An Overview of Electrochemical Sensors Based on Transition Metal Carbides and Oxides: Synthesis and Applications
Abstract
:1. Introduction
2. Syntheses of Transition Metal Carbides and Transition Metal Oxides
2.1. Introduction
2.2. Hydrothermal Method
2.3. Chemical Vapor Deposition Method
2.4. Other Methods
3. Sensing Applications
3.1. Transition Metal Carbides
3.1.1. Gas Electrochemical Sensors
3.1.2. Biological Electrochemical Sensors
3.1.3. Hydrogen Peroxide Electrochemical Sensors
3.2. Transition Metal Oxides
3.2.1. Gas Electrochemical Sensors
| Ref. | Electrode | Analyte | Response Ra/Rg | LOD | Response/Recover Time |
|---|---|---|---|---|---|
| [96] | Ce-TiO2 | NH3 | 23.99 | 140 ppb | 55 s/192 s |
| [97] | TiO2 | H2S | 1120 | 100 ppb | 48 s/5 min |
| [91] | WO3 | NO2 | 12.4 | 400 ppb | - |
| [95] | WO3 | NO2 | - | 25 ppm | 15/0.8 min |
| [97] | WO3/CuO | xylene | - | 15 ppm | 5.5/17.5 s |
| [92] | MoO3 | NO2 | 68 | 10 ppm | 15/150 s |
| [93] | MoO3 | NO2 | 0.05 | 24 ppb | - |
| [66] | Ti3C2Tx | NH3 | 0.21 | 9.27 ppm | - |
| [68] | Ti3C2Tx | Acetone | 0.97 | 50 ppb | - |
| Ethanol | 1.7 | 100 ppb | - | ||
| NH3 | 0.8 | 100 ppb | - | ||
| [72] | Nb2CTx-CTAB | NO2 | 0.66 | 21 ppb | 55 s/60 s |
| [69] | Ti3C2Tx/graphene | NH3 | 0.94 | 10 ppm | - |
| [73] | V2CTx | Acetone | 0.978 | 11.2 ppm | - |
| Methane | 0.983 | 9.4 ppm | 8 min/5.5 min | ||
| H2 | 0.804 | 1.4 ppm | 2 min/7 min | ||
| H2S | 0.995 | 3.5 ppm | - |
3.2.2. Biological Electrochemical Sensors
3.2.3. Hydrogen Peroxide Electrochemical Sensors
4. Summary and Future Outlook
- TMOs and TMCs sensors have not shown good stability at low temperatures (<30 °C) because of their strong oxidation ability. Although the strong oxidation affinity of TMCs and TMOs is good for sensitivity, it might cause serious problems for the stability of the sensors at room temperature, which can be resolved by using different techniques, such as chemical doping and benefiting from composite materials.
- The necessity for rapid reactions is crucial in several applications. However, the sensors stated earlier demonstrated extended response times, indicating a need for additional improvement. This enhancement may be accomplished by optimizing the size, dimension, and material composition of the sensors.
- The manufactured sensors exhibited enhanced durability during extended periods of operation. However, in order to optimize their suitability for industrial applications, it is imperative to further enhance the lifespan of these sensors, particularly for their operation in extreme environmental circumstances.
- It is worthwhile to mention that TMO electrochemical sensor performance can be influenced by capping agents, molecules that are attached to the surface of nanoparticles to prevent them from agglomeration, as they have been used in TMO synthesizing [122]. One of the potential impacts of capping agents might be an improvement in the selectivity and sensitivity of the TMO sensors. This would be due to the fact that capping agents exert an influence on the electronic structure of TMOs, which can change the surface of the sensors bonding to the targeted molecule [123]. Another impact of using capping agents could be reducing the toxicity of the sensors, which is an important parameter, especially in biosensing applications [124]. Capping agents have the potential to enhance the structural and chemical stability of sensors, which can ultimately result in improved stability of sensing performance [122,124]. However, the extent of capping agent influence on the performance of the TMO electrochemical sensors needs further investigation.
