1. Introduction
The global consumption of plant-based beverages has expanded over recent years [
1]. Consumers choose these beverages as alternatives to dairy milk due to health, environmental, and lifestyle preference reasons. Plant-based beverages that are also known as non-dairy milk substitutes are popular for their taste, nutrient value, including vitamins, low-fat content, and ethical advantage, as well as related dietary and environmental aspects [
2,
3]. Investments are currently being made toward the further development and production of such products in order to refine the technology and improve the nutritional value of plant-based drinks, as well as improve their taste and extend their shelf-life [
1]. At the same time, it is important to ensure the safety of these dairy milk alternatives. Plant-based drinks are considered environmentally friendly because of reduced carbon footprint and greenhouse gas emissions, and they also use less land use than the production of conventional dairy milk [
4,
5,
6]. Plant-based food production emits four times less carbon dioxide (CO
2-eq. per kg of product) on average than the production of dairy milk [
6].
Mycotoxins are secondary metabolites of fungi that can be dangerous to human and animal health. The most common mycotoxins are aflatoxins (AFs), trichothecenes, fumonisins (FB1, FB2), zearalenone (ZEA), and ochratoxin A (OTA) [
7,
8]. Raw agricultural products, including cereals, legumes, nuts, fruits, herbs, and other crops used in herbal beverages, can be contaminated with fungi, leading to the occurrence of mycotoxins in processed foods, such as plant-based beverages [
9,
10,
11]. Mycotoxins exhibit acute and chronic toxicity, including genotoxicity, carcinogenicity, immunotoxicity, mutagenicity, nephrotoxicity, and teratogenicity [
7,
8,
12]. Mycotoxins are, therefore, harmful to health when consumed in significant quantities or continuously ingested [
13].
Fusarium is a widespread pathogen affecting cereals, animal feed, and food products worldwide [
14]. Under favorable conditions, the metabolism of
Fusarium species can produce mycotoxins of the hexadepsipeptide type, such as beauvericin (BEA) and enniatins (ENNs), such as ENNA, ENNA1, ENNB, and ENNB1, which are the most common grain contaminants in Europe [
15,
16].
Limited information is available in the scientific literature on the mycotoxin content of plant-based beverages [
9,
10,
11].
The highest concentrations of mycotoxins were found in nut and oat beverages, particularly almond-derived beverages. The concentration of tentoxin ranged from 15 to 98 µg L
−1, the concentration of ENNB ranged from 10 to 109 µg L
−1, and the concentration of ENNB1 ranged from 6 to 60 µg L
−1 in almond-based beverages. Conversely, in oat beverages, the highest concentrations were observed for tentoxin, ENNs, ZEA, and HT-2 toxin [
9,
10,
11].
The authors of the aforementioned publications mostly reported low mycotoxin concentrations, but in a few cases, mycotoxin content reached 109 µg L
−1. For ENN B, alternaria monomethyl ether (AME) and fumonisins were not found in those studies. A study by Hamed et al. on aflatoxins in plant-based beverages could not detect any aflatoxins above quantification levels [
17].
The European Commission still does not have a clear view of the maximum permissible levels of mycotoxins in plant-based beverages, and no risk assessment has been carried out by the European Food Safety Authority [
18].
The European Commission Regulation No. 2023/915 sets maximum levels for the main mycotoxins in similar food commodities, and the Commission Recommendation No. 2013/165 sets the indicative levels for T-2 and HT-2 toxin in cereals and cereal products [
18,
19]. Indicative levels of 200 and 50 μg kg
−1 are recommended for the sum of T-2 and HT-2 toxins in oat bran and flakes and in other cereal milling products. For OTA, DON, ZEA, and AFB1, the maximum levels of 3.0 μg kg
−1, 200 μg kg
−1, 20 μg kg
−1, and 2.0 μg kg
−1 are indicated in the products derived from unprocessed cereal grains and processed cereal foods. The maximum levels for AFB1 and the sum of aflatoxins in oil seeds (such as soybeans, palm nuts, and hemp seeds) and tree nuts (including cashew nuts and cocoa beans) are 2.0 μg kg
−1 and 4.0 μg kg
−1, respectively. For AFB1 in almonds, hazelnuts, and Brazil nuts intended for use as ingredients in food, the maximum levels are between 8 μg kg
−1 and 12 μg kg
−1 [
18,
19].
