Sapovaccarin-S1 and -S2, Two Type I RIP Isoforms from the Seeds of Saponaria vaccaria L.
Abstract
:1. Introduction
2. Results
2.1. Protein Extraction from the Seeds of Saponaria vaccaria L.
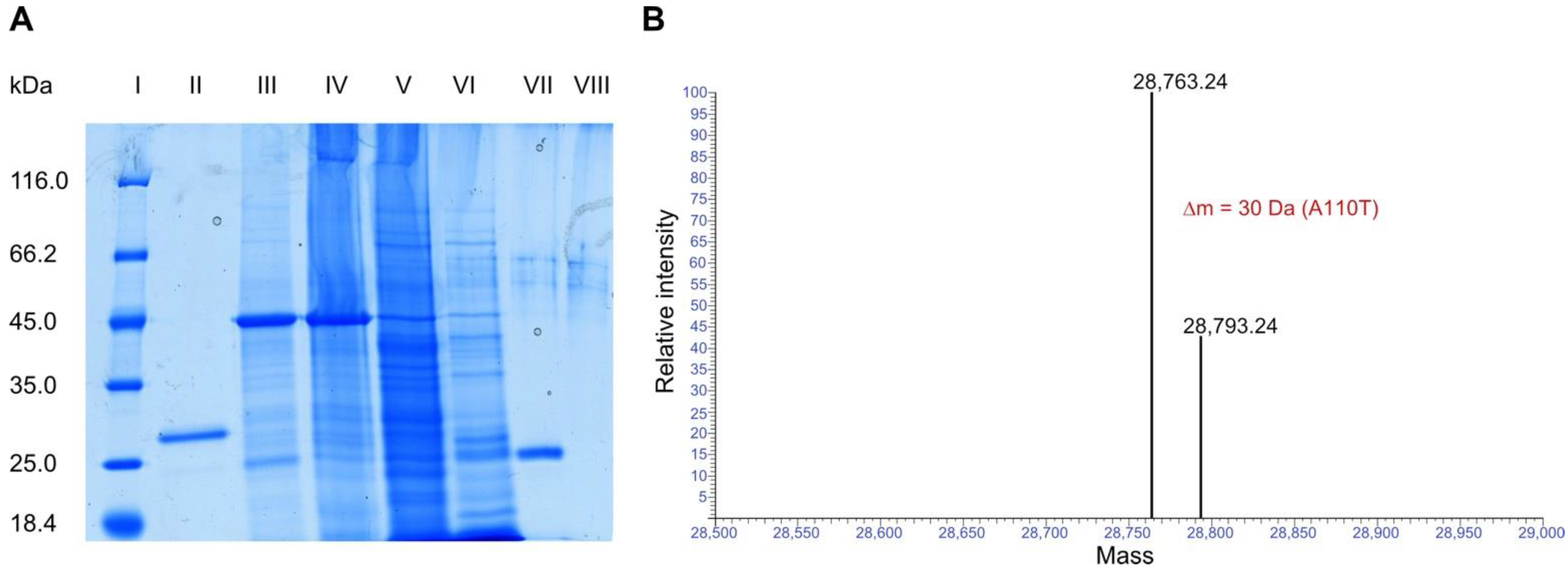
2.2. Protein Sequencing of Sapovaccarin-S1 and -S2
2.3. N-Glycosylase Activity
2.4. Cytotoxicity of Sapovaccarin-S1 and -S2
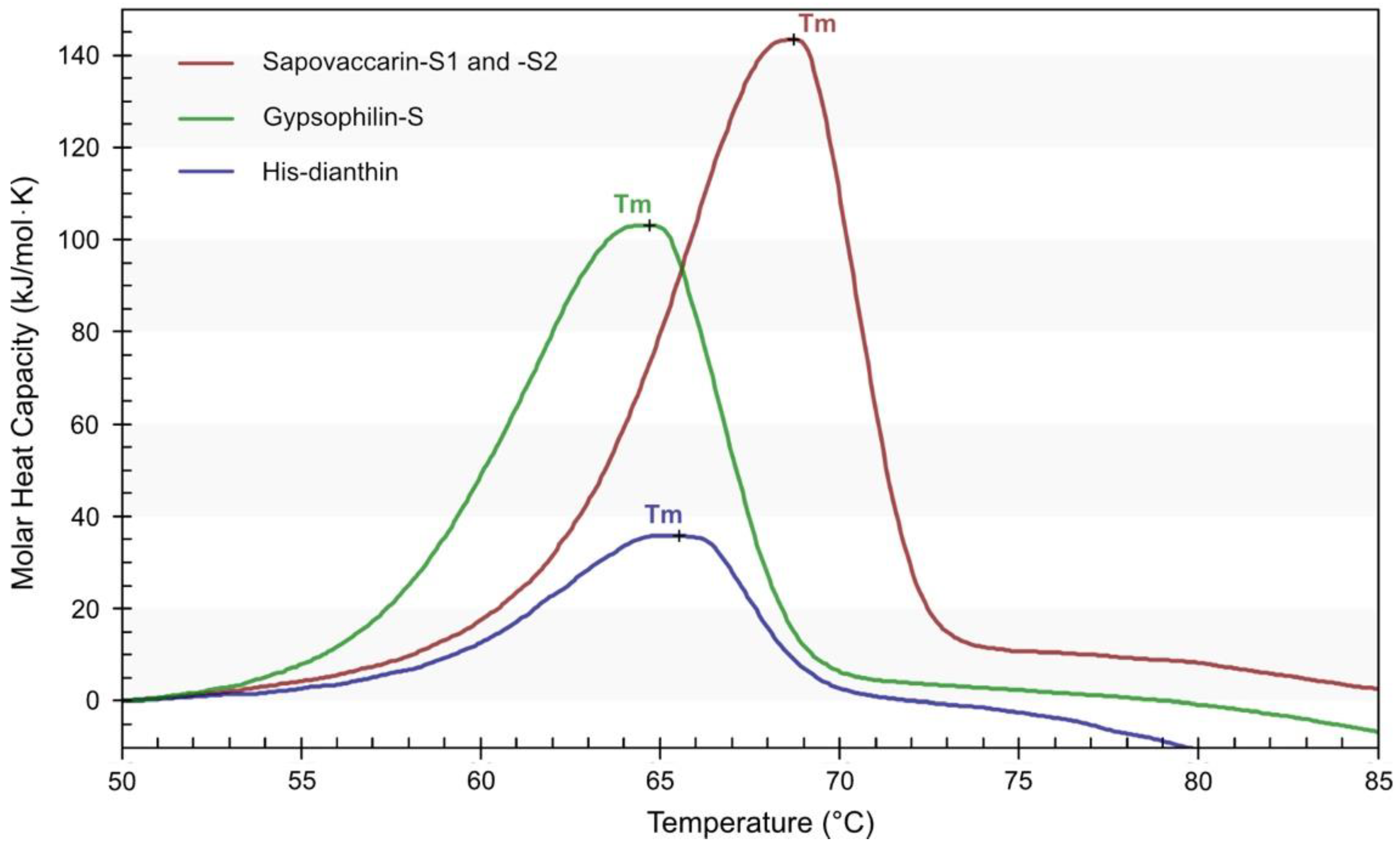
2.5. Thermal Stability
2.6. Distribution of Sapovaccarin-S1 and -S2 in Differently Processed Seed Material from Saponaria vaccaria L.
3. Discussion
4. Materials and Methods
4.1. Seed Material
4.2. Isolation of Sapovaccarin-S1 and -S2
4.3. Recombinant Expression of His-Dianthin and Isolation of Gypsophilin-S
4.4. SDS-PAGE and Protein Quantification
4.5. Protein Mass Spectrometry
4.6. DNA Extraction from the Seeds and Determination of the DNA Sequence by PCR
4.7. Homology Modeling
4.8. Adenine-Releasing Assay
4.9. Cytotoxicity
4.10. Differential Scanning Calorimetry
4.11. Accession Numbers
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, G.; Tang, L.; Wang, T.; Zhou, X.; Kou, Z.; Wang, Z.; Wu, J. Phytochemistry and pharmacological activities of Vaccaria hispanica (Miller) Rauschert: A review. Phytochem. Rev. 2016, 15, 813–827. [Google Scholar] [CrossRef]
- Yun, Y.S.; Morita, H.; Takeya, K.; Itokawa, H. Cyclic peptides from higher plants. 34. Segetalins G and H, structures and estrogen-like activity of cyclic pentapeptides from Vaccaria segetalis. J. Nat. Prod. 1997, 60, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.S.; Shimizu, K.; Morita, H.; Takeya, K.; Itokawa, H.; Shirota, O. Triterpenoid saponin from Vaccaria segetalis. Phytochemistry 1998, 47, 143–144. [Google Scholar] [CrossRef]
- Tian, M.; Huang, Y.; Wang, X.; Cao, M.; Zhao, Z.; Chen, T.; Yuan, C.; Wang, N.; Zhang, B.; Li, C.; et al. Vaccaria segetalis: A Review of Ethnomedicinal, Phytochemical, Pharmacological, and Toxicological Findings. Front. Chem. 2021, 9, 666280. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Olivieri, F.; Battelli, M.G.; Barbieri, L.; Falasca, A.I.; Parente, A.; Del Vecchio Blanco, F.; Stirpe, F. Ribosome-inactivating proteins (RNA N-glycosidases) from the seeds of Saponaria ocymoides and Vaccaria pyramidata. Eur. J. Biochem. 1995, 228, 935–940. [Google Scholar] [CrossRef]