- When operating under high potentials in alkaline environments, the surfaces of TMOs and TMCs may be oxidized and transformed to (oxy)hydroxides [125,126]. Consequently, the coordination and electronic properties of the parent metal will be changed by the introduction of these heteroatoms as interstitials or substitutes. The effect of such surface transformations on sensing performance (e.g., sensitivity, detection limit, and long-term stability) should be further investigated.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Govindaraj, M.; Srivastava, A.; Muthukumaran, M.K.; Tsai, P.-C.; Lin, Y.-C.; Raja, B.K.; Rajendran, J.; Ponnusamy, V.K.; Arockia Selvi, J. Current advancements and prospects of enzymatic and non-enzymatic electrochemical glucose sensors. Int. J. Biol. Macromol. 2023, 253, 126680. [Google Scholar] [CrossRef]
- Li, S.; Ma, Q. Electrochemical nano-sensing interface for exosomes analysis and cancer diagnosis. Biosens. Bioelectron. 2022, 214, 114554. [Google Scholar] [CrossRef]
- Borjian, P.; Chimerad, M.; Pathak, P.; Childs, A.; Rajaraman, S.; Cho, H.J. Electrochemical Sensors for Lead Ion Detection Using Sodium Alginate Crosslinked With 2-Acrylamido-2-Methylpropane Sulfonic Acid and Aluminum Microparticles. IEEE Sens. Lett. 2023, 7, 1–4. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Nasri, A.; Pétrissans, M.; Fierro, V.; Celzard, A. Gas sensing based on organic composite materials: Review of sensor types, progresses and challenges. Mater. Sci. Semicond. Process. 2021, 128, 105744. [Google Scholar] [CrossRef]
- Atta, N.F.; Ahmed, R.A.; Amin, H.M.A.; Galal, A. Monodispersed Gold Nanoparticles Decorated Carbon Nanotubes as an Enhanced Sensing Platform for Nanomolar Detection of Tramadol. Electroanalysis 2012, 24, 2135–2146. [Google Scholar] [CrossRef]
- Agnihotri, A.S.; Varghese, A.; Nidhin, M. Transition metal oxides in electrochemical and bio sensing: A state-of-art review. Appl. Surf. Sci. Adv. 2021, 4, 100072. [Google Scholar] [CrossRef]
- Alwarappan, S.; Nesakumar, N.; Sun, D.; Hu, T.Y.; Li, C.-Z. 2D metal carbides and nitrides (MXenes) for sensors and biosensors. Biosens. Bioelectron. 2022, 205, 113943. [Google Scholar] [CrossRef]
- Soltani, M.; Amin, H.M.A.; Cebe, A.; Ayata, S.; Baltruschat, H. Metal-Supported Perovskite as an Efficient Bifunctional Electrocatalyst for Oxygen Reduction and Evolution: Substrate Effect. J. Electrochem. Soc. 2021, 168, 034504. [Google Scholar] [CrossRef]
- Nada, F.; Atta; Galal, A.; Amin, H.M.A. Synthesis and Photoelectrochemical Behavior of a Hybrid Electrode Composed of Polyaniline Encapsulated in Highly Ordered TiO2 Nanotubes Array. Int. J. Electrochem. Sci. 2012, 7, 3610–3626. [Google Scholar] [CrossRef]
- Daneshnazar, M.; Jaleh, B.; Eslamipanah, M.; Varma, R.S. Optical and gas sensing properties of TiO2/RGO for methanol, ethanol and acetone vapors. Inorg. Chem. Commun. 2022, 145, 110014. [Google Scholar] [CrossRef]
- Jia, Y.; Yi, X.; Li, Z.; Zhang, L.; Yu, B.; Zhang, J.; Wang, X.; Jia, X. Recent advance in biosensing applications based on two-dimensional transition metal oxide nanomaterials. Talanta 2020, 219, 121308. [Google Scholar] [CrossRef] [PubMed]
- Maduraiveeran, G.; Sasidharan, M.; Jin, W. Earth-abundant transition metal and metal oxide nanomaterials: Synthesis and electrochemical applications. Prog. Mater. Sci. 2019, 106, 100574. [Google Scholar] [CrossRef]
- Shahzad, F.; Iqbal, A.; Kim, H.; Koo, C.M. 2D Transition Metal Carbides (MXenes): Applications as an Electrically Conducting Material. Adv. Mater. 2020, 32, 2002159. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Iqbal, A.; Zaidi, S.A.; Hwang, S.-W.; Koo, C.M. Nafion-stabilized two-dimensional transition metal carbide (Ti3C2Tx MXene) as a high-performance electrochemical sensor for neurotransmitter. J. Ind. Eng. Chem. 2019, 79, 338–344. [Google Scholar] [CrossRef]
- Jiang, L.; Gu, S.; Ding, Y.; Jiang, F.; Zhang, Z. Facile and novel electrochemical preparation of a graphene-transition metal oxide nanocomposite for ultrasensitive electrochemical sensing of acetaminophen and phenacetin. Nanoscale 2014, 6, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Hwang, J.-Y.; Sun, Y.-K. Transition metal carbide-based materials: Synthesis and applications in electrochemical energy storage. J. Mater. Chem. A 2016, 4, 10379–10393. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Abbas, A.; Amin, H.M.A. Silver nanoparticles modified electrodes for electroanalysis: An updated review and a perspective. Microchem. J. 2022, 175, 107166. [Google Scholar] [CrossRef]
