Venomics of Scorpion Ananteris platnicki (Lourenço, 1993), a New World Buthid That Inhabits Costa Rica and Panama
Abstract
:1. Introduction
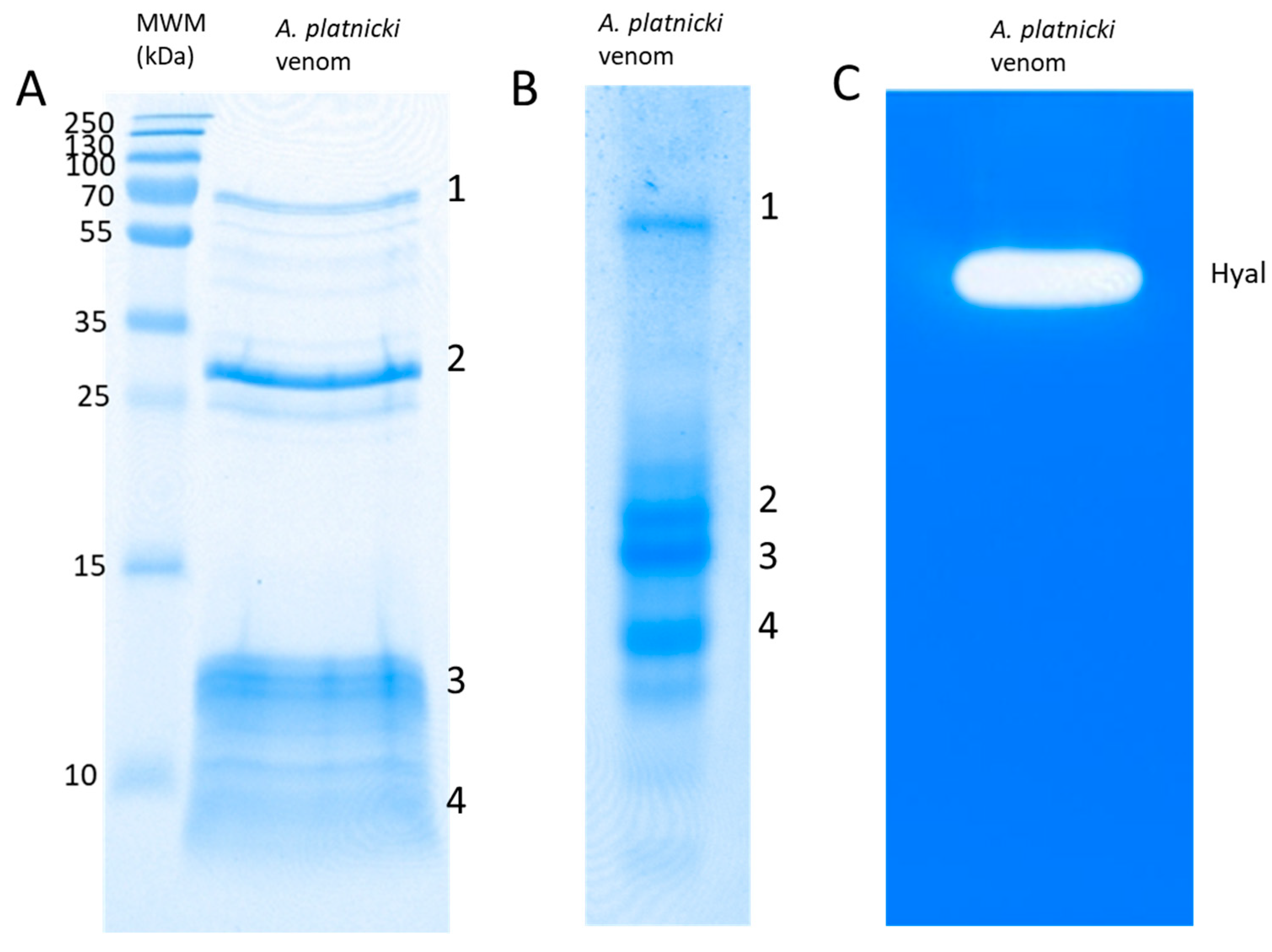
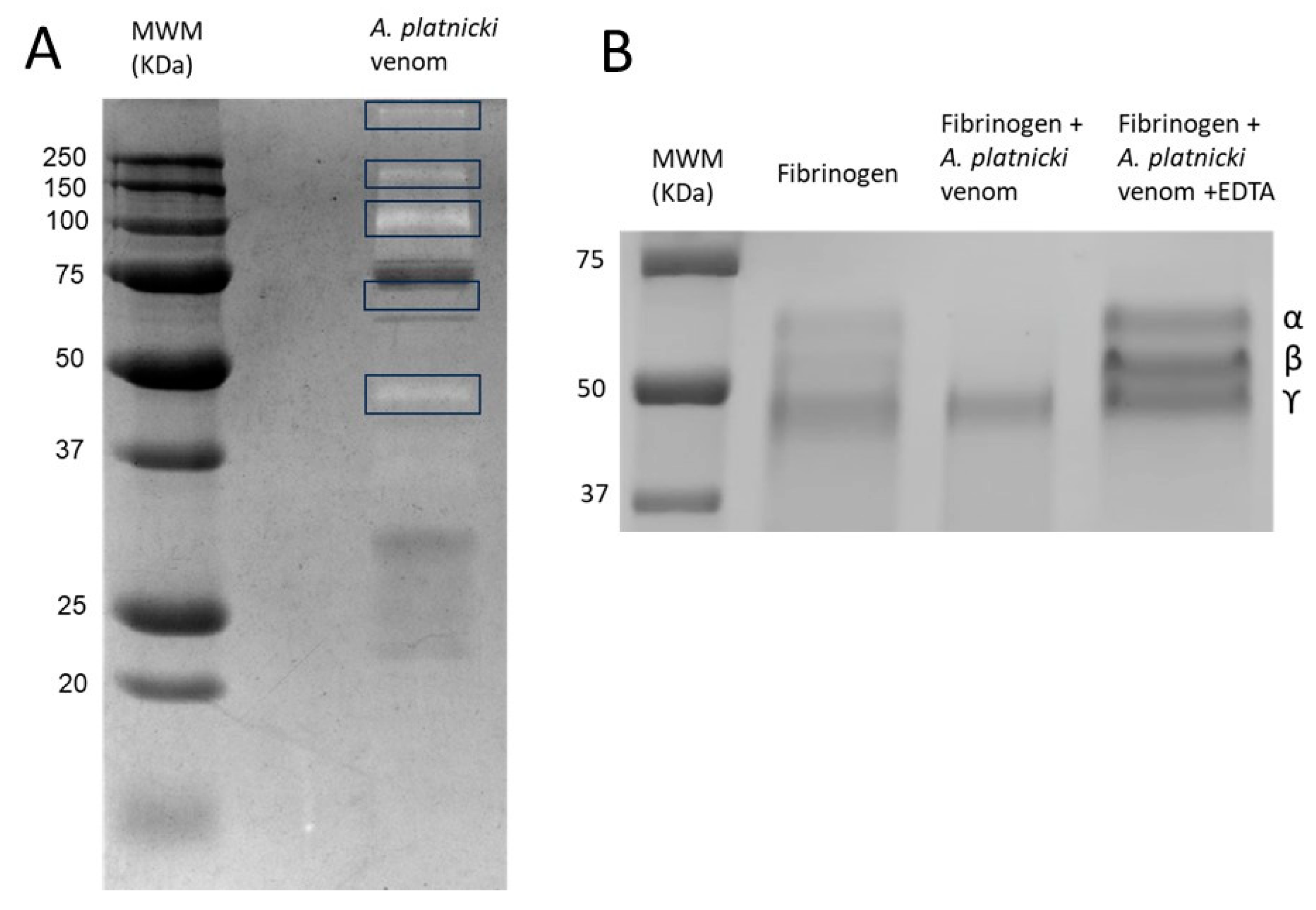
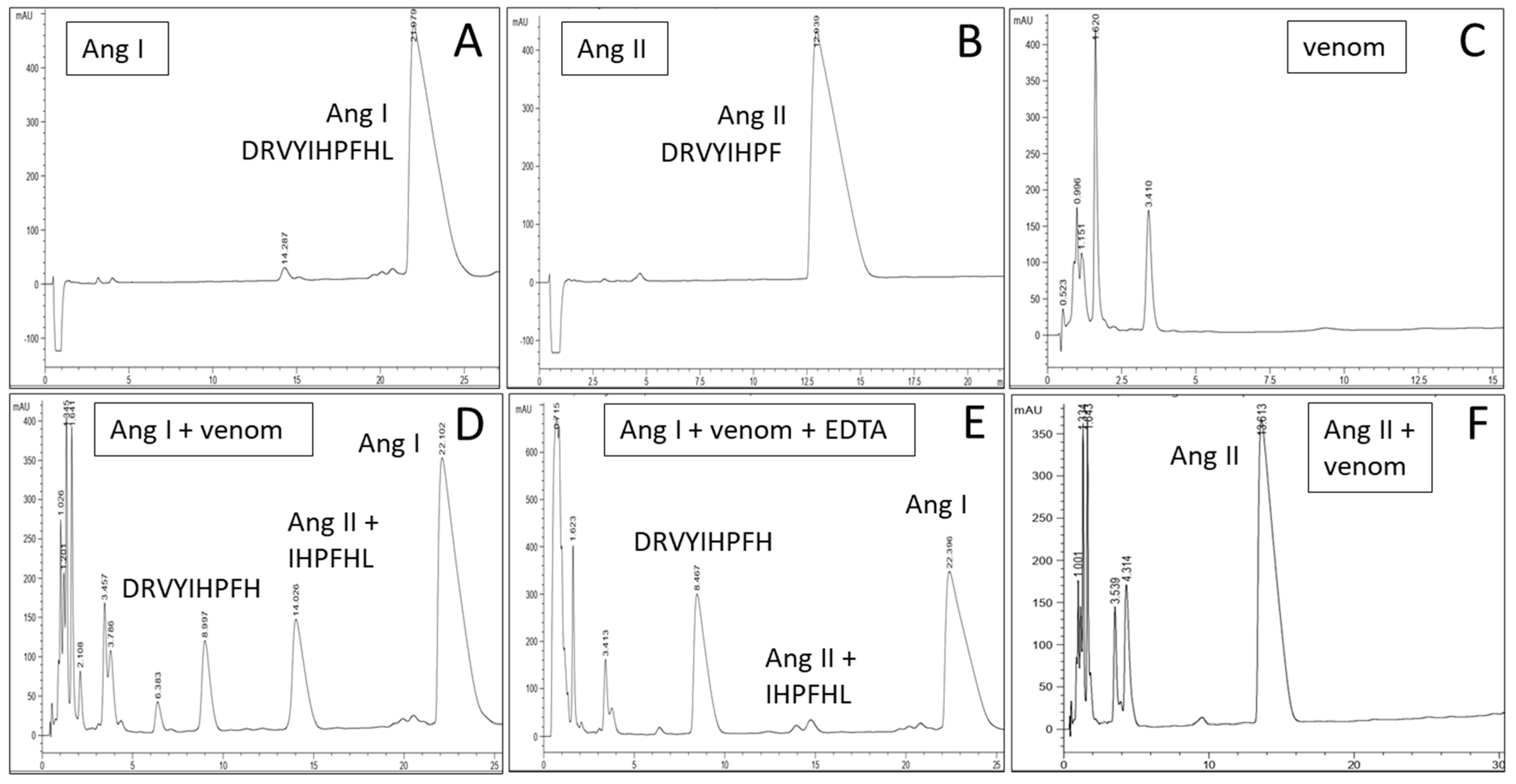
2. Results
3. Discussion
4. Materials and Methods
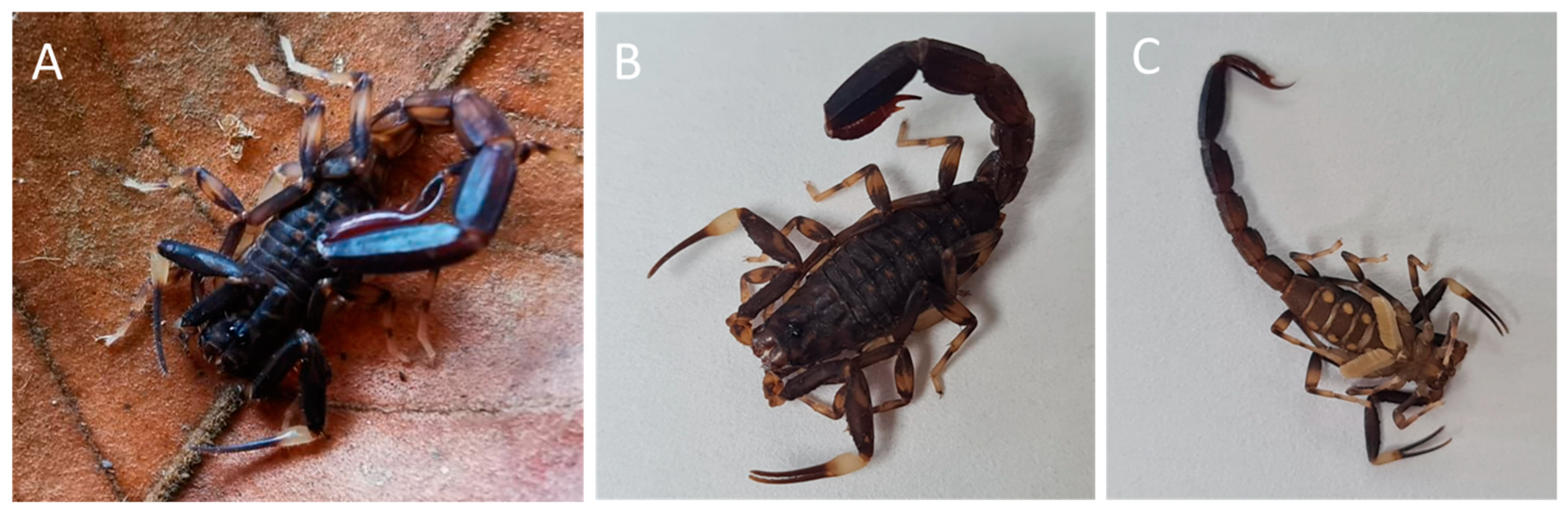
4.1. Scorpion Specimens and Venom
4.2. Electrophoresis and Determination of Enzymatic Activities
4.3. RP-HPLC for Venom Protein Separation
4.4. Mass Spectrometry
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fet, V.; Lowe, G. Family Buthidae C.L. Koch, 1837. In Catalog of the Scorpions of the World; The New York Entomological Society: New York, NY, USA, 2000; pp. 54–286. [Google Scholar]
- Ward, M.J.; Ellsworth, S.A.; Nystrom, G.S. A global accounting of medically significant scorpions: Epidemiology, major toxins, and comparative resources in harmless counterparts. Toxicon 2018, 151, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Fet, V.; Soleglad, M.E.; Lowe, G. A new trichobothrial character for the high-level systematics of Buthoidea (Scorpiones: Buthida). Euscorpious 2005, 23, 1–40. [Google Scholar] [CrossRef]
- Stundlová, J.; Stahlavsky, F.; Opatova, V.; Stundl, J.; Kovarík, K.F.; Dolejs, P.; Smíd, J. Molecular data do not support the traditional morphology-based groupings in the scorpion family Buthidae (Arachnida: Scorpiones). Mol. Phylogenet. Evol. 2022, 173, 107511. [Google Scholar] [CrossRef]
- Santibañez-López, C.E.; Ojanguren-Affilastro, A.A.; Graham, M.R.; Sharma, P.P. Congruence between ultraconserved element-based matrices and phylotranscriptomic datasets in the scorpion Tree of Life. Cladistics 2023, 39, 533–547. [Google Scholar] [CrossRef]
- Pocock, R.I. Arachnida. The Fauna of British India, including Ceylon and Burma; Published under the Authority of the Secretary of State for India in Council; W.T. Blandford: London, UK, 1900; 279p. [Google Scholar]
- Lourenço, W.R. Révision du genre Ananteris Thorell, 1891 (Scorpiones, Buthidae) et déscription de six espèces nouvelles. Bull. Muséum Natl. d’Histoire Nat. Paris (Zool. Biol. Écol. Anim.) 1982, 4, 119–151. [Google Scholar] [CrossRef]
- Lourenço, A. The ‘Ananteris Group’ (Scorpiones: Buthidae), suggested composition and possible links with other Buthids. Bol. Soc. Entom. Arag. 2011, 48, 105–113. [Google Scholar]
- Ythier, E. A new high-altitude scorpion species of the genus Ananteris Thorell, 1891 (Scorpiones: Ananteridae) from the Pico da Neblina, Brazil. Faunitaxys 2024, 12, 1–9. [Google Scholar]