The aim of the present study was to investigate the prevalence of mycotoxins in plant-based beverages, develop and validate a method for mycotoxin detection, and perform a consumer exposure assessment.
In this study, an analytical method using QuEChERS extraction and ultra-high-performance liquid chromatography with tandem mass spectrometry detection (UHPLC-MS/MS) was used for the analysis of 22 mycotoxins in 72 plant-based milk substitutes.
An exposure assessment study was provided based on the assumption of plant-based beverages as a replacement for dairy milk in the daily diet.
In this study, low quantification levels were achieved for this type of matrix, and new compounds (NIV, TEN, 3-ADON, and 15-ADON) not previously studied in plant-based beverages were investigated and new matrices, such as hemp, millet, buckwheat, and pea beverages, have been included in this study.
2. Results and Discussion
2.1. Optimization of the Sample Preparation
2.1.1. Comparison of the SPE and QuEChERS Methodologies
Two sample preparation methods were compared, namely, the “quick, easy, cheap, effective, rugged, and safe” (QuEChERS) method and C18 column-based solid phase extraction (SPE) for the evaluation of the extraction efficiency (EE). The EE was calculated as described in
Section 4.2.5.
The spiking levels used during the validation studies were 0.031 μg kg−1 for aflatoxins AFB2 and AFBG2; 0.125 μg kg−1 for aflatoxins AFB1 and AFG1; 0.5 μg kg−1 for OTA; 0.7 μg kg−1 for ZEA; 1 μg kg−1 for ENNA, ENNA1, ENNB, ENNB1, and BEA; 1.25 μg kg−1 for tentoxin (TEN), T-2, and HT-2 toxins; 2 μg kg−1 for alternariol (AOH) and AME; 2.5 μg kg−1 for DON; 4 μg kg−1 for FB2; 15 μg kg−1 for nivalenol (NIV); 20 μg kg−1 for 3-acetyldeoxynivalenol (3-ADON) and 15-acetyldeoxynivalenol (15-ADON); and 1000 μg kg−1 for FB1.
The extraction efficiencies of both the SPE and C-18 QuEChERS methods were compared based on the EE assessment obtained for eighteen major mycotoxins assessed in the soy matrix in three replicates (see
Figure 1).
For most of the mycotoxins, the EE was higher using the QuEChERS methodology, with the exception of HT-2 toxin and fumonisin B1, for which efficiency was higher in the case of the SPE procedure. A study by Berzina et al. reported that when using SPE C18-E columns, deoxynivalenol (DON) had poor absolute recoveries due to the weak bonding to the C18 sorbent [
20]. Nivalenol (NIV) is a cooccurring
Fusarium metabolite similar in structure and properties to DON, which also presented poor retention on C18 sorbent. The same study described a difficulty in eluting BEA from the SPE C18-E column due to the non-polar nature of this analyte, resulting in strong binding to the sorbent, thus requiring an increased eluent volume [
20]. The QuEChERS method resulted in low analyte signals for the BEA and ENN groups because of inefficient extraction to the organic layer [
21].
Fumonisins and OTA were irreversibly adsorbed using PSA, as described in other publications [
21], because of the strong ionic affinity between the primary and/or secondary amines in the PSA carrier and the carboxyl groups present in the mycotoxin [
22]. For the other mycotoxins, extraction efficiency did not change significantly. It was decided not to use PSA salts in order to allow for effective analysis of acidic mycotoxins.