- Rowley-neale, S.J.; Banks, C.E. Recent advances in portable heavy metal electrochemical sensing platforms. Environ. Sci. Water Res. Technol. 2020, 6, 2676–2690. [Google Scholar] [CrossRef]
- March, G.; Nguyen, T.D.; Piro, B. Modified Electrodes Used for Electrochemical Detection of Metal Ions in Environmental Analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef] [PubMed]
- Saputra, H.A. Electrochemical sensors: Basic principles, engineering, and state of the art. Monatshefte Chem.-Chem. Mon. 2023, 154, 1083–1100. [Google Scholar] [CrossRef]
- Tran, V.A.; Tran, N.T.; Doan, V.D.; Nguyen, T.-Q.; Pham Thi, H.H.; Vo, G.N.L. Application Prospects of MXenes Materials Modifications for Sensors. Micromachines 2023, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.; Wang, M.; Koski, K.J.; Zhang, L.; Sokol, H.; Maric, R.; Lei, Y. Molybdenum Trioxide (α-MoO3) Nanoribbons for Ultrasensitive Ammonia (NH3) Gas Detection: Integrated Experimental and Density Functional Theory Simulation Studies. ACS Appl. Mater. Interfaces 2019, 11, 10697–10706. [Google Scholar] [CrossRef] [PubMed]
- Maciulis, V.; Ramanaviciene, A.; Plikusiene, I. Recent Advances in Synthesis and Application of Metal Oxide Nanostructures in Chemical Sensors and Biosensors. Nanomaterials 2022, 12, 4413. [Google Scholar] [CrossRef]
- Uma, S.; Shobana, M.K. Metal oxide semiconductor gas sensors in clinical diagnosis and environmental monitoring. Sens. Actuators A Phys. 2023, 349, 114044. [Google Scholar] [CrossRef]
- Yuan, C.; Ma, J.; Zou, Y.; Li, G.; Xu, H.; Sysoev, V.V.; Cheng, X.; Deng, Y. Modeling Interfacial Interaction between Gas Molecules and Semiconductor Metal Oxides: A New View Angle on Gas Sensing. Adv. Sci. 2022, 9, 2203594. [Google Scholar] [CrossRef]
- Kannan, P.; Maduraiveeran, G. Metal Oxides Nanomaterials and Nanocomposite-Based Electrochemical Sensors for Healthcare Applications. Biosensors 2023, 13, 542. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.; Shi, F.; Zhan, J.; Tu, J.; Fan, H.J. Transition Metal Carbides and Nitrides in Energy Storage and Conversion. Adv. Sci. 2016, 3, 1500286. [Google Scholar] [CrossRef]
- Gogotsi, Y. Transition metal carbides go 2D. Nat. Mater. 2015, 14, 1079–1080. [Google Scholar] [CrossRef]
- Naguib, M.; Barsoum, M.W.; Gogotsi, Y. Ten Years of Progress in the Synthesis and Development of MXenes. Adv. Mater. 2021, 33, 2103393. [Google Scholar] [CrossRef]
- Brewer, L. A most striking confirmation of the Engel metallic correlation. Acta Metall. 1967, 15, 553–556. [Google Scholar] [CrossRef]
- Nandagudi, A.; Nagarajarao, S.H.; Santosh, M.S.; Basavaraja, B.M.; Malode, S.J.; Mascarenhas, R.J.; Shetti, N.P. Hydrothermal synthesis of transition metal oxides, transition metal oxide/carbonaceous material nanocomposites for supercapacitor applications. Mater. Today Sustain. 2022, 19, 100214. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, W.; Li, Y. Hydrothermal synthesis and controlled growth of hierarchical 3D flower-like MoS2 nanospheres assisted with CTAB and their NO2 gas sensing properties. Appl. Surf. Sci. 2018, 455, 276–282. [Google Scholar] [CrossRef]
- Xiao, B.; Zhao, Q.; Xiao, C.; Yang, T.; Wang, P.; Wang, F.; Chen, X.; Zhang, M. Low-temperature solvothermal synthesis of hierarchical flower-like WO3 nanostructures and their sensing properties for H2S. CrystEngComm 2015, 17, 5710–5716. [Google Scholar] [CrossRef]
- Li, G.; Jiang, L.; Pang, S.; Peng, H.; Zhang, Z. Molybdenum Trioxide Nanostructures: The Evolution from Helical Nanosheets to Crosslike Nanoflowers to Nanobelts. J. Phys. Chem. B 2006, 110, 24472–24475. [Google Scholar] [CrossRef]
- Liu, Y.; Jiao, Y.; Zhang, Z.; Qu, F.; Umar, A.; Wu, X. Hierarchical SnO2 Nanostructures Made of Intermingled Ultrathin Nanosheets for Environmental Remediation, Smart Gas Sensor, and Supercapacitor Applications. ACS Appl. Mater. Interfaces 2014, 6, 2174–2184. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, H.; Zhu, M.; Pei, Z.; Li, H.; Wang, Z.; Huang, Y.; Huang, Y.; Deng, Q.; Zhou, J.; et al. Photoluminescent Ti3C2 MXene Quantum Dots for Multicolor Cellular Imaging. Adv. Mater. 2017, 29, 1604847. [Google Scholar] [CrossRef]
