The Influence of Strain and Sex on High Fat Diet-Associated Alterations of Dopamine Neurochemistry in Mice
Abstract
:1. Introduction
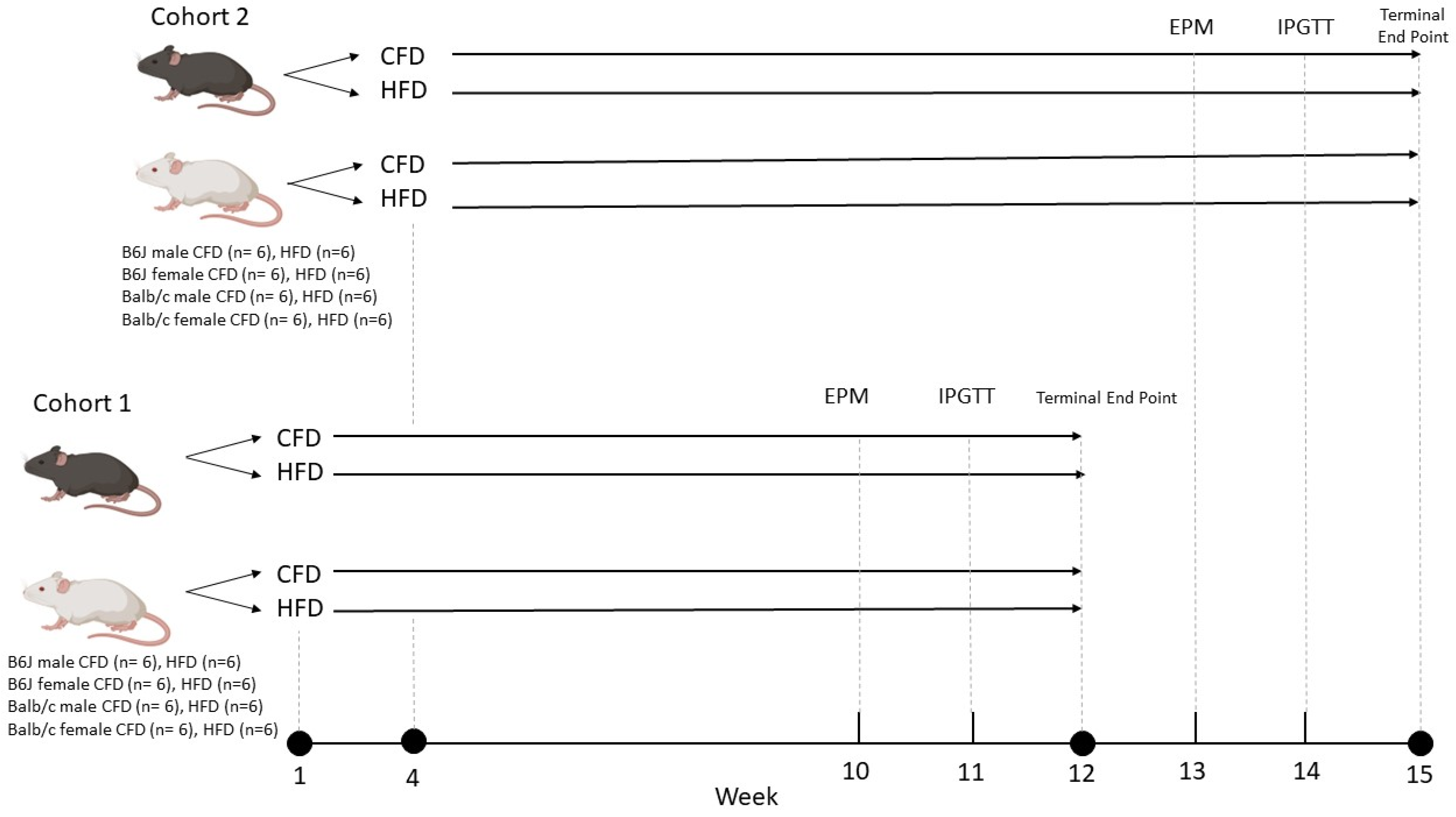
2. Materials and Methods
2.1. Animals and Diet
2.2. Elevated Plus Maze
2.3. Intraperitoneal Glucose Tolerance Test
2.4. Ex Vivo Fast Scan Cyclic Voltammetry
2.5. Transcardiac Perfusion and Fixation
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
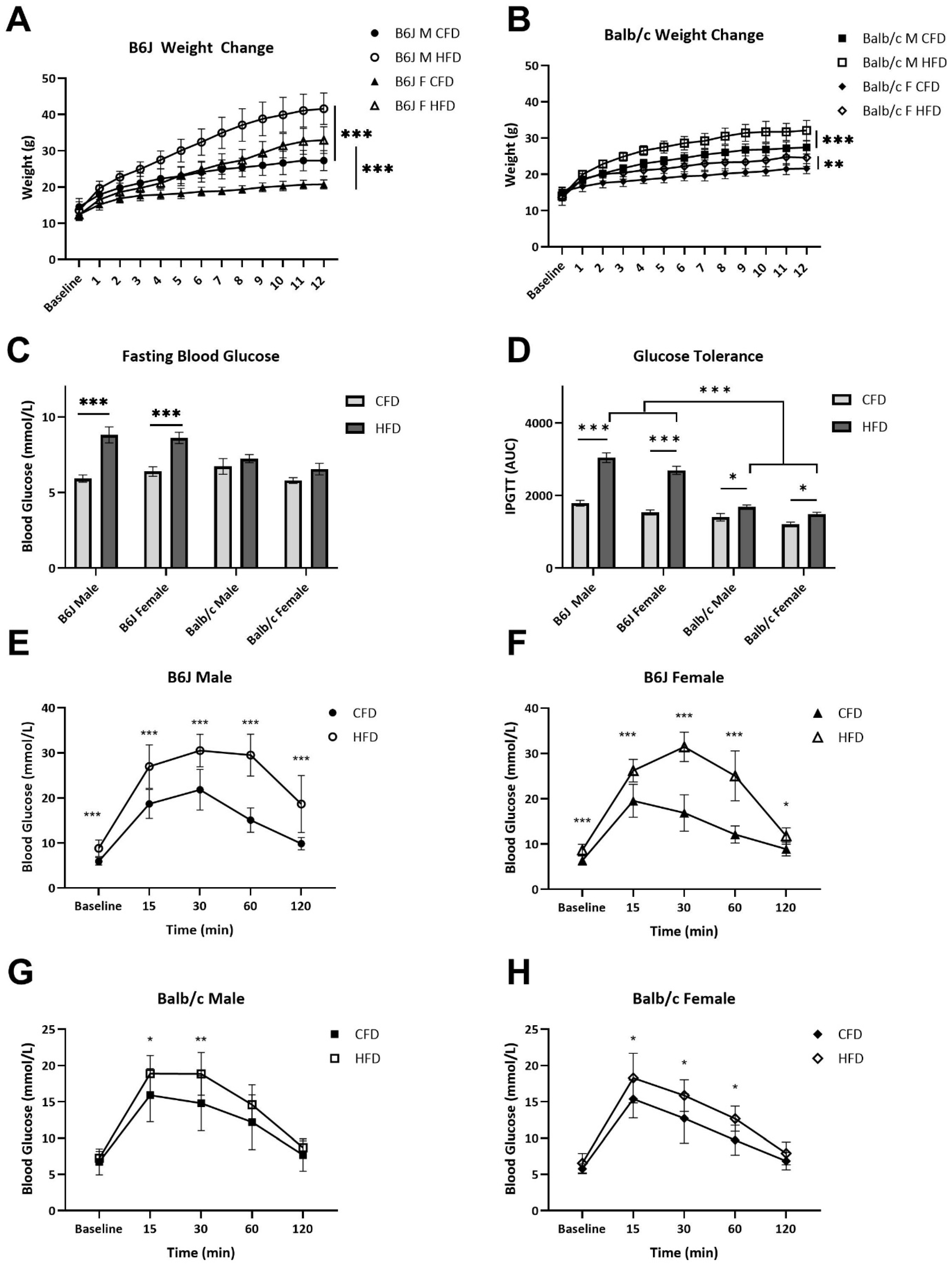
3.1. Strain and Sex Influenced Weight Gain and Glucose Clearance Following 12 Weeks of an HFD
3.2. HFD Influenced Anxiety-like Behavior for the B6J Strain
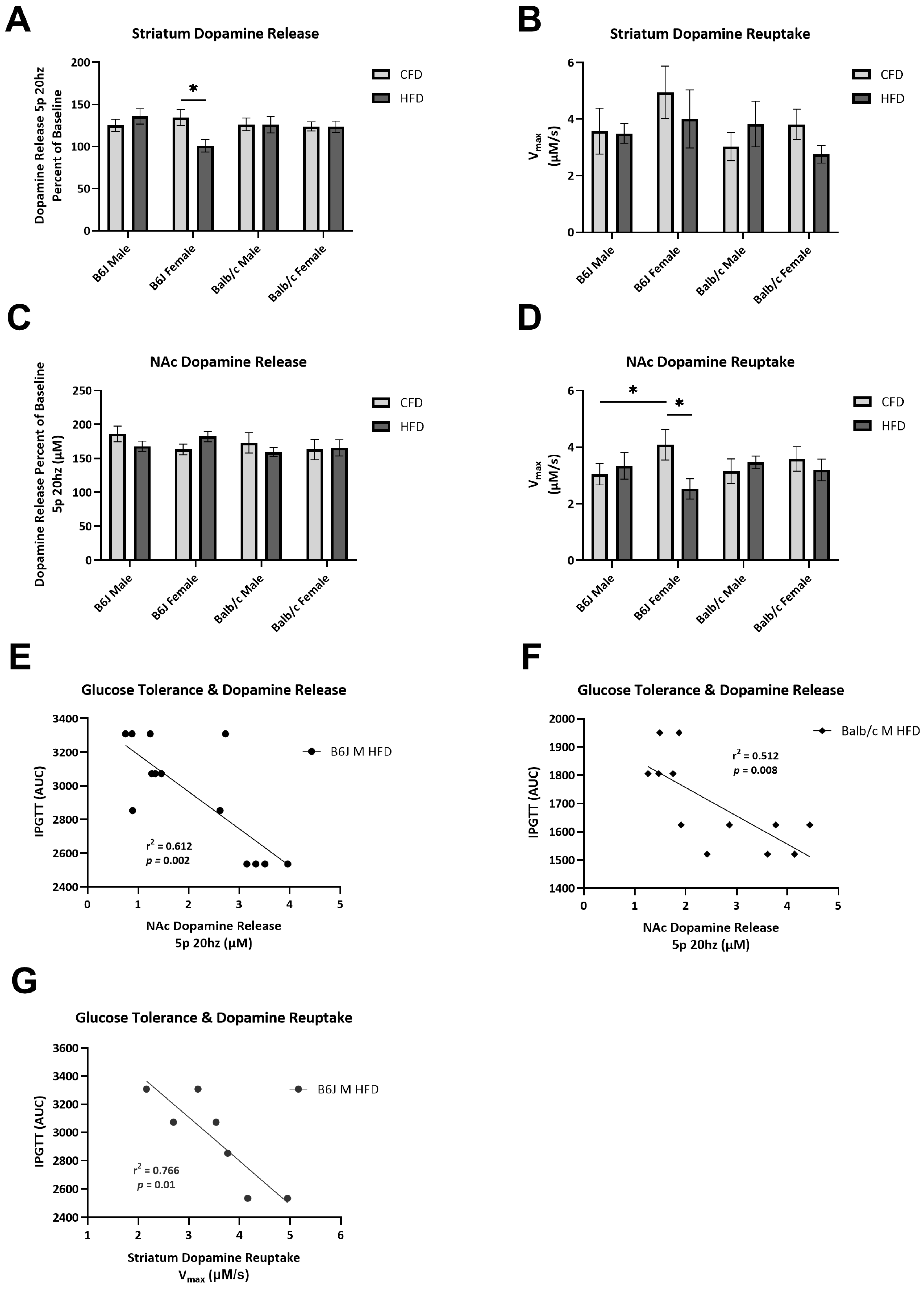
3.3. Impact of Strain, Sex, and Diet on Dopamine Release and Reuptake
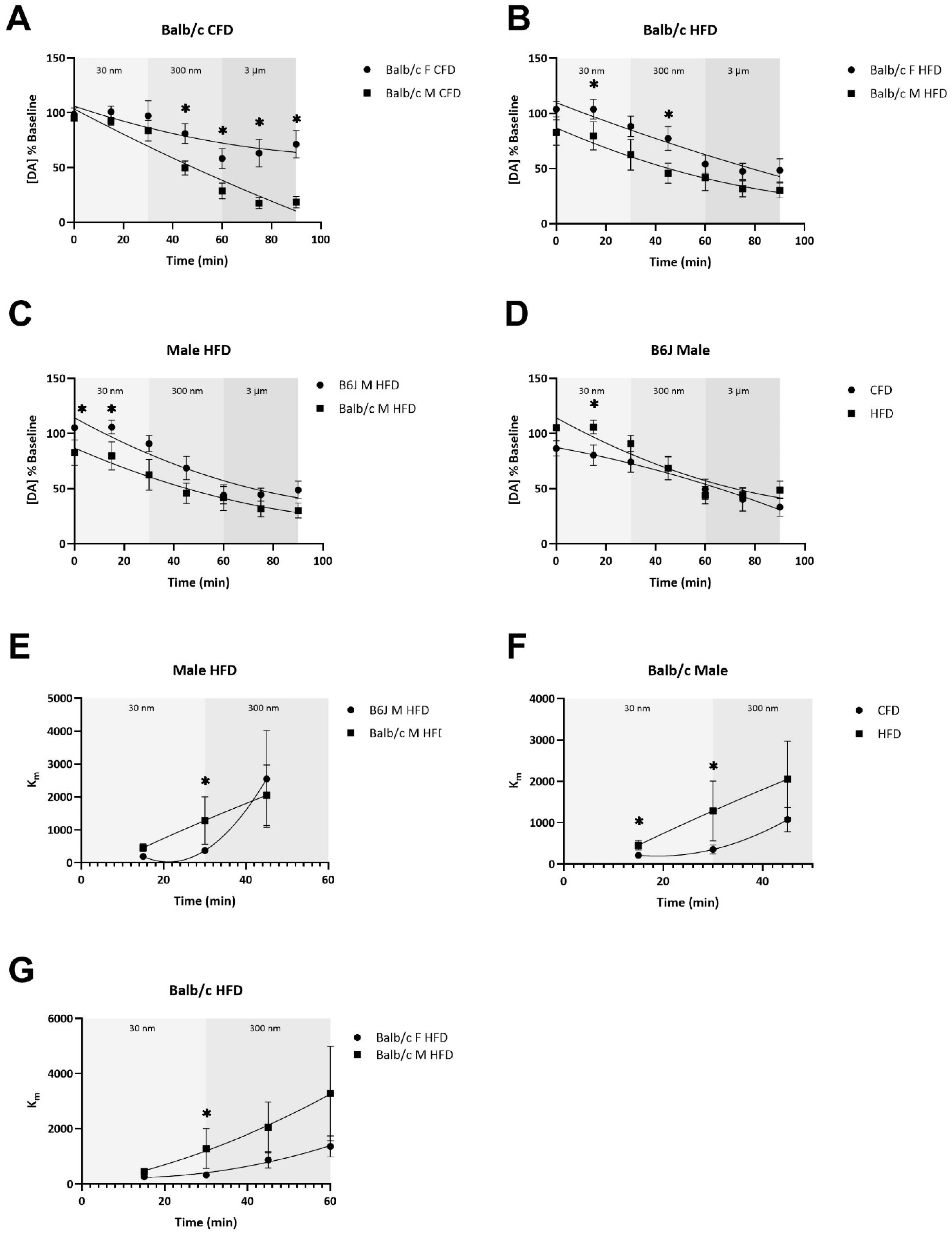
3.4. The Potency of AMPH-Induced Dopamine Uptake Inhibition and the Terminal Depletion of Dopamine Are Dependent upon Strain, Sex, and Diet
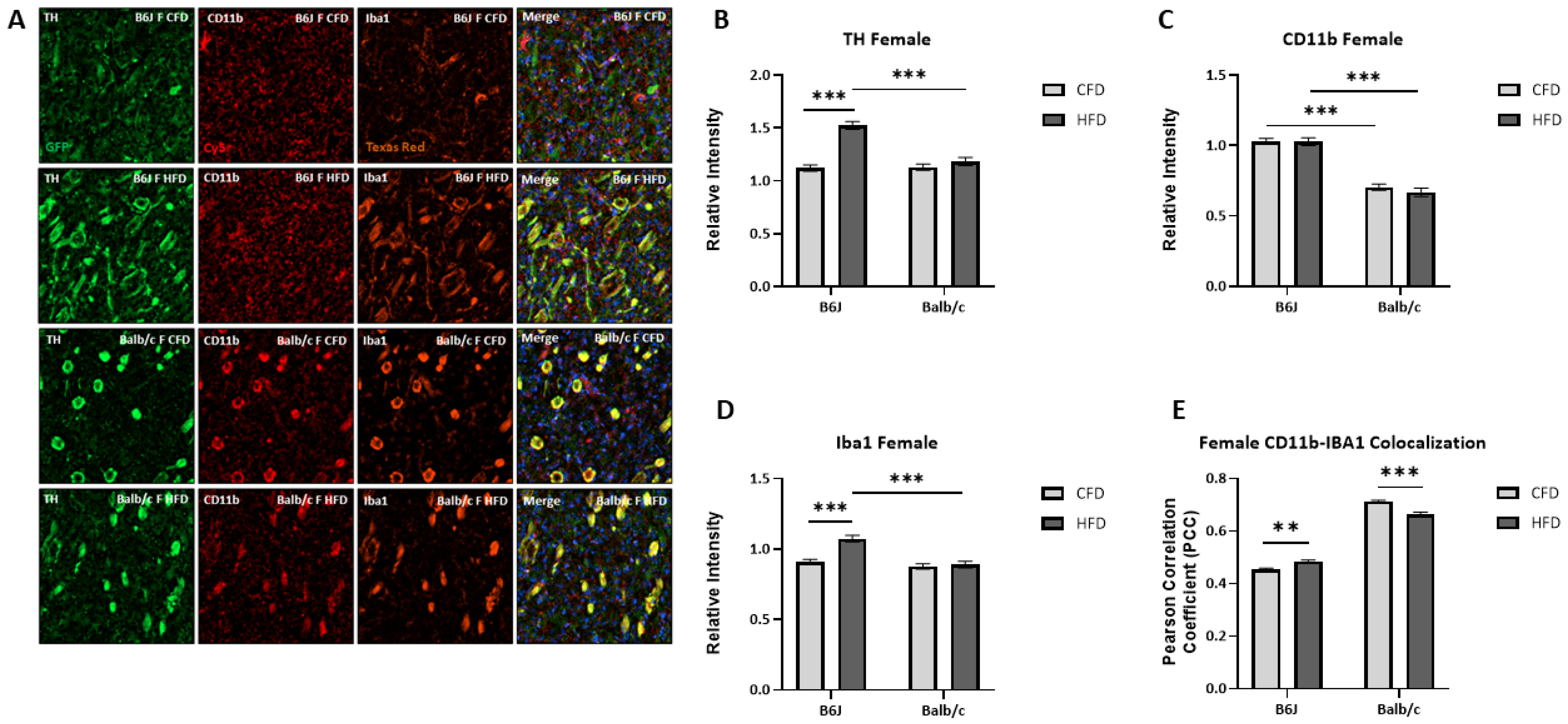
3.5. Influence of Strain and Sex on HFD-Induced Microglial Activation and TH Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fordahl, S.C.; Jones, S.R. High-Fat-Diet-Induced Deficits in Dopamine Terminal Function Are Reversed by Restoring Insulin Signaling. ACS Chem. Neurosci. 2017, 8, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Baufeld, C.; Osterloh, A.; Prokop, S.; Miller, K.R.; Heppner, F.L. High-Fat Diet-Induced Brain Region-Specific Phenotypic Spectrum of CNS Resident Microglia. Acta Neuropathol. 2016, 132, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Spoor, S.; Bohon, C.; Small, D.M. Relation between Obesity and Blunted Striatal Response to Food Is Moderated by TaqIA A1 Allele. Science 2008, 322, 449–452. [Google Scholar] [CrossRef]
- Carlin, J.; Hill-Smith, T.E.; Lucki, I.; Reyes, T.M. Reversal of Dopamine System Dysfunction in Response to High-Fat Diet. Obesity 2013, 21, 2513–2521. [Google Scholar] [CrossRef]
- Cone, J.J.; Chartoff, E.H.; Potter, D.N.; Ebner, S.R.; Roitman, M.F. Prolonged High Fat Diet Reduces Dopamine Reuptake without Altering DAT Gene Expression. PLoS ONE 2013, 8, e58251. [Google Scholar] [CrossRef]
- Estes, M.K.; Bland, J.J.; Ector, K.K.; Puppa, M.J.; Powell, D.W.; Lester, D.B. A High Fat Western Diet Attenuates Phasic Dopamine Release. Neurosci. Lett. 2021, 756, 135952. [Google Scholar] [CrossRef] [PubMed]