Based on the conducted experiments, it was decided to perform the analysis of real samples using a commercially available QuEChERS salt mixture due to its simplicity and comparably high extraction efficiency.
2.1.2. Selection of a Reconstitution Solution and Sample Filtration
In order to reduce the matrix effects and save time, the possibility of concentrating 1 mL of extract was tested instead of 4 mL extract volume as proposed in a former study by Reinholds et al. [
23]. The application of 1 mL evaporated extract caused a significant reduction in the sensitivity and hindered the detection of low mycotoxin concentrations around the level of quantification (LOQ).
The reconstitution solution with high water content is more suitable for the detection of polar compounds, such as DON and NIV. On the other hand, for non-polar compounds, such as BEA and the ENN group, a reconstitution solution with an increased organic solvent content is preferable. Using a solution with high water content reduced the recovery of non-polar compounds for chromatographic analysis. It was, therefore, necessary to find a reconstitution solution with the optimal solubility of both polar and non-polar mycotoxins that would also be acceptable for chromatographic separation. It was also necessary to adjust the injection volume to obtain a satisfactory signal shape.
To find the most effective reconstitution solution, aqueous 15%, 25%, and 50% methanol solutions with 0.5 mM ammonium acetate and 0.1% formic acid, an aqueous 50% methanol solution with 0.5 mM ammonium acetate and 1% formic acid, and aqueous 15% and 25% acetonitrile solutions with 0.5 mM ammonium acetate and 0.1% formic acid were compared. The experiment was prepared in three replicates.
When the organic solvent content exceeded 25% methanol or 15% acetonitrile, asymmetric signal shapes and double signal formations were observed for polar compounds, while enhanced signal intensity was observed for non-polar compounds, like BEA and ENNs. The optimal signal shapes and intensities were obtained by reconstructing the samples in an aqueous 50% methanol solution supplemented with 0.5 mM ammonium acetate and 1% formic acid. When using this reconstitution solution, DON and NIV signal shapes were not optimal, but this fact did not affect the quantification of those signals.
The selected reconstitution solution did not match the initial mobile phase composition; therefore, the injection volumes were tested, and 5 µL was chosen to achieve the optimal signal shape for DON and NIV.
Some mycotoxins were absorbed on polyvinylidene difluoride (PVDF) centrifuge filters, and thus freezing and centrifugation were applied for the final purification of the extract.
2.2. Validation of the Method
The proposed method for mycotoxin analysis based on UHPLC-MS/MS is described in
Section 4.2. and has been used for the assessment of 22 selected mycotoxins. The method was validated using four different types of plant-based (e.g., oat, rice, soya, and almond) beverages according to the current recommendations based on the European Commission Regulation (EC) No. 401/2006 and SANTE 12089/2016 “Guidance on the identification of mycotoxins in food and feed” [
24]. The method characteristics, including recovery, repeatability, selectivity, linearity, method sensitivity, and matrix effects, were evaluated (see
Table S1 in the Supplementary Material). The calculations for the determination of matrix effect (ME) and the method uncertainty are summarized in
Section 4.2.5.
2.2.1. Levels of Quantification, Method Selectivity, and Linearity
The selectivity of the method was tested by analyzing blanks and samples spiked with a standard at the LOQ (see
Table 1).
The retention time difference between the signals in the spiked sample and the signals in the standard solution was less than 1%. No interfering signals were observed in the chromatograms. The method showed good selectivity.
The linearity and correlation coefficients (R2 > 0.98) of the method were tested by a five-point procedural calibration ranging from the LOQ level to 40× LOQ level. Good linearity was maintained over the selected concentration range, with the obtained correlation coefficients being at least ≥0.98.
The level of quantification (LOQ) values (
Table 1) were expressed as the concentrations of mycotoxins corresponding to a signal-to-noise value (S/N) ≥ 10 [
25]. One of the lowest LOQ values compared to other publications was obtained in the present study.
