Hypoallergenic Variant of the Major Egg White Allergen Gal d 1 Produced by Disruption of Cysteine Bridges
Abstract
:1. Introduction
2. Methods
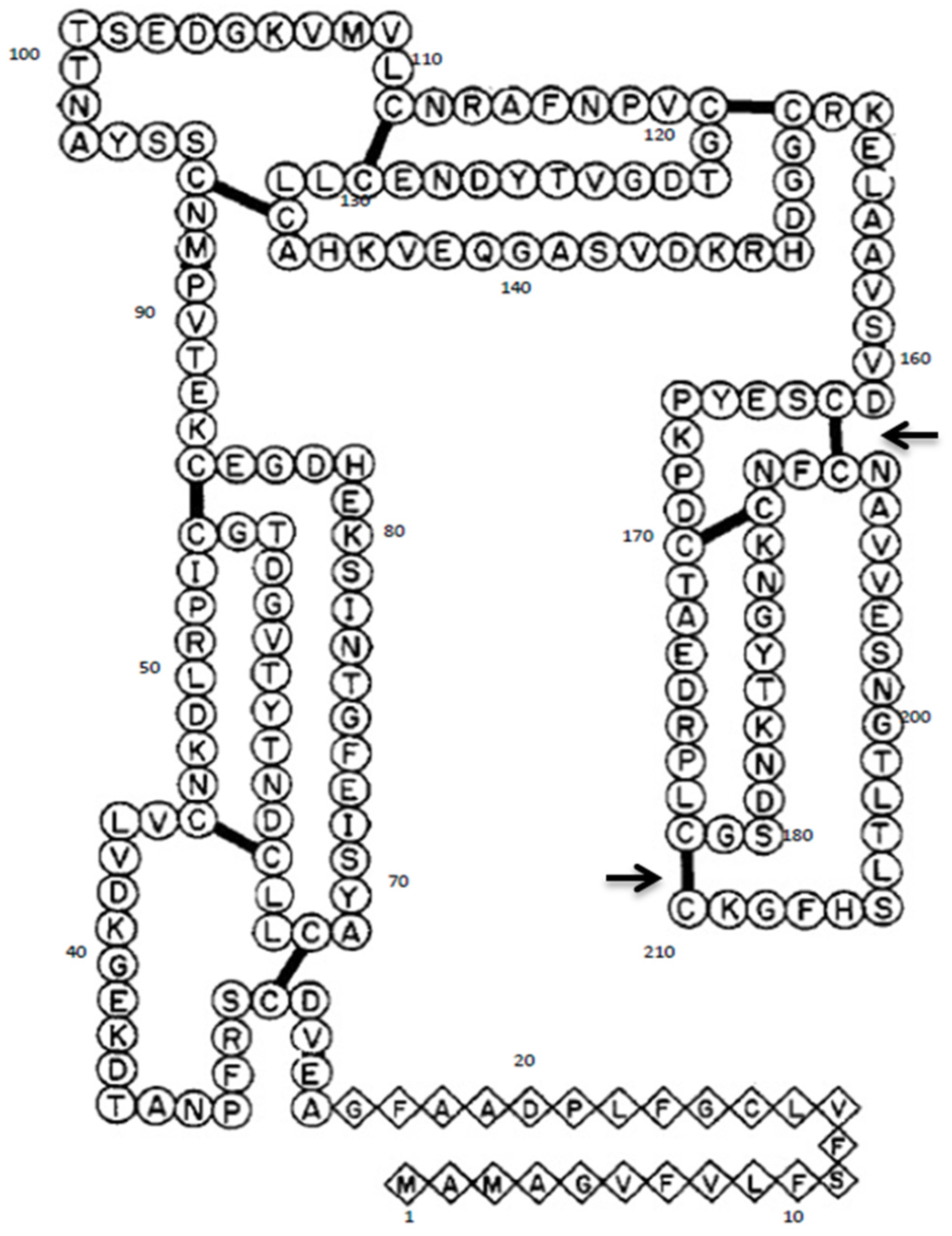
2.1. Site-Directed Mutagenesis of Gal d 1
2.2. Chemical Transformation into E. coli
2.3. Time-Course Expression of Mutant Gal d 1 to Determine Optimum Expression Time
2.4. Expression and Immunoblotting of Wild-Type and Mutant Gal d 1 Using Three Different Detection Antibodies
2.5. Immunological Analysis of Wild-Type vs. Mutant Gal d 1 Using Western Blot
3. Results
3.1. Mutagenesis of Gal d 1
3.2. Time-Course Expression of Mutant Gal d 1 to Determine Optimum Expression Time
3.3. Expression and Immunoblotting of Wild-Type and Mutant Gal d 1 Using Three Different Detection Antibodies
3.4. Immunological Analysis of Wild-Type vs. Mutant Gal d 1 Using Western Blot
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IgE | immunoglobulin E |
| OIT | oral immunotherapy |
| SPT | skin prick tests |
References
- Kato, I.; Schrode, J.; Kohr, W.J.; Laskowski, M. Chicken ovomucoid: Determination of its amino acid sequence, determination of the trypsin reactive site, and preparation of all three of its domains. Biochemistry 1987, 26, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Zhang, J. Identification and Fine Mapping of IgG and IgE Epitopes in Ovomucoid. Biochem. Biophys. Res. Commun. 2002, 292, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Nolan, J.; Zhang, J.W.; Hayakawa, S.; Mine, Y. Immunochemical and Structural Analysis of Pepsin-Digested Egg White Ovomucoid. J. Agric. Food Chem. 2000, 48, 6261–6266. [Google Scholar] [CrossRef] [PubMed]
- Des Roches, A.; Nguyen, M.; Paradis, L.; Primeau, M.N.; Singer, S. Tolerance to cooked egg in an egg allergic population. Allergy 2006, 61, 900–901. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.H.; Matsui, E.C.; Skripak, J.M.; Wood, R.A. The natural history of egg allergy. J. Allergy Clin. Immunol. 2007, 120, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, L.L.; Lutter, C.K.; Bunn, D.A.; Stewart, C.P. Eggs: The uncracked potential for improving maternal and young child nutrition among the world’s poor. Nutr. Rev. 2014, 72, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Kiosseoglou, V.; Paraskevopoulou, A. Eggs. Bakery Products Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 243–258. [Google Scholar]
- Karabus, S.; Gray, C.L.; Goddard, E.; Kriel, M.; Lang, A.C.; Manjra, A.I.; Risenga, S.M.; Terblanche, A.J.; van der Spuy, D.A.; Levin, M.E. Vaccination in food allergic patients: Continuing medical education. S. Afr. Med. J. 2015, 105, 73. [Google Scholar] [CrossRef]