- South, T.; Huang, X.-F. High-Fat Diet Exposure Increases Dopamine D2 Receptor and Decreases Dopamine Transporter Receptor Binding Density in the Nucleus Accumbens and Caudate Putamen of Mice. Neurochem. Res. 2008, 33, 598–605. [Google Scholar] [CrossRef]
- Huang, X.-F.; Zavitsanou, K.; Huang, X.; Yu, Y.; Wang, H.; Chen, F.; Lawrence, A.J.; Deng, C. Dopamine Transporter and D2 Receptor Binding Densities in Mice Prone or Resistant to Chronic High Fat Diet-Induced Obesity. Behav. Brain Res. 2006, 175, 415–419. [Google Scholar] [CrossRef]
- Vucetic, Z.; Carlin, J.L.; Totoki, K.; Reyes, T.M. Epigenetic Dysregulation of the Dopamine System in Diet-Induced Obesity. J. Neurochem. 2012, 120, 891–898. [Google Scholar] [CrossRef]
- Baker, K.D.; Loughman, A.; Spencer, S.J.; Reichelt, A.C. The Impact of Obesity and Hypercaloric Diet Consumption on Anxiety and Emotional Behavior across the Lifespan. Neurosci. Biobehav. Rev. 2017, 83, 173–182. [Google Scholar] [CrossRef]
- Bridgewater, L.C.; Zhang, C.; Wu, Y.; Hu, W.; Zhang, Q.; Wang, J.; Li, S.; Zhao, L. Gender-Based Differences in Host Behavior and Gut Microbiota Composition in Response to High Fat Diet and Stress in a Mouse Model. Sci. Rep. 2017, 7, 10776. [Google Scholar] [CrossRef] [PubMed]
- Totten, M.S.; Wallace, C.W.; Pierce, D.M.; Fordahl, S.C.; Erikson, K.M. The Impact of a High-Fat Diet on Physical Activity and Dopamine Neurochemistry in the Striatum Is Sex and Strain Dependent in C57BL/6J and DBA/2J Mice. Nutr. Neurosci. 2022, 25, 2601–2615. [Google Scholar] [CrossRef] [PubMed]
- Stouffer, M.A.; Woods, C.A.; Patel, J.C.; Lee, C.R.; Witkovsky, P.; Bao, L.; Machold, R.P.; Jones, K.T.; de Vaca, S.C.; Reith, M.E.A.; et al. Insulin Enhances Striatal Dopamine Release by Activating Cholinergic Interneurons and Thereby Signals Reward. Nat. Commun. 2015, 6, 8543. [Google Scholar] [CrossRef]
- Kullmann, S.; Blum, D.; Jaghutriz, B.A.; Gassenmaier, C.; Bender, B.; Häring, H.-U.; Reischl, G.; Preissl, H.; la Fougère, C.; Fritsche, A.; et al. Central Insulin Modulates Dopamine Signaling in the Human Striatum. J. Clin. Endocrinol. Metab. 2021, 106, 2949–2961. [Google Scholar] [CrossRef]
- Patel, J.C.; Stouffer, M.A.; Mancini, M.; Nicholson, C.; Carr, K.D.; Rice, M.E. Interactions between Insulin and Diet on Striatal Dopamine Uptake Kinetics in Rodent Brain Slices. Eur. J. Neurosci. 2019, 49, 794–804. [Google Scholar] [CrossRef]