- Botero-Trujillo, R.; Noriega, J.A. On the identity of Microananteris, with a discussion on pectinal morphology, and description of a new Ananteris from Brazil (Scorpiones, Buthidae). Zootaxa 2011, 2747, 37–52. [Google Scholar] [CrossRef]
- Ojanguren-Affilastro, A.A.; Adilardi, R.S.; Mattoni, C.I.; Ramírez, M.J.; Ceccarelli, F.S. Dated phylogenetic studies of the southernmost American buthids (Scorpiones; Buthidae). Mol. Phylogenet. Evol. 2017, 110, 39–49. [Google Scholar] [CrossRef]
- Sharma, P.P.; Fernández, R.; Esposito, L.A.; González-Santillán, A.; Monod, L. Phylogenomic resolution of scorpions reveals multilevel discordance with morphological phylogenetic signal. Proc. R. Soc. B 2015, 282, 20142953. [Google Scholar] [CrossRef]
- Deshpande, S.; Joshi, M.; Kawai, K.; Deb, A.; Lee, J.D.; Bastawade, D.; Gowande, G.; Sulakhe, S. Molecular and morphological confirmation of Isometrus maculatus (DeGeer, 1778) (Scorpiones: Buthidae) from Northeast India and East Asia. Euscorpius 2023, 374, 1–19. [Google Scholar]
- Teruel, R. Morfología, ecología y distribución de Isometrus maculatus (Degeer 1778) en Cuba (Scorpiones: Buthidae). Bol. Soc. Entom. Arag. 2009, 45, 173–179. [Google Scholar]
- Froy, O.; Gurevitz, M. New insight on scorpion divergence inferred from comparative analysis of toxin structure, pharmacology and distribution. Toxicon 2003, 42, 549–555. [Google Scholar] [CrossRef]
- Lourenço, W.R. Scorpions trapped in amber: A remarkable window on their evolution over time from the Mesozoic period to present days. J. Venom. Anim. Toxins Incl. Trop. Dis. 2023, 29, e20230040. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Runjaic, F.J.M.; Portillo-Quintero, C.; Borges, A. Un nuevo escorpión del género Ananteris Thorell, 1891 (Scorpiones: Buthidae) para la Sierra Perijá, Venezuela. Mem. Fund. Salle Cienc. Nat. 2008, 169, 5–81. [Google Scholar]
- Botero-Trujillo, R.; Florez, E. A revisionary approach of Colombian Ananteris (Scorpiones, Buthidae): Two new species, a new synonymy, and notes on the value of trichobothria and hemispermatophore for the taxonomy of the group. Zootaxa 2011, 2904, 1–44. [Google Scholar] [CrossRef]
- Pucca, M.B.; Oliveira, F.N.; Schwartz, E.F.; Arantes, E.C.; Lira-da-Silva, R.M. Scorpionism and Dangerous Species of Brazil. In Scorpion Venoms; Gopalakrishnakone, P., Possani, L., Schwartz, E.F., Rodríguez de la Vega, R., Eds.; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, Y.; He, Y.; Di, Z.; Wu, Y.; Cao, Z.; Li, W. Comparative venom gland transcriptome analysis of the scorpion Lychas mucronatus reveals intraspecific toxic gene diversity and new venom components. BMC Genom. 2010, 11, 452. [Google Scholar]