- Nurmatov, U.; Devereux, G.; Worth, A.; Healy, L.; Sheikh, A. Effectiveness and safety of orally administered immunotherapy for food allergies: A systematic review and meta-analysis. Br. J. Nutr. 2014, 111, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Burks, A.W.; Dupont, C. State of the art on food allergen immunotherapy: Oral, sublingual, and epicutaneous. J. Allergy Clin. Immunol. 2014, 133, 318–323. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.; Hogan, A. Baseline Specific IgE levels Are Useful to Predict Safety of Oral Immunotherapy in Egg-Allergic Children. Pediatrics 2014, 134 (Suppl. S3), S150–S151. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A. Anaphylaxis and Food Allergy. In Food Allergy; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013; pp. 178–191. [Google Scholar]
- Vazquez-Ortiz, M.; Alvaro-Lozano, M.; Alsina, L.; Garcia-Paba, M.B.; Piquer-Gibert, M.; Giner-Munoz, M.T.; Lozano, J.; Domínguez-Sánchez, O.; Jiménez, R.; Días, M.; et al. Safety and predictors of adverse events during oral immunotherapy for milk allergy: Severity of reaction at oral challenge, specific IgE and prick test. Clin. Exp. Allergy 2013, 43, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.; Steele, P.H.; Vickery, B.P.; Bird, J.A.; Thyagarajan, A.; Scurlock, A.M.; Perry, T.T.; Jones, S.M.; Wesley Burks, A. Adverse Reactions During Peanut Oral Immunotherapy Home Dosing. J. Allergy Clin. Immunol. 2009, 124, 1351–1352. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.Y.Y.; Leung, N.Y.H.; Ho, M.H.K.; Gershwin, L.J.; Shu, S.A.; Leung, P.S.C.; Chu, K.H. Immunization with Hypoallergens of Shrimp Allergen Tropomyosin Inhibits Shrimp Tropomyosin Specific IgE Reactivity. PLoS ONE 2014, 9, e111649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curin, M.; Weber, M.; Thalhamer, T.; Swoboda, I.; Focke-Tejkl, M.; Blatt, K.; Valent, P.; Marth, K.; Garmatiuk, T.; Grönlund, H.; et al. Hypoallergenic derivatives of Fel d 1 obtained by rational reassembly for allergy vaccination and tolerance induction. Clin. Exp. Allergy 2014, 44, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Focke-Tejkl, M.; Weber, M.; Niespodziana, K.; Neubauer, A.; Huber, H.; Henning, R.; Stegfellner, G.; Maderegger, B.; Hauer, M.; Stolz, F.; et al. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J. Allergy Clin. Immunol. 2015, 135, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Drew, A.C.; Eusebius, N.P.; Kenins, L.; de Silva, H.D.; Suphioglu, C.; Rolland, J.M.; O’hehir, R.E. Hypoallergenic Variants of the Major Latex Allergen Hev b 6.01 Retaining Human T Lymphocyte Reactivity. J. Immunol. 2004, 173, 5872–5879. [Google Scholar] [CrossRef] [PubMed]
- Dhanapala, P.; Doran, T.; Tang, M.L.K.; Suphioglu, C. Production and immunological analysis of IgE reactive recombinant egg white allergens expressed in Escherichia coli. Mol. Immunol. 2015, 65, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Fiocchi, A. Is oral immunotherapy the cure for food allergies? Curr. Opin. Allergy Clin. Immunol. 