- Casimiro, I.; Stull, N.D.; Tersey, S.A.; Mirmira, R.G. Phenotypic Sexual Dimorphism in Response to Dietary Fat Manipulation in C57BL/6J Mice. J. Diabetes Complicat. 2021, 35, 107795. [Google Scholar] [CrossRef] [PubMed]
- Stöckli, J.; Fisher-Wellman, K.H.; Chaudhuri, R.; Zeng, X.-Y.; Fazakerley, D.J.; Meoli, C.C.; Thomas, K.C.; Hoffman, N.J.; Mangiafico, S.P.; Xirouchaki, C.E.; et al. Metabolomic Analysis of Insulin Resistance across Different Mouse Strains and Diets. J. Biol. Chem. 2017, 292, 19135–19145. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity Is Associated with Hypothalamic Injury in Rodents and Humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Valdearcos, M.; Robblee, M.M.; Benjamin, D.I.; Nomura, D.K.; Xu, A.W.; Koliwad, S.K. Microglia Dictate the Impact of Saturated Fat Consumption on Hypothalamic Inflammation and Neuronal Function. Cell Rep. 2014, 9, 2124–2138. [Google Scholar] [CrossRef]
- Carvey, P.M.; Chen, E.-Y.; Lipton, J.W.; Tong, C.W.; Chang, Q.A.; Ling, Z.D. Intra-Parenchymal Injection of Tumor Necrosis Factor-Alpha and Interleukin 1-Beta Produces Dopamine Neuron Loss in the Rat. J. Neural Transm. 2005, 112, 601–612. [Google Scholar] [CrossRef]
- Nolan, R.A.; Reeb, K.L.; Rong, Y.; Matt, S.M.; Johnson, H.S.; Runner, K.; Gaskill, P.J. Dopamine Activates NF-κB and Primes the NLRP3 Inflammasome in Primary Human Macrophages. Brain Behav. Immun.—Health 2019, 2, 100030. [Google Scholar] [CrossRef] [PubMed]
- Klawonn, A.M.; Fritz, M.; Castany, S.; Pignatelli, M.; Canal, C.; Similä, F.; Tejeda, H.A.; Levinsson, J.; Jaarola, M.; Jakobsson, J.; et al. Microglial Activation Elicits a Negative Affective State through Prostaglandin-Mediated Modulation of Striatal Neurons. Immunity 2021, 54, 225–234.e6. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell Metab. 2017, 26, 185–197.e3. [Google Scholar] [CrossRef]
- Freire-Regatillo, A.; Diaz-Pacheco, S.; Frago, L.M.; Arévalo, M.-Á.; Argente, J.; Garcia-Segura, L.M.; de Ceballos, M.L.; Chowen, J.A. Sex Differences in Hypothalamic Changes and the Metabolic Response of TgAPP Mice to a High Fat Diet. Front. Neuroanat. 2022, 16, 910477. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Hallahan, N.L.; Brown, S.H.; Liu, M.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Mouse Strain-Dependent Variation in Obesity and Glucose Homeostasis in Response to High-Fat Feeding. Diabetologia 2013, 56, 1129–1139. [Google Scholar] [CrossRef]