- Chen, X.; Sun, X.; Xu, W.; Pan, G.; Zhou, D.; Zhu, J.; Wang, H.; Bai, X.; Dong, B.; Song, H. Ratiometric photoluminescence sensing based on Ti3C2 MXene quantum dots as an intracellular pH sensor. Nanoscale 2018, 10, 1111–1118. [Google Scholar] [CrossRef]
- Liu, Z.; Amin, H.M.A.; Peng, Y.; Corva, M.; Pentcheva, R.; Tschulik, K. Facet-Dependent Intrinsic Activity of Single Co3O4 Nanoparticles for Oxygen Evolution Reaction. Adv. Funct. Mater. 2023, 33, 2210945. [Google Scholar] [CrossRef]
- Roy, M.; Ghosh, S.; Naskar, M.K. Synthesis of morphology controllable porous properties and their catalytic application. Dalt. Trans. 2014, 43, 10248–10257. [Google Scholar] [CrossRef]
- Jaya Prakash, N.; Kandasubramanian, B. Nanocomposites of MXene for industrial applications. J. Alloys Compd. 2021, 862, 158547. [Google Scholar] [CrossRef]
- Ku, C.-A.; Chung, C.-K. Advances in Humidity Nanosensors and Their Application: Review. Sensors 2023, 23, 2328. [Google Scholar] [CrossRef]
- Withanage, S.S.; Khondaker, S.I. Low pressure CVD growth of 2D PdSe2 thin film and its application in PdSe2-MoSe2 vertical heterostructure. 2D Mater. 2022, 9, 25025. [Google Scholar] [CrossRef]
- Aydin, E.; El-Demellawi, J.K.; Yarali, E.; Aljamaan, F.; Sansoni, S.; ur Rehman, A.; Harrison, G.; Kang, J.; El Labban, A.; De Bastiani, M.; et al. Scaled Deposition of Ti3C2Tx MXene on Complex Surfaces: Application Assessment as Rear Electrodes for Silicon Heterojunction Solar Cells. ACS Nano 2022, 16, 2419–2428. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Grützmacher, P.G.; Rosenkranz, A. Novel MXenes—Advanced Synthesis and Tailored Material-Property Design BT—Fundamental Aspects and Perspectives of MXenes. In Engineering Materials; Khalid, M., Grace, A.N., Arulraj, A., Numan, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 325–355. [Google Scholar]
- Wu, S.; Tian, S.; Jian, R.; Wu, T.-N.; Milazzo, T.D.; Luo, T.; Xiong, G. Graphene petal foams with hierarchical micro- and nano-channels for ultrafast spontaneous and continuous oil recovery. J. Mater. Chem. A 2022, 10, 11651–11658. [Google Scholar] [CrossRef]
- Xiong, G.; He, P.; Huang, B.; Chen, T.; Bo, Z.; Fisher, T.S. Graphene nanopetal wire supercapacitors with high energy density and thermal durability. Nano Energy 2017, 38, 127–136. [Google Scholar] [CrossRef]
- Cisquella-Serra, A.; Gamero-Castaño, M.; Ferrer-Argemi, L.; Wardini, J.; Madou, M. Controlled joule-heating of suspended glassy carbon wires for localized chemical vapor deposition. Carbon N. Y. 2020, 156, 329–338. [Google Scholar] [CrossRef]
- Saenz, G.A.; Kaul, A.B. Nanosheets of MoOx crystallites synthesized via chemical vapor deposition and its potential in bolometric applications. Surf. Coat. Technol. 2020, 382, 125031. [Google Scholar] [CrossRef]
- Geng, D.; Zhao, X.; Li, L.; Song, P.; Tian, B.; Liu, W.; Chen, J.; Shi, D.; Lin, M.; Zhou, W.; et al. Controlled growth of ultrathin Mo2C superconducting crystals on liquid Cu surface. 2D Mater. 2017, 4, 11012. [Google Scholar] [CrossRef]
- Geng, D.; Zhao, X.; Chen, Z.; Sun, W.; Fu, W.; Chen, J.; Liu, W.; Zhou, W.; Loh, K.P. Direct Synthesis of Large-Area 2D Mo2C on In Situ Grown Graphene. Adv. Mater. 2017, 29, 1700072. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, L.; Liu, Z.; Chen, L.; Guo, J.; Kang, N.; Ma, X.-L.; Cheng, H.-M.; Ren, W. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Fong, C.-C.; Zhang, X.; Chan, L.L.; Lam, P.K.S.; Chu, P.K.; Wong, K.-Y.; Yang, M. Au Nanoparticles Decorated TiO2 Nanotube Arrays as a Recyclable Sensor for Photoenhanced Electrochemical Detection of Bisphenol A. Environ. Sci. Technol. 2016, 50, 4430–4438. [Google Scholar] [CrossRef] [PubMed]
- Chekin, F.; Vahdat, S.M.; Asadi, M.J. Green synthesis and characterization of cobalt oxide nanoparticles and its electrocatalytic behavior. Russ. J. Appl. Chem. 2016, 89, 816–822. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, J.; Yi, S.; Wan, X.; Tang, J. Biodegradable and photostable Nb2C MXene quantum dots as promising nanofluorophores for metal ions sensing and fluorescence imaging. Sens. Actuators B Chem. 2020, 309, 127735. [Google Scholar] [CrossRef]
