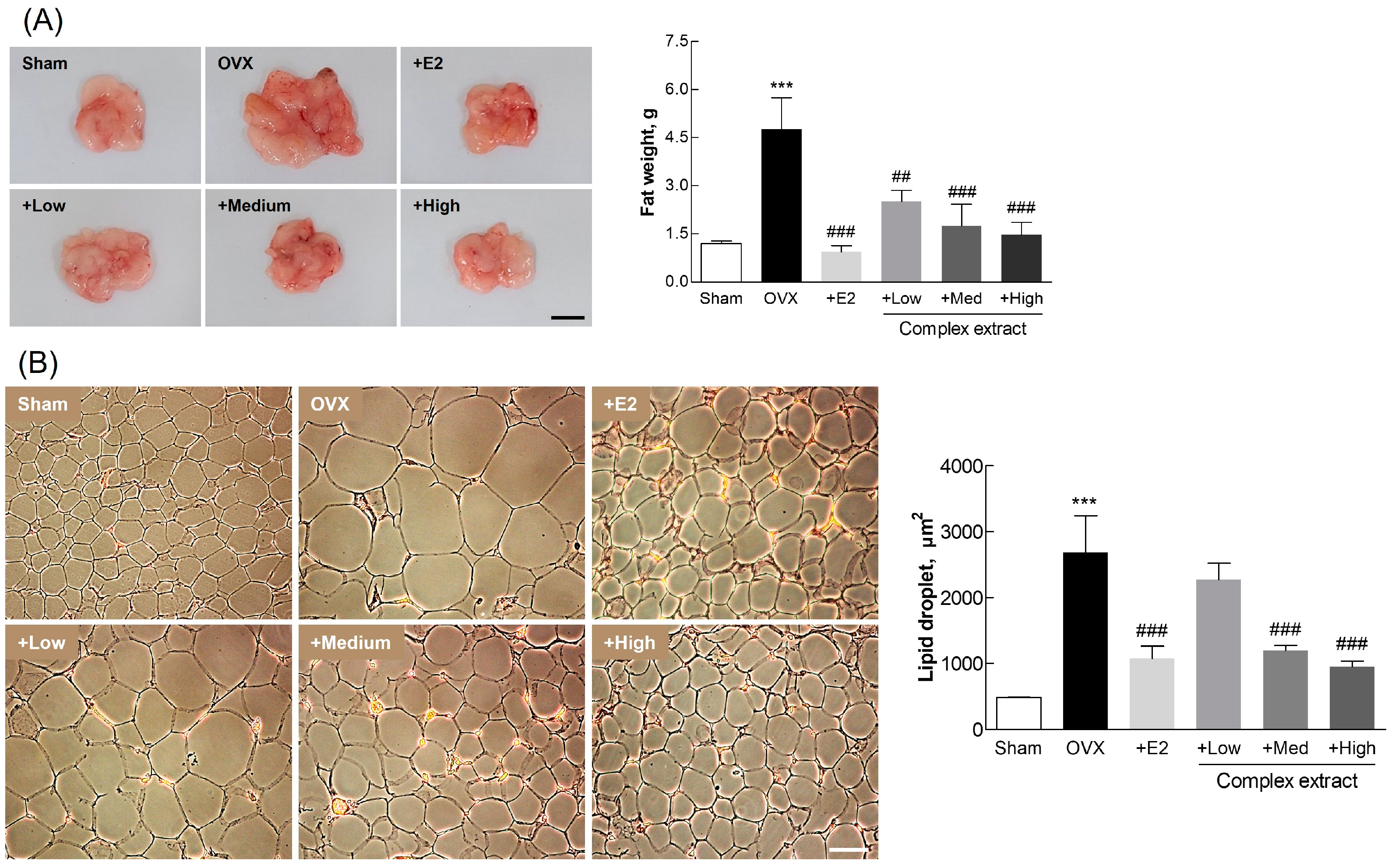
3.6. Postmenopausal Changes in Uterine and Adipose Tissue
3.7. Blood Biochemical Markers
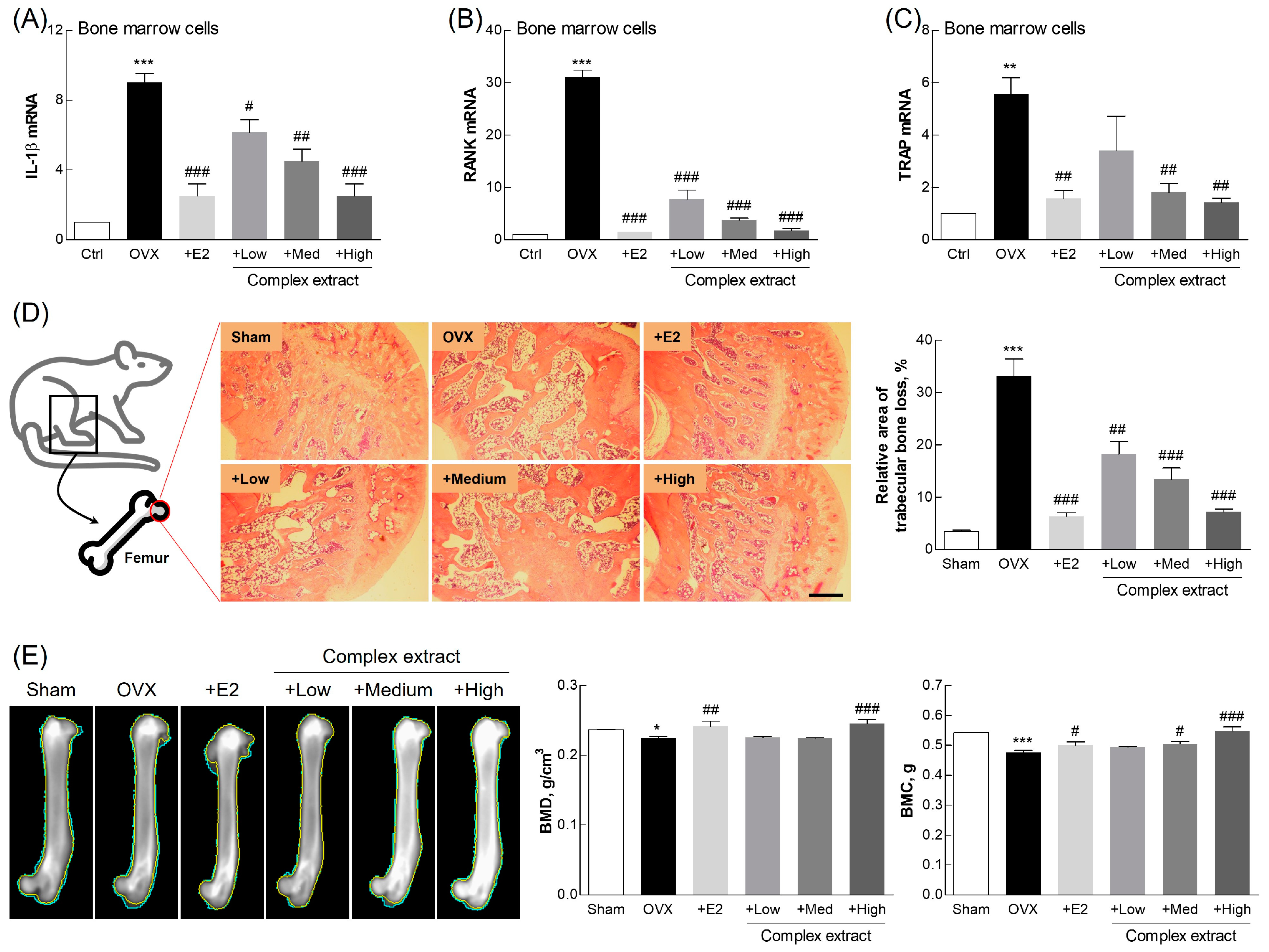
3.8. Evaluation of Femoral Bone Features: Gene Expression, Histological Staining, and Imaging Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duralde, E.R.; Sobel, T.H.; Manson, J.E. Management of perimenopausal and menopausal symptoms. Brit Med. J. 2023, 382, e072612. [Google Scholar] [CrossRef] [PubMed]
- Fait, T. Menopause hormone therapy: Latest developments and clinical practice. Drugs Context 2019, 8, 212551. [Google Scholar] [CrossRef]
- Chen, Z.; Qian, F.; Hu, Y.; Voortman, T.; Li, Y.; Rimm, E.B.; Sun, Q. Dietary phytoestrogens and total and cause-specific mortality: Results from 2 prospective cohort studies. Am. J. Clin. Nutr. 2023, 117, 130–140. [Google Scholar] [CrossRef]
- Rowe, I.J.; Baber, R.J. The effects of phytoestrogens on postmenopausal health. Climacteric 2021, 24, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.R.; Kim, H.J.; Yu, S.H.; Lee, B.S.; Jeon, S.Y.; Lee, J.J.; Lee, Y.C. Combination of red clover and hops extract improved menopause symptoms in an ovariectomized rat model. Evid. Based Complement. Altern. Med. 2020, 2020, 7941391. [Google Scholar] [CrossRef] [PubMed]
- Mahdavian, M.; Mirzaii Najmabadi, K.; Hosseinzadeh, H.; Mirzaeian, S.; Badiee Aval, S.; Esmaeeli, H. Effect of the mixed herbal medicines extract (fennel, chamomile, and saffron) on menopause syndrome: A randomized controlled clinical trial. J. Caring Sci. 2019, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bau, H.M.; Villaume, C.; Méjean, L. Effects of soybean (Glycine max) germination on biologically active components, nutritional values of seeds, and biological characteristics in rats. Nahrung 2000, 44, 2–6. [Google Scholar] [CrossRef]
- Weaver, C.M.; Martin, B.R.; Jackson, G.S.; McCabe, G.P.; Nolan, J.R.; McCabe, L.D.; Barnes, S.; Reinwald, S.; Boris, M.E.; Peacock, M. Antiresorptive effects of phytoestrogen supplements compared with estradiol or risedronate in postmenopausal women using (41)Ca methodology. J. Clin. Endocrinol. Metab. 2009, 94, 3798–3805. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, J.; Kim, S.; Park, S.; Yang, H.; Ahn, B.H.; Jang, C.Y.; Jeong, H.C.; Lee, S.J.; Kim, S.L.; et al. Characterization of Soybean Germinated Embryo Extract as an Estrogen Receptor Subtype-Selective and Tissue-Specific Modulator. J. Med. Food 2019, 22, 186–195. [Google Scholar] [CrossRef]