- Mukherjee, R.; Pandey, S.; Ghosh, A.; Aich, P. Effects of Starch-Rich or Fat-Rich Diets on Metabolism, Adiposity, and Glycemia in Immune-Biased, C57BL/6 and BALB/c Mice. J. Nutr. Biochem. 2022, 108, 109086. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.C.; Mridha, A.R.; Yeh, M.M.; Arsov, T.; Van Rooyen, D.M.; Brooling, J.; Nguyen, T.; Heydet, D.; Delghingaro-Augusto, V.; Nolan, C.J.; et al. Strain Dependence of Diet-Induced NASH and Liver Fibrosis in Obese Mice Is Linked to Diabetes and Inflammatory Phenotype. Liver Int. 2014, 34, 1084–1093. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C.J. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The Use of the Elevated plus Maze as an Assay of Anxiety-Related Behavior in Rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Pawlak, C.R.; Karrenbauer, B.D.; Schneider, P.; Ho, Y.-J. The Elevated Plus-Maze Test: Differential Psychopharmacology of Anxiety-Related Behavior. Emot. Rev. 2012, 4, 98–115. [Google Scholar] [CrossRef]
- Benedé-Ubieto, R.; Estévez-Vázquez, O.; Ramadori, P.; Cubero, F.J.; Nevzorova, Y.A. Guidelines and Considerations for Metabolic Tolerance Tests in Mice. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Ayala, J.E.; Samuel, V.T.; Morton, G.J.; Obici, S.; Croniger, C.M.; Shulman, G.I.; Wasserman, D.H.; McGuinness, O.P.; NIH Mouse Metabolic Phenotyping Center Consortium. Standard Operating Procedures for Describing and Performing Metabolic Tests of Glucose Homeostasis in Mice. Dis. Model. Mech. 2010, 3, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Yorgason, J.T.; España, R.A.; Jones, S.R. Demon Voltammetry and Analysis Software: Analysis of Cocaine-Induced Alterations in Dopamine Signaling Using Multiple Kinetic Measures. J. Neurosci. Methods 2011, 202, 158–164. [Google Scholar] [CrossRef]
- Wu, Q.; Reith, M.E.; Wightman, R.M.; Kawagoe, K.T.; Garris, P.A. Determination of Release and Uptake Parameters from Electrically Evoked Dopamine Dynamics Measured by Real-Time Voltammetry. J. Neurosci. Methods 2001, 112, 119–133. [Google Scholar] [CrossRef]
- Siciliano, C.A.; Calipari, E.S.; Jones, S.R. Amphetamine Potency Varies with Dopamine Uptake Rate across Striatal Subregions. J. Neurochem. 2014, 131, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Cordelières, F.P. A Guided Tour into Subcellular Colocalization Analysis in Light Microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K.; Bomhoff, G.L.; Gorres, B.K.; Davis, V.A.; Kim, J.; Lee, P.-P.; Brooks, W.M.; Gerhardt, G.A.; Geiger, P.C.; Stanford, J.A. Insulin Resistance Impairs Nigrostriatal Dopamine Function. Exp. Neurol. 2011, 231, 171–180. [Google Scholar] [CrossRef]