- Ma, Y.; He, Y.; Zhao, R.; Wu, Y.; Li, W.; Cao, Z. Extreme diversity of scorpion venom peptides and proteins revealed by transcriptomic analysis: Implication for proteome evolution of scorpion venom arsenal. J. Proteom. 2012, 75, 1563–1576. [Google Scholar] [CrossRef]
- Cid-Uribe, J.I.; Veytia-Bucheli, J.I.; Romero-Gutierrez, T.; Ortiz, E.; Possani, L.D. Scorpion venomics: A 2019 overview. Expert Rev. Proteom. 2019, 17, 67–83. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.A.; King, G.F.; Nevalainen, T.J.; Norman, J.; Lewis, R.J.; Norton, R.S.; et al. The Toxicogenomic Multiverse: Convergent Recruitment of Proteins into Animal Venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–551. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, J.; Zhou, X.; Liu, Z. CAP superfamily proteins from venomous animals: Who we are and what to do? Int. J. Biol. Macromol. 2022, 221, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, Z.; Ma, Y. Adaptive evolution of insect selective excitatory β-type sodium channel neurotoxins from scorpion venom. Peptides 2017, 92, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, Y.; Yin, S.; Zhao, R.; Fan, S.; Hu, Y.; Wu, Y.; Cao, Z.; Li, W. Molecular cloning and functional identification of a new K+ channel blocker, LmKTx10, from the scorpion Lychas mucronatus. Peptides 2009, 30, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, H.; Yang, F.; Liang, S.; Cao, Z.; Li, W.; Wu, Y. Functional characterization of two novel scorpion sodium channel toxins from Lychas mucronatus. Toxicon 2014, 90, 318–325. [Google Scholar] [CrossRef]
- Triana, F.; Bonilla, F.; Alfaro-Chinchilla, A.; Víquez, C.; Díaz, C.; Sasa, M. Report of thanatosis in the Central American scorpions Tityus ocelote and Ananteris platnicki (Scorpiones: Buthidae). Euscorpius 2022, 359, 1–5. [Google Scholar]
- Lourenço, A. Review of the geographical distribution of the genus Ananteris Thoreli (Scorpiones: Buthidae), with description of a new species. Rev. Biol. Trop. 1993, 41, 697–701. [Google Scholar]
- Lourenço, W.R. Comments on the Ananterinae Pocock, 1900 (Scorpiones: Buthidae) and description of a new remarkable species of Ananteris from Perú. C.R. Biologies 2015, 330, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Mattoni, C.I.; García-Hernández, S.; Botero-Trujillo, R.; Ochoa, J.A.; Ojanguren-Affilastro, A.A.; Pintoda-Rocha, R.; Prendini, L. Scorpion Sheds ‘Tail’ to Escape: Consequences and Implications of Autotomy in Scorpions (Buthidae: Ananteris). PLoS ONE 2015, 10, e0116639. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Harvey, P.J.; Craik, D.J.; Ronjat, M.; de Waard, M.; Zhu, S. Functional evolution of scorpion venom peptides with an inhibitor cystine knot fold. Biosci. Rep. 2013, 33, e00047. [Google Scholar] [CrossRef] [PubMed]