- Schrot, J.; Weng, A.; Melzig, M.F. Ribosome-inactivating and related proteins. Toxins 2015, 7, 1556–1615. [Google Scholar] [CrossRef]
- Endo, Y.; Tsurugi, K. Mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. Nucleic Acids Symp. Ser. 1986, 17, 187–190. [Google Scholar]
- Brigotti, M.; Rambelli, F.; Zamboni, M.; Montanaro, L.; Sperti, S. Effect of alpha-sarcin and ribosome-inactivating proteins on the interaction of elongation factors with ribosomes. Biochem. J. 1989, 257, 723–727. [Google Scholar] [CrossRef]
- Flexner, S. The Histological Changes Produced by Ricin and Abrin Intoxications. J. Exp. Med. 1897, 2, 197–216. [Google Scholar] [CrossRef] [Green Version]
- Olsnes, S.; Pihl, A. Different biological properties of the two constituent peptide chains of ricin, a toxic protein inhibiting protein synthesis. Biochemistry 1973, 12, 3121–3126. [Google Scholar] [CrossRef]
- Timar, J.; McIntosh, D.P.; Henry, R.; Cumber, A.J.; Parnell, G.D.; Davies, A.J. The effect of ricin B chain on the intracellular trafficking of an A chain immunotoxin. Br. J. Cancer 1991, 64, 655–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandvig, K.; Grimmer, S.; Lauvrak, S.U.; Torgersen, M.L.; Skretting, G.; van Deurs, B.; Iversen, T.G. Pathways followed by ricin and Shiga toxin into cells. Histochem. Cell Biol. 2002, 117, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Battelli, M.G.; Stirpe, F. Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta 1993, 1154, 237–282. [Google Scholar] [CrossRef]
- Bolognesi, A.; Polito, L.; Scicchitano, V.; Orrico, C.; Pasquinelli, G.; Musiani, S.; Santi, S.; Riccio, M.; Bortolotti, M.; Battelli, M.G. Endocytosis and intracellular localisation of type 1 ribosome-inactivating protein saporin-s6. J. Biol. Regul. Homeost. Agents 2012, 26, 97–109. [Google Scholar]