- Lee, K.-S.; Woo, S.-Y.; Lee, M.-J.; Kim, H.Y.; Ham, H.; Lee, D.-J.; Choi, S.-W.; Seo, W.D. Isoflavones and soyasaponins in the germ of Korean soybean [Glycine max (L.) Merr.] cultivars and their compound-enhanced BMP-2-induced bone formation. Appl. Biol. Chem. 2020, 63, 26. [Google Scholar] [CrossRef]
- Kamo, S.; Suzuki, S.; Sato, T. Comparison of bioavailability (I) between soyasaponins and soyasapogenols, and (II) between group A and B soyasaponins. Nutrition 2014, 30, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Liao, J.; Duan, T.; Wu, Q.; Huang, X.; Pei, X.; Wang, C. Chemical composition and pharmacological properties of Flos sophorae immaturus, Flos sophorae and Fructus sophorae: A review. J. Future Foods 2023, 3, 330–339. [Google Scholar] [CrossRef]
- Ahn, J.W.; Jang, S.K.; Jo, B.R.; Kim, H.S.; Park, J.Y.; Park, H.Y.; Yoo, Y.M.; Joo, S.S. A therapeutic intervention for Alzheimer’s disease using ginsenoside Rg3: Its role in M2 microglial activation and non-amyloidogenesis. J. Physiol. Pharmacol. 2021, 72, 185–193. [Google Scholar] [CrossRef]
- Stout Steele, M.; Bennett, R.A. Clinical technique: Dorsal ovariectomy in rodents. J. Exot. Pet. Med. 2011, 20, 222–226. [Google Scholar] [CrossRef]
- Muschter, D.; Göttl, C.; Vogel, M.; Grifka, J.; Straub, R.H.; Grässel, S. Reactivity of rat bone marrow-derived macrophages to neurotransmitter stimulation in the context of collagen II-induced arthritis. Arthritis Res. Ther. 2015, 17, 169. [Google Scholar] [CrossRef]
- Leanza, G.; Conte, C.; Cannata, F.; Isgrò, C.; Piccoli, A.; Strollo, R.; Quattrocchi, C.C.; Papalia, R.; Denaro, V.; Maccarrone, M.; et al. Oxidative stress in postmenopausal women with or without obesity. Cells 2023, 12, 1137. [Google Scholar] [CrossRef]
- Doshi, S.B.; Agarwal, A. The role of oxidative stress in menopause. J. Midlife Health 2013, 4, 140–146. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Are oxidative stress and inflammation mediators of bone loss due to estrogen deficiency? A review of current evidence. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, Y.M.; Chin, Y.W.; Kang, K.S. Schisandrol A exhibits estrogenic activity via estrogen receptor α-dependent signaling pathway in estrogen receptor-positive breast cancer cells. Pharmaceutics 2021, 13, 1082. [Google Scholar] [CrossRef]