- Geiger, B.M.; Haburcak, M.; Avena, N.M.; Moyer, M.C.; Hoebel, B.G.; Pothos, E.N. Deficits of Mesolimbic Dopamine Neurotransmission in Rat Dietary Obesity. Neuroscience 2009, 159, 1193–1199. [Google Scholar] [CrossRef]
- Fritz, B.M.; Muñoz, B.; Yin, F.; Bauchle, C.; Atwood, B.K. A High-Fat, High-Sugar ‘Western’ Diet Alters Dorsal Striatal Glutamate, Opioid, and Dopamine Transmission in Mice. Neuroscience 2018, 372, 1–15. [Google Scholar] [CrossRef]
- Fordahl, S.C.; Locke, J.L.; Jones, S.R. High Fat Diet Augments Amphetamine Sensitization in Mice: Role of Feeding Pattern, Obesity, and Dopamine Terminal Changes. Neuropharmacology 2016, 109, 170–182. [Google Scholar] [CrossRef]
- Plaza-Briceño, W.; Velásquez, V.B.; Silva-Olivares, F.; Ceballo, K.; Céspedes, R.; Jorquera, G.; Cruz, G.; Martínez-Pinto, J.; Bonansco, C.; Sotomayor-Zárate, R. Chronic Exposure to High Fat Diet Affects the Synaptic Transmission That Regulates the Dopamine Release in the Nucleus Accumbens of Adolescent Male Rats. Int. J. Mol. Sci. 2023, 24, 4703. [Google Scholar] [CrossRef] [PubMed]
- Walker, Q.D.; Rooney, M.B.; Wightman, R.M.; Kuhn, C.M. Dopamine Release and Uptake Are Greater in Female than Male Rat Striatum as Measured by Fast Cyclic Voltammetry. Neuroscience 2000, 95, 1061–1070. [Google Scholar] [CrossRef]
- Walker, Q.D.; Ray, R.; Kuhn, C.M. Sex Differences in Neurochemical Effects of Dopaminergic Drugs in Rat Striatum. Neuropsychopharmacology 2006, 31, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Hasbi, A.; Nguyen, T.; Rahal, H.; Manduca, J.D.; Miksys, S.; Tyndale, R.F.; Madras, B.K.; Perreault, M.L.; George, S.R. Sex Difference in Dopamine D1-D2 Receptor Complex Expression and Signaling Affects Depression- and Anxiety-like Behaviors. Biol. Sex Differ. 2020, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L.; Rutstein, M.; Benzo, J.M.; Hostetter, J.C.; Teicher, M.H. Sex Differences in Dopamine Receptor Overproduction and Elimination. Neuroreport 1997, 8, 1495–1498. [Google Scholar] [CrossRef]
- Williams, O.O.F.; Coppolino, M.; George, S.R.; Perreault, M.L. Sex Differences in Dopamine Receptors and Relevance to Neuropsychiatric Disorders. Brain Sci. 2021, 11, 1199. [Google Scholar] [CrossRef]