- Yu, X.; Cai, X.; Cui, H.; Lee, S.-W.; Yu, X.-F.; Liu, B. Fluorine-free preparation of titanium carbide MXene quantum dots with high near-infrared photothermal performances for cancer therapy. Nanoscale 2017, 9, 17859–17864. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, C.; Duan, M.; Tang, Y.; Zhu, J. TiO2 nanoparticle modified organ-like Ti3C2 MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances. Biosens. Bioelectron. 2015, 74, 1022–1028. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Pandey, R.P.; Jabbar, K.A.; Ponraj, J.; Mahmoud, K.A. Sensitive electrochemical detection of l-cysteine based on a highly stable Pd@Ti3C2Tx (MXene) nanocomposite modified glassy carbon electrode. Anal. Methods 2019, 11, 3851–3856. [Google Scholar] [CrossRef]
- Yan, X.; Ma, J.; Yu, K.; Li, J.; Yang, L.; Liu, J.; Wang, J.; Cai, L. Highly green fluorescent Nb2C MXene quantum dots for Cu2+ ion sensing and cell imaging. Chin. Chem. Lett. 2020, 31, 3173–3177. [Google Scholar] [CrossRef]
- Ramki, S.; Sukanya, R.; Chen, S.-M.; Sakthivel, M. Hierarchical multi-layered molybdenum carbide encapsulated oxidized carbon nanofiber for selective electrochemical detection of antimicrobial agents: Inter-connected path in multi-layered structure for efficient electron transfer. Inorg. Chem. Front. 2019, 6, 1680–1693. [Google Scholar] [CrossRef]
- Jia, Z.; Li, Z.; Ma, S.; Zhang, W.; Chen, Y.; Luo, Y.; Jia, D.; Zhong, B.; Razal, J.M.; Wang, X.; et al. Constructing conductive titanium carbide nanosheet (MXene) network on polyurethane/polyacrylonitrile fibre framework for flexible strain sensor. J. Colloid Interface Sci. 2021, 584, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vovusha, H.; Amorim, R.G.; Bae, H.; Lee, S.; Hussain, T.; Lee, H. Sensing of sulfur containing toxic gases with double transition metal carbide MXenes. Mater. Today Chem. 2023, 30, 101543. [Google Scholar] [CrossRef]
- Obodo, K.O.; Ouma, C.N.M.; Obodo, J.T.; Gebreyesus, G.; Rai, D.P.; Ukpong, A.M.; Bouhafs, B. Sn3C2 monolayer with transition metal adatom for gas sensing: A density functional theory studies. Nanotechnology 2021, 32, 355502. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Y.; Cheng, J.; Liu, Z.; Li, Q.; Li, W.; Yang, X.; Xiao, B. Monolayer Ti2CO2: A Promising Candidate for NH3 Sensor or Capturer with High Sensitivity and Selectivity. ACS Appl. Mater. Interfaces 2015, 7, 13707–13713. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; VahidMohammadi, A.; Prorok, B.C.; Yoon, Y.S.; Beidaghi, M.; Kim, D.-J. Room Temperature Gas Sensing of Two-Dimensional Titanium Carbide (MXene). ACS Appl. Mater. Interfaces 2017, 9, 37184–37190. [Google Scholar] [CrossRef] [PubMed]
- Kahn, N.; Lavie, O.; Paz, M.; Segev, Y.; Haick, H. Dynamic Nanoparticle-Based Flexible Sensors: Diagnosis of Ovarian Carcinoma from Exhaled Breath. Nano Lett. 2015, 15, 7023–7028. [Google Scholar] [CrossRef]
- Kim, S.J.; Koh, H.-J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.-Y.; Anasori, B.; Kim, C.-K.; Choi, Y.-K.; Kim, J.; et al. Metallic Ti3C2Tx MXene Gas Sensors with Ultrahigh Signal-to-Noise Ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef]
- Lee, S.H.; Eom, W.; Shin, H.; Ambade, R.B.; Bang, J.H.; Kim, H.W.; Han, T.H. Room-Temperature, Highly Durable Ti3C2Tx MXene/Graphene Hybrid Fibers for NH3 Gas Sensing. ACS Appl. Mater. Interfaces 2020, 12, 10434–10442. [Google Scholar] [CrossRef]
- Li, L.; Cao, H.; Liang, Z.; Cheng, Y.; Yin, T.; Liu, Z.; Yan, S.; Jia, S.; Li, L.; Wang, J.; et al. First-Principles Study of Ti-Deficient Ti3C2 MXene Nanosheets as NH3 Gas Sensors. ACS Appl. Nano Mater. 2022, 5, 2470–2475. [Google Scholar] [CrossRef]
- Yang, D.; Fan, X.; Zhao, D.; An, Y.; Hu, Y.; Luo, Z. Sc2CO2 and Mn-doped Sc2CO2 as gas sensor materials to NO and CO: A first-principles study. Phys. E Low-Dimens. Syst. Nanostruct. 2019, 111, 84–90. [Google Scholar] [CrossRef]
- Rathi, K.; Arkoti, N.K.; Pal, K. Fabrication of Delaminated 2D Metal Carbide MXenes (Nb2CTx) by CTAB-based NO2 Gas Sensor with Enhanced Stability. Adv. Mater. Interfaces 2022, 9, 2200415. [Google Scholar] [CrossRef]