In a paper by Hamed et al., Afs LOQ values were estimated to be 0.5 µg L
−1 [
17], whereas in the present study, the LOQ values for the Afs were 0.0078–0.063 µg kg
−1. The LOQ values for the ENNs and BEA obtained by Arroyo-Manzanares et al. were 0.3–0.8 µg L
−1, depending on the type of the plant-based beverage [
11]. In the present study, the LOQ values for BEA and the ENNS were three to eight times lower (0.10 µg kg
−1) for all of these mycotoxins in all types of tested beverages. The LOQ values for all mycotoxins described by Miro-Abella et al. (2017) ranged from 0.05 to 15 µg L
−1 [
9], while the LOQ values in the study by Juan et al. ranged from 0.3 to 18 µg L
−1 [
10].
2.2.2. Repeatability and Recovery
The method repeatability and recovery were tested for each matrix sample spiked with a mycotoxin standard at the LOQ level and at 2–10 times the LOQ level.
In addition, for all beverages, the two main concentration levels tested were the LOQ and 5× LOQ levels in five replicates for each level (the number of replicates was different for the aflatoxnis because four aflatoxins were in the mixture). Additionally, data from quality control experiments (spiked samples) for the calculation of method performance characteristics (for example, uncertainty) were used.
Each sample series included at least one quality control sample for each matrix. Detailed recovery values for mycotoxins are given in the
Supplementary Material (Table S1). The average mycotoxin recoveries for all matrices are given in
Table 2.
2.2.3. Matrix Effect and Extraction Efficiency
The matrix effects (MEs, %) were tested by calibrating the standard solution and calibrating the matrix by adding mycotoxin standards to the extracts during the evaporation step. Calibration with standard solutions cannot be applied to the quantification of mycotoxins in real samples due to matrix effects. In order to compensate for matrix effects, it is necessary to use an internal standard or matrix-matched calibration (
Figure 2).
The matrix effects were the highest for soy drinks, except for the enniatin group, BEA, OTA, and fumonisins. In all matrices, the matrix effects for fumonisins were positive, suggesting ion enhancement.
The EE was tested for all types of plant-based beverages in three replicates. For most mycotoxins, the EE was greater than 50%. In the case of oat and rice beverages, reduced extraction efficiencies were obtained for BEA and ENNA1, probably due to the applied reconstitution solution. However, for DON and NIV, a reconstitution solution containing more than 50% organic solvent was not suitable, and SPE columns, such as C18-E, were unable to retain DON and NIV due to the polarity of these analytes.
2.3. Method Application
The developed methodology was applied to the analysis of plant-based beverages obtained from the retail market. The results are shown in
Table S2.
Aflatoxins (except for AFB2), fumonisins, NIV, 3-ADON, and 15-ADON were not observed in oat beverages. The most frequently detected mycotoxins were ENNB, ENNB1, DON, BEA, ENNA, HT-2, and T-2-toxin. ENNB and ENNB1 were detected in 20 samples. The mycotoxin burden in oat drinks was dominated by a high percentage of ENN B and ENN B1, which was in agreement with the publication by Juan et al. (2022) [
10].
The highest concentrations were found for DON, ENNB1, AME, and TEN (9 ± 2, 3.2 ± 0.8, 3.0 ± 0.4, and 2.7 ± 0.5 µg kg−1, respectively).
Only two mycotoxins were detected in nut beverages: AFB1 and AFB2. AFB1 was detected at the highest concentration of 0.07 ± 0.02 μg kg−1.
The paper by Juan et al. mentioned other mycotoxins in almond drinks, such as ENNs, whereas the occurrence of AFs in this type of drink was only determined in 5% of tested samples [
10]. In this study, no other mycotoxins were detected above the LOQ value in nut drinks. However, traces of AFB1 and AFB2 were detected at very low levels. Other authors have also reported low levels of AFs in nut-based beverages [
17].