- Moriarty, K.; Kim, K.H.; Bender, J.R. Estrogen receptor-mediated rapid signaling. Endocrinology 2006, 147, 5557–5563. [Google Scholar] [CrossRef]
- Echeverria, V.; Echeverria, F.; Barreto, G.E.; Echeverría, J.; Mendoza, C. Estrogenic plants: To prevent neurodegeneration and memory loss and other symptoms in women after menopause. Front. Pharmacol. 2021, 12, 644103. [Google Scholar] [CrossRef]
- Movérare-Skrtic, S.; Börjesson, A.E.; Farman, H.H.; Sjögren, K.; Windahl, S.H.; Lagerquist, M.K.; Andersson, A.; Stubelius, A.; Carlsten, H.; Gustafsson, J.; et al. The estrogen receptor antagonist ICI 182,780 can act both as an agonist and an inverse agonist when estrogen receptor α AF-2 is modified. Proc. Natl. Acad. Sci. USA 2014, 111, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Bai, Y.; Zhao, Z.; Wang, X.; Fang, J.; Huang, L.; Zeng, M.; Zhang, Q.; Zhang, Y.; Zheng, X. Local and traditional uses, phytochemistry, and pharmacology of Sophora japonica L.: A review. J. Ethnopharmacol. 2016, 187, 160–182. [Google Scholar] [CrossRef]
- Canivenc-Lavier, M.C.; Bennetau-Pelissero, C. Phytoestrogens and health effects. Nutrients 2023, 15, 317. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.H.; Elbadry, A.M.M.; El-Shazly, M.; Hwang, T.-L. Exploring the potential role of genus Sophora in the management of osteoporosis: A phytochemical and biological review. Front. Nat. Prod. 2023, 2, 1302371. [Google Scholar] [CrossRef]
- Guang, C.; Chen, J.; Sang, S.; Cheng, S. Biological functionality of soyasaponins and soyasapogenols. J. Agric. Food Chem. 2014, 62, 8247–8255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Liu, L.; Cui, H. Ovariectomy induces abdominal fat accumulation by improving gonadotropin-releasing hormone secretion in mouse. Biochem. Biophys. Res. Commun. 2022, 588, 111–117. [Google Scholar] [CrossRef]
- Wellberg, E.A.; Corleto, K.A.; Checkley, L.A.; Jindal, S.; Johnson, G.; Higgins, J.A.; Obeid, S.; Anderson, S.M.; Thor, A.D.; Schedin, P.J.; et al. Preventing ovariectomy-induced weight gain decreases tumor burden in rodent models of obesity and postmenopausal breast cancer. Breast Cancer Res. 2022, 24, 42. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tamura, M.; Hayashi, M.; Katsuura, Y.; Tanabe, H.; Ohta, T.; Komoriya, K. Elevation of tail skin temperature in ovariectomized rats in relation to menopausal hot flushes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R863–R869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; DiVittorio, J.R.; Joseph, A.M.; Correa, S.M. The effects of estrogens on neural circuits that control temperature. Endocrinology 2021, 162, bqab087. [Google Scholar] [CrossRef]