- Yochum, C.L.; Medvecky, C.M.; Cheh, M.A.; Bhattacharya, P.; Wagner, G.C. Differential Development of Central Dopaminergic and Serotonergic Systems in BALB/c and C57BL/6J Mice. Brain Res. 2010, 1349, 97–104. [Google Scholar] [CrossRef]
- Fleckenstein, A.E.; Volz, T.J.; Riddle, E.L.; Gibb, J.W.; Hanson, G.R. New Insights into the Mechanism of Action of Amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 681–698. [Google Scholar] [CrossRef]
- Jones, S.R.; Gainetdinov, R.R.; Wightman, R.M.; Caron, M.G. Mechanisms of Amphetamine Action Revealed in Mice Lacking the Dopamine Transporter. J. Neurosci. 1998, 18, 1979–1986. [Google Scholar] [CrossRef]
- Richfield, E.K.; Penney, J.B.; Young, A.B. Anatomical and Affinity State Comparisons between Dopamine D1 and D2 Receptors in the Rat Central Nervous System. Neuroscience 1989, 30, 767–777. [Google Scholar] [CrossRef]
- Daberkow, D.P.; Brown, H.D.; Bunner, K.D.; Kraniotis, S.A.; Doellman, M.A.; Ragozzino, M.E.; Garris, P.A.; Roitman, M.F. Amphetamine Paradoxically Augments Exocytotic Dopamine Release and Phasic Dopamine Signals. J. Neurosci. 2013, 33, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Ferris, M.J.; Calipari, E.S.; Mateo, Y.; Melchior, J.R.; Roberts, D.C.; Jones, S.R. Cocaine Self-Administration Produces Pharmacodynamic Tolerance: Differential Effects on the Potency of Dopamine Transporter Blockers, Releasers, and Methylphenidate. Neuropsychopharmacology 2012, 37, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, C.A.; Calipari, E.S.; Ferris, M.J.; Jones, S.R. Adaptations of Presynaptic Dopamine Terminals Induced by Psychostimulant Self-Administration. ACS Chem. Neurosci. 2015, 6, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Calipari, E.S.; Ferris, M.J.; Salahpour, A.; Caron, M.G.; Jones, S.R. Methylphenidate Amplifies the Potency and Reinforcing Effects of Amphetamines by Increasing Dopamine Transporter Expression. Nat. Commun. 2013, 4, 2720. [Google Scholar] [CrossRef]
- Li, Y.; South, T.; Han, M.; Chen, J.; Wang, R.; Huang, X.-F. High-Fat Diet Decreases Tyrosine Hydroxylase mRNA Expression Irrespective of Obesity Susceptibility in Mice. Brain Res. 2009, 1268, 181–189. [Google Scholar] [CrossRef]
- Chou, M.-C.; Lee, H.-C.; Liu, Y.-C.; Yen, P.S.-Y.; Liu, C.-K.; Chen, C.-H.; Hsieh, T.-H.; Chen, S.-L. Long-Term High-Fat Diet Consumption Depletes Glial Cells and Tyrosine Hydroxylase-Containing Neurons in the Brain of Middle-Aged Rats. Cells 2022, 11, 295. [Google Scholar] [CrossRef]