- Jaenicke, E.; Pairet, B.; Hartmann, H.; Decker, H. Crystallization and Preliminary Analysis of Crystals of the 24-Meric Hemocyanin of the Emperor Scorpion (Pandinus imperator). PLoS ONE 2012, 7, e32548. [Google Scholar] [CrossRef]
- Cunningham, M.; Laino, A.; Romero, S.; García, F. Arachnid hemocyanins. In Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and Other Body Fluid Proteins, Subcellular Biochemistry 94; Hoeger, U., Harris, J.R., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 219–231. [Google Scholar]
- Barth, T.; Coelho Mandacaru, S.; Charneau, S.; Valle de Souza, M.; Ornelas Ricart, C.A.; Ferreira Noronha, E.; Araújo Souza, A.; de Freitas, S.M.; Roepstorff, P.; Fontes, W.; et al. Biochemical and structural characterization of a protein complex containing a hyaluronidase and a CRISP-like protein isolated from the venom of the spider Acanthoscurria natalensis. J. Proteom. 2019, 192, 102–113. [Google Scholar] [CrossRef]
- Cajado-Carvalho, D.; Kazuo Kuniyoshi, A.; Duzzi, B.; Kei Iwai, L.; Castro de Oliveira, U.; Junqueira de Azevedo, I.L.M.; Tadashi Kodama, R.; Vieira Portaro, F. Insights into the hypertensive effects of Tityus serrulatus scorpion venom: Purification of an Angiotensin-converting enzyme-like peptide. Toxins 2016, 8, 348. [Google Scholar] [CrossRef]
- Oliveira, I.S.; Malachize Alano-da-Silva, N.; Gobbo Ferreira, I.; Cerni, F.A.; de Almeida Goncalves Sachett, J.; Monteiro, W.M.; Pucca, M.B.; Candiani Arantes, E. Understanding the complexity of Tityus serrulatus venom: A focus on high molecular weight components. J. Venom. Anim. Toxins Incl. Trop. Dis. 2024, 30, e20230046. [Google Scholar] [CrossRef]
- Gao, B.; Zhu, S. Mesobuthus venom-derived antimicrobial peptides possess intrinsic multifunctionality and differential potential as drugs. Front. Microbiol. 2018, 9, 320. [Google Scholar] [CrossRef]
- Sunagar, K.; Undheim, E.A.B.; Chan, A.H.C.; Koludarov, I.; Muñoz-Gómez, S.A.; Antunes, A.; Fry, B.G. Evolution stings: The origin and diversification of scorpion toxin peptide scaffolds. Toxins 2013, 5, 2456–2487. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.J.; de Armas, L.F.; Cambra, R.A. Predation of Ananteris spp. (Scorpions: Buthidae) by ants and social wasps (Hymenoptera, Formicidae, Vespidae) in Panama, Central America. Euscorpius 2021, 329, 1–4. [Google Scholar]