- Lethaby, A.; Marjoribanks, J.; Kronenberg, F.; Roberts, H.; Eden, J.; Brown, J. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst. Rev. 2013, 2013, CD001395. [Google Scholar] [CrossRef] [PubMed]
- Angelou, K.; Grigoriadis, T.; Diakosavvas, M.; Zacharakis, D.; Athanasiou, S. The genitourinary syndrome of menopause: An overview of the recent data. Cureus 2020, 12, e7586. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jung, D.H.; Park, M.; Yeon, S.W.; Jung, S.H.; Yun, S.I.; Park, H.O.; Yoo, W. The effect of Lactobacillus gasseri BNR17 on postmenopausal symptoms in ovariectomized rats. J. Microbiol. Biotechnol. 2021, 31, 1281–1287. [Google Scholar] [CrossRef]
- Parhizkar, S.; Latiff, L.A. Supplementary health benefits of linoleic acid by improvement of vaginal cornification of ovariectomized rats. Adv. Pharm. Bull. 2013, 3, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Marathe, J.; Pudney, J. The structure of the human vaginal stratum corneum and its role in immune defense. Am. J. Reprod. Immunol. 2014, 71, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Hebbar, S.; Chaya, V.; Rai, L.; Ramachandran, A. Factors influencing endometrial thickness in postmenopausal women. Ann. Med. Health Sci. Res. 2014, 4, 608–614. [Google Scholar] [CrossRef]
- Yu, K.; Huang, Z.Y.; Xu, X.L.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen receptor function: Impact on the human endometrium. Front. Endocrinol. 2022, 13, 827724. [Google Scholar] [CrossRef]
- Babaei, P.; Mehdizadeh, R.; Ansar, M.M.; Damirchi, A. Effects of ovariectomy and estrogen replacement therapy on visceral adipose tissue and serum adiponectin levels in rats. Menopause Int. 2010, 16, 100–104. [Google Scholar] [CrossRef]
- Domínguez-López, I.; Yago-Aragón, M.; Salas-Huetos, A.; Tresserra-Rimbau, A.; Hurtado-Barroso, S. Effects of dietary phytoestrogens on hormones throughout a human lifespan: A review. Nutrients 2020, 12, 2456. [Google Scholar] [CrossRef]