- Han, J.; Fan, Y.; Zhou, K.; Blomgren, K.; Harris, R.A. Uncovering Sex Differences of Rodent Microglia. J. Neuroinflamm. 2021, 18, 74. [Google Scholar] [CrossRef]
- Komine, O.; Ohnuma, S.; Hinohara, K.; Hara, Y.; Shimada, M.; Akashi, T.; Watanabe, S.; Sobue, A.; Kawade, N.; Ogi, T.; et al. Genetic Background Variation Impacts Microglial Heterogeneity and Disease Progression in Amyotrophic Lateral Sclerosis Model Mice. iScience 2024, 27, 108872. [Google Scholar] [CrossRef]
- Kopec, A.M.; Smith, C.J.; Ayre, N.R.; Sweat, S.C.; Bilbo, S.D. Microglial Dopamine Receptor Elimination Defines Sex-Specific Nucleus Accumbens Development and Social Behavior in Adolescent Rats. Nat. Commun. 2018, 9, 3769. [Google Scholar] [CrossRef]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783.e6. [Google Scholar] [CrossRef]
- Lynch, M.A. Exploring Sex-Related Differences in Microglia May Be a Game-Changer in Precision Medicine. Front. Aging Neurosci. 2022, 14, 868448. [Google Scholar] [CrossRef] [PubMed]
- Pepping, J.K.; Freeman, L.R.; Gupta, S.; Keller, J.N.; Bruce-Keller, A.J. NOX2 Deficiency Attenuates Markers of Adiposopathy and Brain Injury Induced by High-Fat Diet. Am. J. Physiol.—Endocrinol. Metab. 2013, 304, E392–E404. [Google Scholar] [CrossRef] [PubMed]
- Buckman, L.B.; Hasty, A.H.; Flaherty, D.K.; Buckman, C.T.; Thompson, M.M.; Matlock, B.K.; Weller, K.; Ellacott, K.L.J. Obesity Induced by a High-Fat Diet Is Associated with Increased Immune Cell Entry into the Central Nervous System. Brain. Behav. Immun. 2014, 35, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Elabi, O.F.; Cunha, J.P.M.C.M.; Gaceb, A.; Fex, M.; Paul, G. High-Fat Diet-Induced Diabetes Leads to Vascular Alterations, Pericyte Reduction, and Perivascular Depletion of Microglia in a 6-OHDA Toxin Model of Parkinson Disease. J. Neuroinflamm. 2021, 18, 175. [Google Scholar] [CrossRef]
- Villa, A.; Vegeto, E.; Poletti, A.; Maggi, A. Estrogens, Neuroinflammation, and Neurodegeneration. Endocr. Rev. 2016, 37, 372–402. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, W.; Huang, M.; Lin, X.; Chiou, J. Prolonged High-Fat Diet Consumption throughout Adulthood in Mice Induced Neurobehavioral Deterioration via Gut-Brain Axis. Nutrients 2023, 15, 392. [Google Scholar] [CrossRef]