Eleven mycotoxins (DON, AOH, BEA, ENN A, ENB, ENNB1, ZEA, T-2, TEN, AFB1, and AFB2) were detected in rice beverages. In general, rice beverages have relatively low mycotoxin content, according to other publications [
9,
10,
11,
17].
In this study, ENNs, DON, AFB1, and AFB2 are frequently found in rice beverages. Juan et al. also mentioned that the enniatin group is predominant in rice beverages.
In the present study, DON, ZEA, ENNB, and ENNB1 were detected in soy beverages at low concentrations. The highest concentration was revealed for DON at 2.0 ± 0.3 μg kg−1.
A total of ten mycotoxins were detected above the LOQ value in other tested plant- beverages based on hemp, millet, buckwheat, and peas. The most frequently detected among these mycotoxins were ENN B1, OTA, and TEN. The highest concentrations were quantified for NIV in the buckwheat drink, for AOH in the pea drink, and for TEN in the hemp drink. Prior to our study, there was no data in the literature on the occurrence of mycotoxins in hemp, millet, buckwheat, and pea-based beverages.
2.4. Results of Thermal Stability Testing
The results of thermal processing tests for the plant beverages are presented in
Figure 3 and
Figure 4.
Mycotoxin concentrations decreased with heat treatment, except for AFB1. In the almond drink, AFB1 concentrations slightly increased during heating.
The heating of plant-based beverages in a coffee machine reduced mycotoxin concentrations more than boiling, despite the fact that the treatment temperature in the coffee machine simulation was lower compared to the boiling simulation. This was probably caused by the fact that the beverage heated up much faster in the coffee machine than during boiling. The steam temperature in the coffee machine can reach 130 °C, according to the user manual. This could have a major effect on the reduction in mycotoxin content.
In order to correctly assess the thermal treatment effects on mycotoxin concentrations, the resulting mycotoxin concentrations must be outside the uncertainty limit of the compound analysis. Otherwise, the apparent changes in mycotoxin concentrations may be caused by random fluctuations. The change in mycotoxin concentration after thermal treatment exceeded the method uncertainty for the oat drink.
The boiling of the oat drink resulted in an 8–18% reduction in mycotoxin concentrations. In contrast, processing the same drink in a coffee machine resulted in a 49–100% reduction in mycotoxin concentrations.
2.5. Exposure Risk Studies
2.5.1. Consumption of Milk in Latvia
Dietary exposure to mycotoxins was assessed based on the available data on mycotoxin contamination in beverages and the estimated acute intake data. First, the Comprehensive Food Consumption database of the European Food Safety Authority (EFSA) was checked to find some data regarding the consumption of non-dairy milk substitutes in Latvia and other Baltic states. However, there was no data available regarding the Latvian population. Thus, it was decided to use recent data from a national survey by Siksna et al. (2020) for salt and iodine consumption in the adult population in Latvia, which also presented the average consumption data over a three-day period for different milk products, including pasteurized dairy milk, among adults in Latvia [
26].
These data are summarized in
Table 3. Due to the absence of direct data on plant-based milk substitute consumption, we elected to use data on dairy milk intake due to the assumption that certain populations may fully replace their dairy milk intake with plant-based non-dairy alternatives. The provided prediction of dairy milk replacement allows us to characterize at least the potential worst-case exposure to plant-based milk substitute beverages, as well as anticipate the likely future trends in this direction.
It must be emphasized that there was a 1.7 to 1.5 times lower milk consumption rate among females compared to males. Furthermore, the milk consumption level among males decreased by almost two times with an increase in age, whereas for females, there was little difference in age, as the consumption rate was almost the same. This may be attributed to the cultural habits of older people, as plant-based beverages are still not very popular among elderly people.
It was indicated from the data that male toddlers and younger males between 19 and 34 years were the major groups that could be exposed to mycotoxin contamination by replacing milk with plant-based milk alternatives.