- Lee, E.; VahidMohammadi, A.; Yoon, Y.S.; Beidaghi, M.; Kim, D.-J. Two-Dimensional Vanadium Carbide MXene for Gas Sensors with Ultrahigh Sensitivity Toward Nonpolar Gases. ACS Sens. 2019, 4, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Vatandoost, N.; Ghanbari, J.; Mojaver, M.; Avan, A.; Ghayour-Mobarhan, M.; Nedaeinia, R.; Salehi, R. Early detection of colorectal cancer: From conventional methods to novel biomarkers. J. Cancer Res. Clin. Oncol. 2016, 142, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Wang, F.; Zhang, Y.; Wang, H.; Liu, Y. Ti3C2 MXene mediated Prussian blue in situ hybridization and electrochemical signal amplification for the detection of exosomes. Talanta 2021, 224, 121879. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Singhal, A.; Kumar, N.; Khan, R.; Khan, M.A.; Srivastava, A.K. Next-Generation Intelligent MXene-Based Electrochemical Aptasensors for Point-of-Care Cancer Diagnostics. Nano-Micro Lett. 2022, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. MXenes for Cancer Therapy and Diagnosis: Recent Advances and Current Challenges. ACS Biomater. Sci. Eng. 2021, 7, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wei, Y.; Jiao, S.; Zhu, S.; Liu, X. A novel label-free strategy for the ultrasensitive miRNA-182 detection based on MoS2/Ti3C2 nanohybrids. Biosens. Bioelectron. 2019, 137, 45–51. [Google Scholar] [CrossRef]
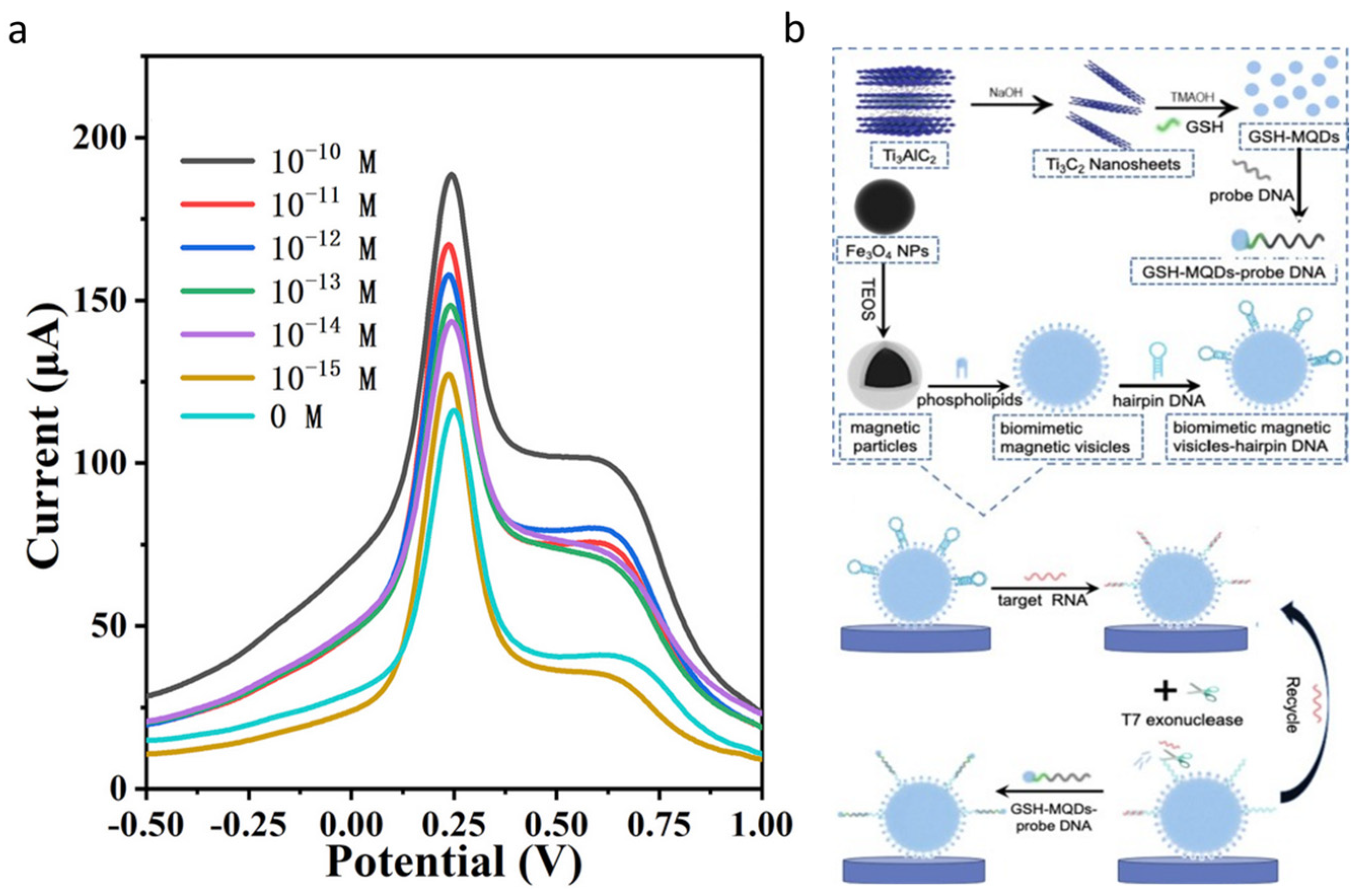
- Li, Z.; Wang, Z.; Nie, Y.; Wang, P.; Ma, Q. A novel GSH-capping MXene QD-based ECL biosensor for the detection of miRNA221 in triple-negative breast cancer tumor. Chem. Eng. J. 2022, 448, 137636. [Google Scholar] [CrossRef]
- Ibrahim, M.R.; Greish, Y.E. MOF-Based Biosensors for the Detection of Carcinoembryonic Antigen: A Concise Review. Molecules 2023, 28, 5970. [Google Scholar] [CrossRef]
- Kumar, S.; Lei, Y.; Alshareef, N.H.; Quevedo-Lopez, M.A.; Salama, K.N. Biofunctionalized two-dimensional Ti3C2 MXenes for ultrasensitive detection of cancer biomarker. Biosens. Bioelectron. 2018, 121, 243–249. [Google Scholar] [CrossRef]
- Li, G.; Zhu, X.; Liu, J.; Li, S.; Liu, X. Metal Oxide Semiconductor Gas Sensors for Lung Cancer Diagnosis. Chemosensors 2023, 11, 251. [Google Scholar] [CrossRef]
- Wang, H.; Shi, X.; Liu, F.; Duan, T.; Sun, B. Non-Invasive Rapid Detection of Lung Cancer Biomarker Toluene with a Cataluminescence Sensor Based on the Two-Dimensional Nanocomposite Pt/Ti3C2Tx-CNT. Chemosensors 2022, 10, 333. [Google Scholar] [CrossRef]
- Reji, R.P.; Balaji, S.K.C.; Sivalingam, Y.; Kawazoe, Y.; Velappa Jayaraman, S. First-Principles Density Functional Theory Calculations on the Potential of Sc2CO2 MXene Nanosheets as a Dual-Mode Sensor for Detection of Volatile Organic Compounds in Exhaled Human Breath. ACS Appl. Nano Mater. 2023, 6, 5345–5356. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W. Recent advances in graphene-based nanomaterials for fabricating electrochemical hydrogen peroxide sensors. Biosens. Bioelectron. 2017, 89, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Annalakshmi, M.; Balasubramanian, P.; Chen, S.-M.; Chen, T.-W. Enzyme-free electrocatalytic sensing of hydrogen peroxide using a glassy carbon electrode modified with cobalt nanoparticle-decorated tungsten carbide. Microchim. Acta 2019, 186, 265. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, C.; Liang, T.; Tang, C.; Lv, X.; Tang, K.; Li, C.M. Porous Molybdenum Carbide Nanostructured Catalyst toward Highly Sensitive Biomimetic Sensing of H2O2. Electroanalysis 2020, 32, 1243–1250. [Google Scholar] [CrossRef]
- Li, B.; Kong, D.-R.; Liu, L.-H.; Yang, M.; Zhang, X.-F.; Deng, Z.-P.; Huo, L.-H.; Gao, S. Facile synthesis of copper and carbon co-doped peanut shell-like Mo2C/Mo3P electrocatalysts for ultra-sensitive amperometric detection of hydrogen peroxide. Microchem. J. 2022, 181, 107795. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, X.; Yang, X.; Zhao, C.; Zhang, Y.; Qu, S.; Wu, S.; Ji, W. Analytical Methods Reasonable design of an MXene-based enzyme- free amperometric sensing interface for highly sensitive hydrogen peroxide detection. J. Electrochem. Soc. 2021, 13, 2512–2518. [Google Scholar] [CrossRef]
- Pula, P.; Leniart, A.A.; Krol, J.; Gorzkowski, M.T.; Suster, M.C.; Wrobel, P.; Lewera, A.; Majewski, P.W. Block Copolymer-Templated, Single-Step Synthesis of Transition Metal Oxide Nanostructures for Sensing Applications. ACS Appl. Mater. Interfaces 2023, 15, 57970–57980. [Google Scholar] [CrossRef]