- Stirpe, F.; Williams, D.G.; Onyon, L.J.; Legg, R.F.; Stevens, W.A. Dianthins, ribosome-damaging proteins with anti-viral properties from Dianthus caryophyllus L. (carnation). Biochem. J. 1981, 195, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Stirpe, F.; Gasperi-Campani, A.; Barbieri, L.; Falasca, A.; Abbondanza, A.; Stevens, W.A. Ribosome-inactivating proteins from the seeds of Saponaria officinalis L. (soapwort), of Agrostemma githago L. (corn cockle) and of Asparagus officinalis L. (asparagus), and from the latex of Hura crepitans L. (sandbox tree). Biochem. J. 1983, 216, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Ferreras, J.M.; Citores, L.; Iglesias, R.; Jiménez, P.; Girbés, T.; Lord, J.M.; Hartley, M.R. Sambucus ribosome-inactivating proteins and lectins. In Toxic Plant Proteins; Springer: Berline, Germany, 2010; Volume 18, pp. 107–131. [Google Scholar]
- Girbes, T.; Ferreras, J.M.; Arias, F.J.; Stirpe, F. Description, distribution, activity and phylogenetic relationship of ribosome-inactivating proteins in plants, fungi and bacteria. Mini Rev. Med. Chem. 2004, 4, 461–476. [Google Scholar] [CrossRef]
- De Zaeytijd, J.; Chen, P.; Scheys, F.; Subramanyam, K.; Dubiel, M.; De Schutter, K.; Smagghe, G.; Van Damme, E.J. Involvement of OsRIP1, a ribosome-inactivating protein from rice, in plant defense against Nilaparvata lugens. Phytochemistry 2020, 170, 112190. [Google Scholar] [CrossRef]
- Stirpe, F.; Barbieri, L.; Gorini, P.; Valbonesi, P.; Bolognesi, A.; Polito, L. Activities associated with the presence of ribosome-inactivating proteins increase in senescent and stressed leaves. FEBS Lett. 1996, 382, 309–312. [Google Scholar] [CrossRef] [Green Version]
- Gilabert-Oriol, R.; Weng, A.; Mallinckrodt, B.; Melzig, M.F.; Fuchs, H.; Thakur, M. Immunotoxins constructed with ribosome-inactivating proteins and their enhancers: A lethal cocktail with tumor specific efficacy. Curr. Pharm. Des. 2014, 20, 6584–6643. [Google Scholar] [CrossRef] [Green Version]
- Citores, L.; Iglesias, R.; Ferreras, J.M. Antiviral Activity of Ribosome-Inactivating Proteins. Toxins 2021, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; McDonald, K.A.; Dandekar, A.M.; Jackman, A.P.; Falk, B. Expression of recombinant trichosanthin, a ribosome-inactivating protein, in transgenic tobacco. J. Biotechnol. 2002, 97, 69–88. [Google Scholar] [CrossRef]