2.5.2. Exposure Assessment
Section 4.4 describes the methodology used for the exposure estimation based on the assumption of conventional milk replacement with plant beverages.
Many studies have been dedicated to mycotoxin exposure assessment via the consumption of teas, as well as fruit and vegetable juices, but there is still a lack of data about human exposure from plant-based milk substitute beverages. Considering that plant-based milk substitutes are mostly used in addition to tea and coffee, the PDI values were calculated based on this assumption. The calculated PDI values for males and females based on the upper bound (UB) average values of contamination and the highest concentration levels determined in the studied plant-based beverages are summarized in
Table 4.
The calculated PDI values for the average UB mycotoxin contamination levels ranged between 0.01 and 7.62 ng kg
−1 body weight per day for all age groups of males, whereas the highest levels were determined for the exposure of 19–39 years old males to NIV, and the lowest value was determined for AFB1 based on the average contamination levels in nut-based beverages. For females, the PDI for the average UB levels ranged between 0.01 and 4.29 ng kg
−1 body weight per day, and the highest levels were found in 19- to 34-year-old females in the case of exposure to NIV. It must be emphasized that compared to the studies by other authors on the overall mycotoxin exposure rates in the European population, our study revealed PDI values that were quite low for most of the EU-regulated mycotoxins. A recent study of PDI outputs simulated for adults in Northern Europe indicated the average UB levels of the PDI for OTA, DON, and ZEN to be 0.6764, 0.48282, and 0.00149 µg kg
−1 body weight per day, respectively [
27]. It can be expected that the determined levels of mycotoxin contamination in plant-based milk substitute beverages are very low compared to these simulated values.
It can be pointed out that compared to males, the PDI data for all age groups of females did not exhibit major differences between the groups.
The PDI values of the UB contamination levels were used for the calculation of the potential exposure (%TDI values) that are shown in
Table 5.
For the commonly found Fusarium mycotoxins, such as DON and ZEA, as well as the emerging mycotoxins (a sum of ENNs, BEA, and TEN), the values of determined PDI were shown to be below 1% of the determined TDI or threshold of the concern, considering the determined average UB levels. For NIV and AFB2, the levels were slightly above 1% of the recommended TDI by the EFSA.
The %TDI values for the sum of T-2 and HT-2 toxins were almost two times higher for males in the age group of 19–34 years compared to females of the same age, whereas the average values for all age groups ranged between 4.66 and 6.84%. It was determined that for the carcinogenic mycotoxins, such as AFB1, the sum of determined AFs, OTA, the %TDI values were also below 10%, indicating quite low risks. While the %TDI values for AOH and AME were quite high and reached 35.1 to 43.5% of the actual exposure levels for males and 1.5 times lower values (29.7 and 23.9%) for females, it must be emphasized that these mycotoxins are considered to have low acute toxicity, whereas their chronic effects can be quite severe. as these mycotoxins are reported to cause esophageal cancer [
28].
Overall, the UB level studies indicated that all the studied beverages are safe for human consumption. However, it was also evaluated whether the highest concentration levels determined in the tested beverages may have some impact on the %TDI. Similar to the UB levels, the PDI values were also calculated for the maximum concentration levels (data summarized in
Table 6).
It can be seen that DON and its derivative NIV had the highest PDI levels compared to other mycotoxins due to their higher concentrations. For example, the PDIO ranged between 10.5 and 7.1 ng kg−1 body weight per day for DON when comparing the data for all male and female age groups. It must be emphasized that if we divide the PDI values at the maximum contamination levels and the UB average data assessment for the younger age group or even for all age groups of males, then the ratio of PDI at the maximum mycotoxin concentration is 5 to 7 times higher compared to the average contamination levels considering the UB approach. For example, for DON, TEN, the sum of ENNs, OTA, and AME, the PDI (max) values were 6.9, 6.6, 5.0, 4.8, and 4.2 times higher compared to the PDI values for the average contamination levels for all male age groups. The same tendency can be seen in the case of the females. Obviously, this indicates the notable impact of contamination levels on the probable impact of mycotoxins during daily consumption. Even for the other tested mycotoxins, the PDI values considering the highest mycotoxin concentrations were about 1.5 to 2 times higher compared to the average mycotoxin concentration, indicating that the frequency of mycotoxin contamination and its prevalence was very different in the tested beverages.