- Shim, J.J.; Kim, J.W.; Oh, C.H.; Lee, Y.R.; Lee, J.S.; Park, S.Y.; Kim, B.H.; Oh, I.H. Serum alanine aminotransferase level and liver-related mortality in patients with chronic hepatitis B: A large national cohort study. Liver Int. 2018, 38, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Rodriguez Polanco, S.; Bousvarou, M.D.; Papakonstantinou, E.J.; Peña Genao, E.; Guzman, E.; Kostara, C.E. The triglyceride/high-density lipoprotein cholesterol (TG/HDL-C) ratio as a risk marker for metabolic syndrome and cardiovascular disease. Diagnostics 2023, 13, 929. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, D.; Wu, C.; Zhang, L.; Mei, Q.; Hu, G.; Long, G.; Sun, W. Prognostic significance of serum lactate dehydrogenase in patients with breast cancer: A meta-analysis. Cancer Manag. Res. 2019, 11, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Schini, M.; Vilaca, T.; Gossiel, F.; Salam, S.; Eastell, R. Bone turnover markers: Basic biology to clinical applications. Endocr. Rev. 2023, 44, 417–473. [Google Scholar] [CrossRef]
- Goretti Penido, M.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef]
- Møller, A.M.J.; Delaissé, J.M.; Olesen, J.B.; Madsen, J.S.; Canto, L.M.; Bechmann, T.; Rogatto, S.R.; Søe, K. Aging and menopause reprogram osteoclast precursors for aggressive bone resorption. Bone Res. 2020, 8, 27. [Google Scholar] [CrossRef]
- Jin Hee, P.; Na Kyung, L.; Soo Young, L. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef]
- Hayman, A.R. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity 2008, 41, 218–223. [Google Scholar] [CrossRef]
- Wang, L.T.; Chen, L.R.; Chen, K.H. Hormone-related and drug-induced osteoporosis: A cellular and molecular overview. Int. J. Mol. Sci. 2023, 24, 5814. [Google Scholar] [CrossRef]
- Abdi, F.; Alimoradi, Z.; Haqi, P.; Mahdizad, F. Effects of phytoestrogens on bone mineral density during the menopause transition: A systematic review of randomized, controlled trials. Climacteric 2016, 19, 535–545. [Google Scholar] [CrossRef]
- Sansai, K.; Na Takuathung, M.; Khatsri, R.; Teekachunhatean, S.; Hanprasertpong, N.; Koonrungsesomboon, N. Effects of isoflavone interventions on bone mineral density in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2020, 31, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Y.; Xu, Q.; Shikov, A.N.; Pozharitskaya, O.N.; Flisyuk, E.V.; Liu, M.; Li, H.; Vargas-Murga, L.; Duez, P. Flavonoids and saponins: What have we got or missed? Phytomedicine 2023, 109, 154580. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [PubMed]



| Activity | SJFE | GSEE | SJFE and GSEE Combinations | ||
|---|---|---|---|---|---|
| 1:1 | 1:2 | 2:1 | |||