- Botero-Trujillo, R. Anuran predators of Scorpions: Bufo marinus (Linnaeus, 1758) (Anura: Bufonidae), first known natural enemy of Tityus nematochirus Mello-Leitão, 1940 (Scorpiones: Buthidae). Rev. Iber. Aracnol. 2006, 13, 199–202. [Google Scholar]
- Salabi, F.; Vazirianzadeh, B.; Baradaran, M. Identification, classification, and characterization of alpha and beta subunits of LVP1 protein from the venom gland of four Iranian scorpion species. Sci. Rep. 2023, 13, 22277. [Google Scholar] [CrossRef]
- de Oliveira, U.C.; Nishiyama, M.Y., Jr.; dos Santos, M.B.V.; Santos-da-Silva, A.d.P.; Chalkidis, H.d.M.; Souza-Imberg, A.; Candido, D.M.; Yamanouye, N.; Dorce, A.C.; Junqueira-de-Azevedo, I.d.L.M. Proteomic endorsed transcriptomic profiles of venom glands from Tityus obscurus and Tserrulatus scorpions. PLoS ONE 2018, 13, e0193739. [Google Scholar] [CrossRef]
- Kalapothakis, Y.; Miranda, K.; Pereira, A.H.; Witt, A.S.A.; Marani, C.; Martins, A.P.; Gomes Leal, H.; Campos-Júnior, E.; Pimenta, A.M.C.; Borges, A.; et al. Novel components of Tityus serrulatus venom: A transcriptomic approach. Toxicon 2021, 189, 91–104. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, B. Molecular characterization of a possible progenitor sodium channel toxin from the Old World scorpion Mesobuthus martensii. FEBS Lett. 2006, 580, 5979–5987. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, W.; Zeng, X.; Jiang, D.; Mao, X.; Liu, H. Molecular cloning and sequencing of two ‘short chain’ K+ channel blocking peptides from the Chinese scorpion Buthus martensii Karsch. FEBS Lett. 1999, 457, 509–514. [Google Scholar] [CrossRef] [PubMed]
- So, W.L.; Leung, T.C.N.; Nong, W.; Bendena, W.G.; Ngai, S.M.; Hui, J.H.L. Transcriptomic and proteomic analyses of venom glands from scorpions Liocheles australasiae, Mesobuthus martensii, and Scorpio maurus palmatus. Peptides 2021, 146, 170643. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Chen, L.; Wei, J.-F.; Yang, X.; Ma, D.; Xu, X.; Xu, X.; He, S.; Lu, J.; Laiz, R. Purification and characterization of two new allergens from the venom of Vespa magnifica. PLoS ONE 2012, 7, e31920. [Google Scholar] [CrossRef] [PubMed]