- Iglesias, R.; Perez, Y.; de Torre, C.; Ferreras, J.M.; Antolin, P.; Jimenez, P.; Rojo, M.A.; Mendez, E.; Girbes, T. Molecular characterization and systemic induction of single-chain ribosome-inactivating proteins (RIPs) in sugar beet (Beta vulgaris) leaves. J. Exp. Bot. 2005, 56, 1675–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertholdo-Vargas, L.R.; Martins, J.N.; Bordin, D.; Salvador, M.; Schafer, A.E.; Barros, N.M.; Barbieri, L.; Stirpe, F.; Carlini, C.R. Type 1 ribosome-inactivating proteins-entomotoxic, oxidative and genotoxic action on Anticarsia gemmatalis (Hubner) and Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). J. Insect Physiol. 2009, 55, 51–58. [Google Scholar] [CrossRef]
- Di Massimo, A.M.; Di Loreto, M.; Pacilli, A.; Raucci, G.; D’Alatri, L.; Mele, A.; Bolognesi, A.; Polito, L.; Stirpe, F.; De Santis, R. Immunoconjugates made of an anti-EGF receptor monoclonal antibody and type 1 ribosome-inactivating proteins from Saponaria ocymoides or Vaccaria pyramidata. Br. J. Cancer 1997, 75, 822–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, W.W.; Mak, A.N.; Wong, K.B.; Shaw, P.C. Structures and Ribosomal Interaction of Ribosome-Inactivating Proteins. Molecules 2016, 21, 1588. [Google Scholar] [CrossRef]
- Weng, A. A novel adenine-releasing assay for ribosome-inactivating proteins. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1072, 300–304. [Google Scholar] [CrossRef]
- Kokorin, A.; Weise, C.; Sama, S.; Weng, A. A new type 1 ribosome-inactivating protein from the seeds of Gypsophila elegans M.Bieb. Phytochemistry 2019, 157, 121–127. [Google Scholar] [CrossRef]
- Legname, G.; Bellosta, P.; Gromo, G.; Modena, D.; Keen, J.N.; Roberts, L.M.; Lord, J.M. Nucleotide sequence of cDNA coding for dianthin 30, a ribosome inactivating protein from Dianthus caryophyllus. Biochim. Biophys. Acta 1991, 1090, 119–122. [Google Scholar] [CrossRef]
- Maras, B.; Ippoliti, R.; De Luca, E.; Lendaro, E.; Bellelli, A.; Barra, D.; Bossa, F.; Brunori, M. The amino acid sequence of a ribosome-inactivating protein from Saponaria officinalis seeds. Biochem. Int. 1990, 21, 831–838. [Google Scholar]