This can be illustrated by comparing the %TDI values and taking into consideration the highest mycotoxin contamination levels determined in the tested plant beverages (
Table 7).
Despite the relatively lower %TDI for females, we can see a rather important increase for the male subgroup at the age of 19–34. The %TDI for AFB1 and the sum of the two determined AFs were 10.5% and 13.5%, respectively. It can be seen that in the case of OTA, this ratio is even five times higher than %TDI for the OTA average levels and is also very high for the sum of T-2 and HT-2 toxins (24%) in this subgroup of younger males. It must be emphasized that this group may have an even higher risk in the case of an additional use of plant beverages as additives to cereals or other food products, not only by adding them to hot drinks. For the female groups, these values are up to two times lower but still present some concern about the impact of mycotoxins.
As noted previously, AOH and AME may be more relevant to the long-term exposure risks, whereas the %TDI exceeded the threshold of toxicological concern (TTC), indicating that these mycotoxins may present actual risks. Obviously, more studies about their presence are needed.
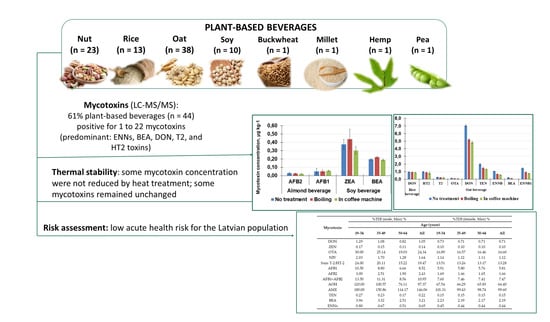
3. Conclusions
An analytical methodology based on QuEChERS extraction and HPLC-MS/MS was developed for the analysis of 22 mycotoxins in plant-based milk substitute beverages. The method parameters (correlation coefficient ≥ 0.98, LOQ 0.0078–5.0 μg kg
−1, method recovery 79–119%) complied with the European Union Commission Regulation No. 401/2006 [
24]. A total of sixteen mycotoxins were detected in the tested beverages, and 61% of all analyzed beverages contained at least one mycotoxin. The most frequently detected mycotoxins were enniatin B1 in soy drinks, enniatins B and B1, deoxynivalenol, beauvericin, enniatin A, HT-2 and T-2 toxins in oat drinks, aflatoxin B2 in nut drinks, deoxynivalenol, enniatins B and B1, aflatoxins B1 and B2 in rice drinks, and enniatin B1 in hemp, millet, buckwheat, and pea beverages.
The oat drink was one of the most contaminated plant-based drinks, with the highest detected deoxynivalenol concentration at 9 ± 2 μg kg−1. Thermal treatment of plant beverages did not consistently reduce mycotoxin concentrations, with several mycotoxins remaining unchanged. The highest reduction of 49–100% was observed when the oat drink was processed in a coffee machine. This study found that mycotoxin contamination in plant-based beverages generally poses a low acute health risk for the Latvian population. Most mycotoxin exposure levels were well below the recommended thresholds. However, exposure to AOH and AME may require further investigation due to potential chronic health concerns. Additionally, higher mycotoxin exposure in specific population groups, especially young males, could lead to elevated risks. Overall, plant-based milk substitute beverages can be considered safe, but further research is needed to fully understand the long-term effects and potential risks associated with mycotoxin exposure.