- Díaz, C.; Rivera, J.; Lomonte, B.; Bonilla, F.; Diego-García, E.; Camacho, E.; Tytgat, J.; Sasa, M. Venom characterization of the bark scorpion Centruroides edwardsii (Gervais 1843): Composition, biochemical activities and in vivo toxicity for potential prey. Toxicon 2019, 171, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Coates, C.J.; Nairn, J. Diverse immune functions of hemocyanins. Dev. Comp. Immunol. 2014, 45, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Diego-García, E.; Peigneur, S.; Clynen, E.; Marien, T.; Czech, L.; Schoofs, L.; Tytgat, J. Molecular diversity of the telson and venom components from Pandinus cavimanus (Scorpionidae Latreille 1802): Transcriptome, venomics and function. Proteomics 2012, 12, 313–328. [Google Scholar] [CrossRef]
- Salabi, F.; Jafari, H. Differential venom gland gene expression analysis of juvenile and adult scorpions Androctonus crassicauda. BMC Genom. 2022, 23, 636. [Google Scholar] [CrossRef]
- Khamtorn, P.; Rungsa, P.; Jangpromma, N.; Klaynongsruang, S.; Daduang, J.; Tessiri, T.; Daduang, S. Partial proteomic analysis of brown widow spider (Latrodectus geometricus) venom to determine the biological activities. Toxicon X 2020, 8, 100062. [Google Scholar] [CrossRef]
- Liang, S. Proteome and peptidome profiling of spider venoms. Expert Rev. Proteom. 2008, 5, 731–746. [Google Scholar] [CrossRef]
- Dai, C.; Ma, Y.; Zhao, Z.; Zhao, R.; Wang, Q.; Wu, Y.; Cao, Z.; Li, W. Mucroporin, the First Cationic Host Defense Peptide from the Venom of Lychas mucronatus. Antimicrob. Agents Chemother. 2008, 52, 3967–3972. [Google Scholar] [CrossRef]
- Zobel-Thropp, P.A.; Bulger, E.A.; Cordes, M.H.J.; Binord, G.J.; Gillespie, R.G.; Brewer, M.S. Sexually dimorphic venom proteins in long-jawed orb-weaving spiders (Tetragnatha) comprise novel gene families. PeerJ 2018, 6, e4691. [Google Scholar] [CrossRef]
- Verano-Braga, T.; Dutra, A.A.A.; León, I.R.; Melo-Braga, M.N.; Roepstorff, P.; Pimenta, A.M.C.; Kjeldsen, F. Moving Pieces in a Venomic Puzzle: Unveiling Post-translationally Modified Toxins from Tityus serrulatus. J. Proteome Res. 2013, 12, 3460–3470. [Google Scholar] [CrossRef] [PubMed]
- Bodiga, V.; Bodiga, S. Renin-Angiotensin System in Cognitive Function and Dementia. Asian J. Neurosci. 2013, 2013, 102602. [Google Scholar] [CrossRef]
- Isaac, R.E.; Bland, N.D.; Shirras, A.D. Neuropeptides and metabolic inactivation of insect neuropeptides. Gen. Comp. Endocrinol. 2009, 162, 8–17. [Google Scholar] [CrossRef]
- Rastogi, A.; Sarkar, A.; Chakrabarty, D. Partial purification and identification of a metalloproteinase with anticoagulant activity from Rhizostoma pulmo (Barrel Jellyfish). Toxicon 2017, 132, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Murgas, I.; Bermúdez, S.; Miranda, R. First report of accidental envenomation by Ananteris platnicki Lourenço, 1993 (Scorpiones: Buthidae) in Panama. Rev. Med. Pan. 2020, 40, 163–164. [Google Scholar] [CrossRef]