- Lee-Huang, S.; Kung, H.F.; Huang, P.L.; Huang, P.L.; Li, B.Q.; Huang, P.; Huang, H.I.; Chen, H.C. A new class of anti-HIV agents: GAP31, DAPs 30 and 32. FEBS Lett. 1991, 291, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Fermani, S.; Falini, G.; Ripamonti, A.; Polito, L.; Stirpe, F.; Bolognesi, A. The 1.4 anstroms structure of dianthin 30 indicates a role of surface potential at the active site of type 1 ribosome inactivating proteins. J. Struct. Biol. 2005, 149, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Savino, C.; Federici, L.; Ippoliti, R.; Lendaro, E.; Tsernoglou, D. The crystal structure of saporin SO6 from Saponaria officinalis and its interaction with the ribosome. FEBS Lett. 2000, 470, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Moolten, F.; Zajdel, S.; Cooperband, S. Immunotherapy of experimental animal tumors with antitumor antibodies conjugated to diphtheria toxin or ricin. Ann. N. Y. Acad. Sci. 1976, 277, 690–699. [Google Scholar] [CrossRef]
- Barbieri, L.; Valbonesi, P.; Bonora, E.; Gorini, P.; Bolognesi, A.; Stirpe, F. Polynucleotide:adenosine glycosidase activity of ribosome-inactivating proteins: Effect on DNA, RNA and poly(A). Nucleic Acids Res. 1997, 25, 518–522. [Google Scholar] [CrossRef]
- Barbieri, L.; Valbonesi, P.; Righi, F.; Zuccheri, G.; Monti, F.; Gorini, P.; Samori, B.; Stirpe, F. Polynucleotide:Adenosine glycosidase is the sole activity of ribosome-inactivating proteins on DNA. J. Biochem. 2000, 128, 883–889. [Google Scholar] [CrossRef]
- Ferreras, J.M.; Barbieri, L.; Girbes, T.; Battelli, M.G.; Rojo, M.A.; Arias, F.J.; Rocher, M.A.; Soriano, F.; Mendez, E.; Stirpe, F. Distribution and properties of major ribosome-inactivating proteins (28 S rRNA N-glycosidases) of the plant Saponaria officinalis L. (Caryophyllaceae). Biochim. Biophys. Acta 1993, 1216, 31–42. [Google Scholar] [CrossRef]
- Bolognesi, A.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Polito, L. Ribosome-Inactivating Proteins from Plants: A Historical Overview. Molecules 2016, 21, 1627. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, M.; Scire, A.; Tanfani, F.; Ausili, A. The thermal unfolding of the ribosome-inactivating protein saporin-S6 characterized by infrared spectroscopy. Biochim. Biophys. Acta 2015, 1854, 1357–1364. [Google Scholar] [CrossRef]