- Maciak, E.; Opilski, Z. Transition metal oxides covered Pd film for optical H2 gas detection. Thin Solid Films 2007, 515, 8351–8355. [Google Scholar] [CrossRef]
- Mane, A.A.; Moholkar, A. V Orthorhombic MoO3 nanobelts based NO2 gas sensor. Appl. Surf. Sci. 2017, 405, 427–440. [Google Scholar] [CrossRef]
- Li, W.; Xing, K.; Liu, P.; Chuang, C.; Lu, Y.-R.; Chan, T.-S.; Tesfamichael, T.; Motta, N.; Qi, D.-C. Ultrasensitive NO2 Gas Sensors Based on Layered α-MoO3 Nanoribbons. Adv. Mater. Technol. 2022, 7, 2100579. [Google Scholar] [CrossRef]
- Giancaterini, L.; Emamjomeh, S.M.; De Marcellis, A.; Palange, E.; Resmini, A.; Anselmi-Tamburini, U.; Cantalini, C. The influence of thermal and visible light activation modes on the NO2 response of WO3 nanofibers prepared by electrospinning. Sens. Actuators B Chem. 2016, 229, 387–395. [Google Scholar] [CrossRef]
- Morais, P.V.; Suman, P.H.; Silva, R.A.; Orlandi, M.O. High gas sensor performance of WO3 nanofibers prepared by electrospinning. J. Alloys Compd. 2021, 864, 158745. [Google Scholar] [CrossRef]
- Wu, K.; Debliquy, M.; Zhang, C. Room temperature gas sensors based on Ce doped TiO2 nanocrystals for highly sensitive NH3 detection. Chem. Eng. J. 2022, 444, 136449. [Google Scholar] [CrossRef]
- Nagmani; Pravarthana, D.; Tyagi, A.; Jagadale, T.C.; Prellier, W.; Aswal, D.K. Highly sensitive and selective H2S gas sensor based on TiO2 thin films. Appl. Surf. Sci. 2021, 549, 149281. [Google Scholar] [CrossRef]
- Kamble, C.; Panse, M.; Nimbalkar, A. Ag decorated WO3 sensor for the detection of sub-ppm level NO2 concentration in air. Mater. Sci. Semicond. Process. 2019, 103, 104613. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Y.; Kong, F.; Wang, S.; Zhu, B.; Guo, X.; Zhang, J.; Wang, Y.; Wu, S. Au-doped WO3-based sensor for NO2 detection at low operating temperature. Sens. Actuators B Chem. 2008, 134, 133–139. [Google Scholar] [CrossRef]
- Guo, M.; Luo, N.; Chen, Y.; Fan, Y.; Wang, X.; Xu, J. Fast-response MEMS xylene gas sensor based on CuO/WO3 hierarchical structure. J. Hazard. Mater. 2022, 429, 127471. [Google Scholar] [CrossRef]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of Nanoparticles in Electrochemical Sensors and Biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- Jalil, O.; Pandey, C.M.; Kumar, D. Electrochemical biosensor for the epithelial cancer biomarker EpCAM based on reduced graphene oxide modified with nanostructured titanium dioxide. Microchim. Acta 2020, 187, 275. [Google Scholar] [CrossRef] [PubMed]
- Scremin, J.; Barbosa, E.C.M.; Salamanca-Neto, C.A.R.; Camargo, P.H.C.; Sartori, E.R. Amperometric determination of ascorbic acid with a glassy carbon electrode modified with TiO2-gold nanoparticles integrated into carbon nanotubes. Microchim. Acta 2018, 185, 251. [Google Scholar] [CrossRef] [PubMed]
- Anshori, I.; Kepakisan, K.A.A.; Nuraviana Rizalputri, L.; Rona Althof, R.; Nugroho, A.E.; Siburian, R.; Handayani, M. Facile synthesis of graphene oxide/Fe3O4 nanocomposite for electrochemical sensing on determination of dopamine. Nanocomposites 2022, 8, 155–166. [Google Scholar] [CrossRef]
- Riahifar, V.; Haghnazari, N.; Keshavarzi, F.; Ahmadi, E. A sensitive voltammetric sensor for methamphetamine determination based on modified glassy carbon electrode using Fe3O4@poly pyrrole core-shell and graphene oxide. Microchem. J. 2021, 170, 106748. [Google Scholar] [CrossRef]
- Dinani, H.S.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Ebrahimi, S.A.S.; Shayeh, J.S.; Ghorbani, M. Fabrication of Au/Fe3O4/RGO based aptasensor for measurement of miRNA-128, a biomarker for acute lymphoblastic leukemia (ALL). Eng. Life Sci. 2022, 22, 519–534. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Chen, Z.; Zuo, X. Innovative Electrochemical Sensor Using TiO2 Nanomaterials to Detect Phosphopeptides. Anal. Chem. 2021, 93, 10635–10643. [Google Scholar] [CrossRef]
- Kiranmai, S.; Kuchi, C.; Sravani, B.; Ƚuczak, T.; Kim, M.J.; Madhavi, G.; Veera Manohara Reddy, Y. Construction of ultrasensitive electrochemical sensor using TiO2-reduced graphene oxide nanofibers nanocomposite for epinephrine detection. Surf. Interfaces 2022, 35, 102455. [Google Scholar] [CrossRef]