- Cevallos, M.A.; Navarro-Duque, C.; Varela-Julia, M.; Alagon, A.C. Molecular mass determination and assay of venom hyaluronidases by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Toxicon 1992, 30, 925–930. [Google Scholar] [CrossRef]
- Delafontaine, M.; Villas-Boas, I.M.; Mathiew, L.; Josset, P.; Blomet, J.; Tambourgi, D.V. Enzymatic and pro-inflammatory activities of Bothrops lanceolatus venom: Relevance for envenomation. Toxins 2017, 9, 244. [Google Scholar] [CrossRef]
- Araújo Tenorio, H.; da Costa Marques, M.E.; Salgueiro Machado, S.; Juárez Vieira Pereira, H. Angiotensin processing activities in the venom of Thalassophryne nattereri. Toxicon 2015, 98, 49–53. [Google Scholar] [CrossRef]
- Lomonte, B.; Fernández, J. Solving the microheterogeneity of Bothrops asper myotoxin-II by high-resolution mass spectrometry: Insights into C-terminal region variability in Lys49-phospholipase A2 homologs. Toxicon 2022, 210, 123–1314. [Google Scholar] [CrossRef]


| # | Matching Protein, Species | Protein Family | Accession |
|---|---|---|---|
| 1 | Toxin, Mesobuthus eupeus | K+ channel/Ca+2 channel | A0A5P8U2N2 |
| 2 | Toxin, Mesobuthus eupeus | K+ channel | A0A5P8U2Q6 |
| 3 | Toxin, Tityus obscurus | K+ channel | A0A1E1WVV4 |
| 4 | Neurotoxin LmNaTx9, Lychas mucronatus | Na+ channel | A0A0U1S5U0 |
| 5 | Neurotoxin LmNaTx12, Lychas mucronatus | Na+ channel | A0A0U1SJ71 |
| 6 | Neurotoxin LmNaTx18, Lychas mucronatus | Na+ channel | A0A0U1SN99 |
| 7 | LCN-type CS-alpha/beta domain-containing protein, Isometrus maculatus/LmNaTx19, Lychas mucronatus | Na+ channel, LAPβ | A0A0U1S617 |
| 8 | LCN-type CS-alpha/beta domain-containing protein, Isometrus maculatus/ LmNaTx25 Lychas mucronatus | Na+ channel, LAPα | A0A0U1SPD0 |
| 9 | LCN-type CS-alpha/beta domain-containing protein, Isometrus maculatus/LmNaTx30, Lychas mucronatus | Na+ channel | A0A0U1TZ19 |
| 10 | LCN-type CS-alpha/beta domain-containing protein, Isometrus maculatus | Na+ channel | A0A0U1S617 |
| 11 | Venom peptide meuPep27, Mesobuthus eupeus | Na+ channel, LAPα | A0A146CJ25 |
| 12 | Putative Na+ channel toxin, Superstitionia donensis | Na+ channel | A0A1V1WBQ9 |
| 13 | Sodium channel toxin Ts1, Tityus serrulatus | Na+ channel | A0A7S8RFZ4 |
| 14 | Neurotoxin, Tityus obscurus | Na+ channel | A0A1E1WW03 |
| 15 | Alpha-amylase, Tityus obscurus | Amylase | A0A1E1WVL9 |
| 16 | Alpha-amylase, Hadrurus spadix | Amylase | A0A1W7RB82 |
| 17 | Cysteine-rich secretory protein, Centruroides hentzi | CRISP | A0A2I9LNV7 |
| 18 | CAP-Lyc-1, Lychas buchari | CRISP | T1DPC1 |