| DPPH scavenging (IC50, µg) | 61.42 ± 11.23 | 293.75 ± 15.71 | 117.56 ± 12.04 | 84.48 ± 13.74 | 77.72 ± 14.13 |
| NO inhibition (ED50, µg/mL) | 206.71 ± 18.11 | 34.22 ± 5.71 | 64.82 ± 1.95 | 48.19 ± 1.88 | 59.37 ± 1.44 |
| RANKL inhibition (p < 0.001, µg/mL) | 250 | 10 | 100 | 10 | 10 |
| Parameter | Sham | OVX | +E2 | Complex Extract | ||
|---|---|---|---|---|---|---|
| +Low | +Medium | +High | ||||
| E2 (pg/mL) | 24.68 ± 1.14 | 14.4 ± 0.63 * | 29.72 ± 1.4 ## | 19.86 ± 1.5 | 22.47 ± 2.29 | 33.59 ± 1.88 ## |
| CTx (pg/mL) | 292.97 ± 33.6 | 548.31 ± 28.81 ** | 293.39 ± 21.57 ## | 365.98 ± 49.36 # | 264.35 ± 12.44 ## | 196.32 ± 19.91 ## |
| OC (pg/mL) | 7.44 ± 0.34 | 13.31 ± 0.51 ** | 5.11 ± 0.7 ### | 9.44 ± 0.57 # | 7.57 ± 0.58 ## | 6.29 ± 0.17 ### |
| Ca (mg/dL) | 10.45 ± 0.05 | 10.55 ± 0.55 | 11.15 ± 1.25 | 10.2 ± 0.1 | 10.45 ± 0.05 | 10.8 ± 0.5 |
| P (mg/dL) | 3.65 ± 0.05 | 2.15 ± 0.15 ** | 3.4 ± 0.2 # | 2.3 ± 0.2 | 3.35 ± 0.15 # | 3.75 ± 0.25 ## |
| TG (mg/dL) | 49 ± 5 | 139.25 ± 0.75 ** | 82.9 ± 7.1 # | 94.4 ± 13.6 | 74.05 ± 13.45 | 56.1 ± 2.1 # |
| HDL (mg/dL) | 48.3 ± 1.8 | 41.75 ± 5.25 | 40.03 ± 1.02 | 36.5 ± 1.5 | 43.5 ± 1.5 | 50.4 ± 2.6 |
| LDL (mg/dL) | 5.5 ± 0.5 | 10.5 ± 0.5 ** | 5.5 ± 0.5 ## | 10.5 ± 0.5 | 10 ± 1 | 7.5 ± 0.5 # |
| ALT (U/L) | 70.3 ± 2.8 | 102.05 ± 2.15 * | 88.55 ± 6.15 | 105.95 ± 4.05 | 87.95 ± 9.25 | 63.35 ± 2.05 # |
| LDH (U/L) | 318.25 ± 6.25 | 945.25 ± 80.25 ** | 684.5 ± 79.5 | 396.25 ± 20.25 ## | 210.5 ± 9.5 ### | 227.5 ± 57.5 ### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.-W.; Kim, H.-S.; Damodar, K.; Shin, H.-H.; Kim, K.-M.; Park, J.-Y.; Jang, S.-K.; Yoo, Y.-M.; Jung, J.-C.; Joo, S.-S. Styphnolobium japonicum Fruit and Germinated Soybean Embryo Complex Extract for Postmenopausal-Symptom Relief. Nutrients 2024, 16, 3297. https://doi.org/10.3390/nu16193297
Ahn J-W, Kim H-S, Damodar K, Shin H-H, Kim K-M, Park J-Y, Jang S-K, Yoo Y-M, Jung J-C, Joo S-S. Styphnolobium japonicum Fruit and Germinated Soybean Embryo Complex Extract for Postmenopausal-Symptom Relief. Nutrients. 2024; 16(19):3297. https://doi.org/10.3390/nu16193297
Chicago/Turabian StyleAhn, Jeong-Won, Hyun-Soo Kim, Kongara Damodar, Hee-Hyun Shin, Kyung-Mi Kim, Jung-Youl Park, Su-Kil Jang, Yeong-Min Yoo, Jae-Chul Jung, and Seong-Soo Joo. 2024. "Styphnolobium japonicum Fruit and Germinated Soybean Embryo Complex Extract for Postmenopausal-Symptom Relief" Nutrients 16, no. 19: 3297. https://doi.org/10.3390/nu16193297