2010, 10, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Linhart, B.; Swoboda, I.; Niederberger, V. Recombinant allergens for allergen-specific immunotherapy: 10 years anniversary of immunotherapy with recombinant allergens. Allergy 2011, 66, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, V.; Horak, F.; Vrtala, S.; Spitzauer, S.; Krauth, M.-T.; Valent, P.; Reisinger, J.; Pelzmann, M.; Hayek, B.; Kronqvist, M.; et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc. Natl. Acad. Sci. USA 2004, 101 (Suppl. S2), 14677–14682. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.; Wells, J. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science 1989, 244, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Mine, Y. Characterization of IgE and IgG Epitopes on Ovomucoid Using Egg-White-Allergic Patients’ Sera. Biochem. Biophys. Res. Commun. 1998, 253, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Rolland, J.; O’Hehir, R. Immunotherapy of allergy: Anergy, deletion, and immune deviation. Curr. Opin. Immunol. 1998, 10, 640–645. [Google Scholar] [CrossRef]
- Palomares, O. The role of regulatory T cells in IgE-mediated food allergy. J. Investig. Allergol. Clin. Immunol. 2013, 23, 371–382. [Google Scholar] [PubMed]
- Van Gramberg, J.L.; de Veer, M.J.; O’Hehir, R.E.; Meeusen, E.N.T.; Bischof, R.J. Use of Animal Models to Investigate Major Allergens Associated with Food Allergy. J. Allergy 2013. [Google Scholar] [CrossRef] [PubMed]




| Reaction Component | Volume Used (µL) |
|---|---|
| 10× QuickChange Lightning Multi reaction buffer | 2.5 |
| Double-distilled water | 15.5 |
| Template DNA | 1 (50 ng) |
| Mutagenic primers | 1 of each primer (100 ng of each primer) |
| Deoxy-nucleoside triphosphate (dNTP) mix | 1 |
| QuickChange Lightning Multi enzyme blend | 1 |
| Total | 25 |
| Segment | Cycles | Temperature | Time |
|---|---|---|---|
| 1 | 1 | 95 °C | 2 min |
| 2 | 30 | 95 °C | 20 s |
| 55 °C | 30 s | ||
| 65 °C | 3 min (30 s/kb of plasmid length) | ||
| 3 | 1 | 65 °C | 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhanapala, P.; Withanage-Dona, D.; Tang, M.L.K.; Doran, T.; Suphioglu, C. Hypoallergenic Variant of the Major Egg White Allergen Gal d 1 Produced by Disruption of Cysteine Bridges. Nutrients 2017, 9, 171. https://doi.org/10.3390/nu9020171
Dhanapala P, Withanage-Dona D, Tang MLK, Doran T, Suphioglu C. Hypoallergenic Variant of the Major Egg White Allergen Gal d 1 Produced by Disruption of Cysteine Bridges. Nutrients. 2017; 9(2):171. https://doi.org/10.3390/nu9020171
Chicago/Turabian StyleDhanapala, Pathum, Dulashi Withanage-Dona, Mimi L. K. Tang, Tim Doran, and Cenk Suphioglu. 2017. "Hypoallergenic Variant of the Major Egg White Allergen Gal d 1 Produced by Disruption of Cysteine Bridges" Nutrients 9, no. 2: 171. https://doi.org/10.3390/nu9020171
APA StyleDhanapala, P., Withanage-Dona, D., Tang, M. L. K., Doran, T., & Suphioglu, C. (2017). Hypoallergenic Variant of the Major Egg White Allergen Gal d 1 Produced by Disruption of Cysteine Bridges. Nutrients, 9(2), 171. https://doi.org/10.3390/nu9020171