| 19 | Hyaluronidase, Androctonus crassicauda | Hyaluronidase | A0A7T9L322 |
| 20 | Glyceraldehyde-3-phosphate dehydrogenase, Hadrurus spadix | Oxidorreductase | A0A1W7RAH3 |
| 21 | Putative peptidyl-glycine alpha-hydroxylating monooxygenase, Tityus serrulatus | Oxidase | A0A7S8RGB6 |
| 22 | Angiotensin-converting enzyme, Tityus serrulatus | Peptidase | A0A1E1WWB8 |
| 23 | Neprilysn, Hadrurus spadix | Peptidase | A0A1W7RA38 |
| 24 | Metalloproteinase, Centruroides hentzi | Metalloproteinase | A0A2I9LP89 |
| 25 | Metalloproteinase, Centruroides hentzi | Metalloproteinase | A0A2I9LP68 |
| 26 | Metalloproteinase, Tityus obscurus | Metalloproteinase | A0A1E1WVW3 |
| 27 | Peptidase M14, Isometrus maculatus | Carboxypeptidase | A0A0U1SF04 |
| 28 | Cathepsin spartic protease, Centruroides hentzi | Aspartic protease | A0A2I9LNV0 |
| 29 | Peptidase S1 domain-containing protein, Isometrus maculatus | Serine protease | A0A0U1S633 |
| 30 | Aminopeptidase, Hadrurus spadix | Aminopeptidase | A0A1W7RAL3 |
| 31 | Phospholipase A2 Tityus obscurus | Phospholipase A2 | A0A1E1WVV6 |
| 32 | Phospholipase A2, Hadrurus spadix | Phospholipase A2 | A0A1W7RA16 |
| 33 | Venom protein, Centruroides hentzi/Isometrus maculatus | unclassified | A0A2I9LPX8 |
| 34 | Venom protein, Isometrus maculatus | unclassified | A0A0U1S614 |
| 35 | Venom protein, Mesobuthus eupeus | unclassified | E4VP36 |
| 36 | Ectonucleotide pyrophosphatase/phosphodiesterase, Tityus obscurus | Phosphodiesterase | A0A1E1WVL0 |
| 37 | Hemocyanins, Tityus obscurus/Hadrurus spadix/Pandinus imperator | Hemocyanin | A0A1E1WWC5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, C.; Lomonte, B.; Chang-Castillo, A.; Bonilla, F.; Alfaro-Chinchilla, A.; Triana, F.; Angulo, D.; Fernández, J.; Sasa, M. Venomics of Scorpion Ananteris platnicki (Lourenço, 1993), a New World Buthid That Inhabits Costa Rica and Panama. Toxins 2024, 16, 327. https://doi.org/10.3390/toxins16080327
Díaz C, Lomonte B, Chang-Castillo A, Bonilla F, Alfaro-Chinchilla A, Triana F, Angulo D, Fernández J, Sasa M. Venomics of Scorpion Ananteris platnicki (Lourenço, 1993), a New World Buthid That Inhabits Costa Rica and Panama. Toxins. 2024; 16(8):327. https://doi.org/10.3390/toxins16080327
Chicago/Turabian StyleDíaz, Cecilia, Bruno Lomonte, Arturo Chang-Castillo, Fabián Bonilla, Adriana Alfaro-Chinchilla, Felipe Triana, Diego Angulo, Julián Fernández, and Mahmood Sasa. 2024. "Venomics of Scorpion Ananteris platnicki (Lourenço, 1993), a New World Buthid That Inhabits Costa Rica and Panama" Toxins 16, no. 8: 327. https://doi.org/10.3390/toxins16080327