- Scire, A.; Tanfani, F.; Ausili, A. A Spectroscopic Study on Secondary Structure and Thermal Unfolding of the Plant Toxin Gelonin Confirms Some Typical Structural Characteristics and Unravels the Sequence of Thermal Unfolding Events. Toxins 2019, 11, 483. [Google Scholar] [CrossRef] [Green Version]
- Gilabert-Oriol, R.; Weng, A.; Trautner, A.; Weise, C.; Schmid, D.; Bhargava, C.; Niesler, N.; Wookey, P.J.; Fuchs, H.; Thakur, M. Combinatorial approach to increase efficacy of Cetuximab, Panitumumab and Trastuzumab by dianthin conjugation and co-application of SO1861. Biochem. Pharm. 2015, 97, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Neuhoff, V.A.; Arold, N.; Taube, D.; Erhardt, W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brillant Blue G-250 and R-250. Electrophoresis 1988, 9, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef]
- Suckau, D.; Resemann, A.; Schuerenberg, M.; Hufnagel, P.; Franzen, J.; Holle, A. A novel MALDI LIFT-TOF/TOF mass spectrometer for proteomics. Anal. Bioanal. Chem. 2003, 376, 952–965. [Google Scholar] [CrossRef] [PubMed]




| Seed Fraction | Adenine Release (µg/mg Total Protein) |
|---|---|
| Whole seeds | 720 |
| EEF | 99 |
| PSF | 851 |
| Mesh size < 200 | 487 |
| Mesh size 200 | 639 |
| Mesh size 100 | 614 |
| Mesh size 80 | 556 |
| Mesh size 60 | 285 |
| Mesh size 40 | 381 |
| Primer Pairs | Primer Sequence | Annealing Temperature (°C) | |
|---|---|---|---|
| A | Forward | 5′-AAT GCT AAG ATT ACA CAA GGG-3′ | 59 |
| Reverse | 5′-GCC CAA ATA CAT AAG GAG TCC C-3′ | ||
| B | Forward | 5′-CAT TAA ATC TCG CAA ATC C-3′ | 55 |
| Reverse | 5′-GAC TCC ATC AAT TGA CGT TAC-3′ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlaak, L.; Weise, C.; Kuropka, B.; Weng, A. Sapovaccarin-S1 and -S2, Two Type I RIP Isoforms from the Seeds of Saponaria vaccaria L. Toxins 2022, 14, 449. https://doi.org/10.3390/toxins14070449
Schlaak L, Weise C, Kuropka B, Weng A. Sapovaccarin-S1 and -S2, Two Type I RIP Isoforms from the Seeds of Saponaria vaccaria L. Toxins. 2022; 14(7):449. https://doi.org/10.3390/toxins14070449
Chicago/Turabian StyleSchlaak, Louisa, Christoph Weise, Benno Kuropka, and Alexander Weng. 2022. "Sapovaccarin-S1 and -S2, Two Type I RIP Isoforms from the Seeds of Saponaria vaccaria L." Toxins 14, no. 7: 449. https://doi.org/10.3390/toxins14070449