- Faith, M.S.; Butryn, M.; Wadden, T.A.; Fabricatore, A.; Nguyen, A.M.; Heymsfield, S.B. Evidence for Prospective Associations among Depression and Obesity in Population-Based Studies. Obes. Rev. 2011, 12, e438–e453. [Google Scholar] [CrossRef]
- Amiri, S.; Behnezhad, S. Obesity and Anxiety Symptoms: A Systematic Review and Meta-Analysis. Neuropsychiatr. Klin. Diagn. Ther. Rehabil. Organ Ges. Osterreichischer Nervenarzte Psychiater 2019, 33, 72–89. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Asai, M.; Hara, T. High-Fat Diet Enhances Working Memory in the Y-Maze Test in Male C57BL/6J Mice with Less Anxiety in the Elevated Plus Maze Test. Nutrients 2020, 12, 2036. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.; Zhou, Y.; Du, H.; Zhang, C.; Zhao, Z.; Chen, Y.; Zhou, Z.; Mei, J.; Wu, W.; et al. High Fat Diet-Induced Obesity Leads to Depressive and Anxiety-like Behaviors in Mice via AMPK/mTOR-Mediated Autophagy. Exp. Neurol. 2022, 348, 113949. [Google Scholar] [CrossRef]
- Bouwknecht, J.A.; Paylor, R. Behavioral and Physiological Mouse Assays for Anxiety: A Survey in Nine Mouse Strains. Behav. Brain Res. 2002, 136, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Ryu, S.; Suk, J.-G.; Park, C. Comparative Analysis of the Anxiety-Related Behaviors in Four Inbred Mice. Behav. Processes 2002, 60, 181–190. [Google Scholar] [CrossRef] [PubMed]
- An, X.-L.; Zou, J.-X.; Wu, R.-Y.; Yang, Y.; Tai, F.-D.; Zeng, S.-Y.; Jia, R.; Zhang, X.; Liu, E.-Q.; Broders, H. Strain and Sex Differences in Anxiety-Like and Social Behaviors in C57BL/6J and BALB/cJ Mice. Exp. Anim. 2011, 60, 111–123. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagarty-Waite, K.A.; Emmons, H.A.; Fordahl, S.C.; Erikson, K.M. The Influence of Strain and Sex on High Fat Diet-Associated Alterations of Dopamine Neurochemistry in Mice. Nutrients 2024, 16, 3301. https://doi.org/10.3390/nu16193301
Hagarty-Waite KA, Emmons HA, Fordahl SC, Erikson KM. The Influence of Strain and Sex on High Fat Diet-Associated Alterations of Dopamine Neurochemistry in Mice. Nutrients. 2024; 16(19):3301. https://doi.org/10.3390/nu16193301
Chicago/Turabian StyleHagarty-Waite, Kristen A., Heather A. Emmons, Steve C. Fordahl, and Keith M. Erikson. 2024. "The Influence of Strain and Sex on High Fat Diet-Associated Alterations of Dopamine Neurochemistry in Mice" Nutrients 16, no. 19: 3301. https://doi.org/10.3390/nu16193301