- Madhu, S.; Ramasamy, S.; Manickam, P.; Nagamony, P.; Chinnuswamy, V. TiO2 anchored carbon fibers as non-invasive electrochemical sensor platform for the cortisol detection. Mater. Lett. 2022, 308, 131238. [Google Scholar] [CrossRef]
- Chen, Z.; Li, B.; Liu, J.; Li, H.; Li, C.; Xuan, X.; Li, M. A label-free electrochemical immunosensor based on a gold–vertical graphene/TiO2 nanotube electrode for CA125 detection in oxidation/reduction dual channels. Microchim. Acta 2022, 189, 257. [Google Scholar] [CrossRef]
- Mohamad Nor, N.; Abdul Razak, K.; Lockman, Z. Physical and Electrochemical Properties of Iron Oxide Nanoparticles-modified Electrode for Amperometric Glucose Detection. Electrochim. Acta 2017, 248, 160–168. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, X.; Yang, D.; Yuwen, L.; Zhu, X.; Weng, L.; Wang, L. Synthesis of highly dispersed titanium dioxide nanoclusters on reduced graphene oxide for increased glucose sensing. Carbon 2013, 57, 470–476. [Google Scholar] [CrossRef]
- Arvand, M.; Orangpour, S.; Ghodsi, N. RSC Advances determination of the antipsychotic medication olanzapine at a magnetic nano-composite with a. RSC Adv. 2015, 5, 46095–46103. [Google Scholar] [CrossRef]
- Teymourian, H.; Salimi, A.; Khezrian, S. Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens. Bioelectron. 2013, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhao, W.; Zhang, Y.; Jiang, Q.; He, J.-H.; Baeumner, A.J.; Wolfbeis, O.S.; Wang, Z.L.; Salama, K.N.; Alshareef, H.N. A MXene-Based Wearable Biosensor System for High-Performance In Vitro Perspiration Analysis. Small 2019, 15, 1901190. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Alam, M.M.; Asiri, A.M.; Alsaiari, M.; Saad Alruwais, R.; Jalalah, M.; Madkhali, O.; Rahman, M.M.; Harraz, F.A. Detection of hydrogen peroxide with low-dimensional silver nanoparticle-decorated PPy-C/TiO2 nanocomposites by electrochemical approach. J. Electroanal. Chem. 2023, 928, 117030. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, G.; Yi, H.; Jia, G.; Li, Z.; Yuan, C.; Bai, Y.; Fu, D. Interfacial Engineering of Hierarchical Transition Metal Oxide Heterostructures for Highly Sensitive Sensing of Hydrogen Peroxide. Small 2018, 14, 1703713. [Google Scholar] [CrossRef] [PubMed]
- Sobahi, N.; Imran, M.; Khan, M.E.; Mohammad, A.; Alam, M.M.; Yoon, T.; Mehedi, I.M.; Hussain, M.A.; Abdulaal, M.J.; Jiman, A.A. Electrochemical Sensing of H2O2 by Employing a Flexible Fe3O4/Graphene/Carbon Cloth as Working Electrode. Materials 2023, 16, 2770. [Google Scholar] [CrossRef]
- Kaplan, S.; Suna Karatekin, R.; Kahya Dudukcu, M.; Avcı, G. A novel Ni–Fe3O4@s-rGO/GCE electrode for electrochemical detection of H2O2. Mater. Chem. Phys. 2023, 294, 127051. [Google Scholar] [CrossRef]
- Rathinam, R.; Singh, D.P.; Dutta, A.; Rudresha, S.; Ali, S.R.; Chatterjee, P. TiO2 Nanoparticles Based Peroxidase Mimics for Colorimetric Sensing of Cholesterol and Hydrogen Peroxide. Adv. Sci. Technol. 2022, 117, 85–90. [Google Scholar] [CrossRef]
- Suna Karatekin, R. TiO2 nanoparticles supported on N–S co-doped rGO as electrocatalyst for non-enzymatic H2O2 sensing. J. Appl. Electrochem. 2023, 53, 2273–2284. [Google Scholar] [CrossRef]
- Pooja; Barman, P.B.; Hazra, S.K. Role of Capping Agent in Palladium Nanoparticle Based Hydrogen Sensor. J. Clust. Sci. 2018, 29, 1209–1216. [Google Scholar] [CrossRef]
- Munyayi, T.A.; Vorster, B.C.; Mulder, D.W. The Effect of Capping Agents on Gold Nanostar Stability, Functionalization, and Colorimetric Biosensing Capability. Nanomaterials 2022, 12, 2470. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; ul Ain, N.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Bera, S.; Woo, H.; Kim, H.G.; Baek, J.-H.; Hong, W.; Park, J.-Y.; Oh, S.-J.; Kwon, S.-H. In Situ Engineering of a Metal Oxide Protective Layer into Pt/Carbon Fuel-Cell Catalysts by Atomic Layer Deposition. Chem. Mater. 2022, 34, 5949–5959. [Google Scholar] [CrossRef]
- Ledendecker, M.; Krick Calderón, S.; Papp, C.; Steinrück, H.-P.; Antonietti, M.; Shalom, M. The Synthesis of Nanostructured Ni5P4 Films and their Use as a Non-Noble Bifunctional Electrocatalyst for Full Water Splitting. Angew. Chem. Int. Ed. 2015, 54, 12361–12365. [Google Scholar] [CrossRef]
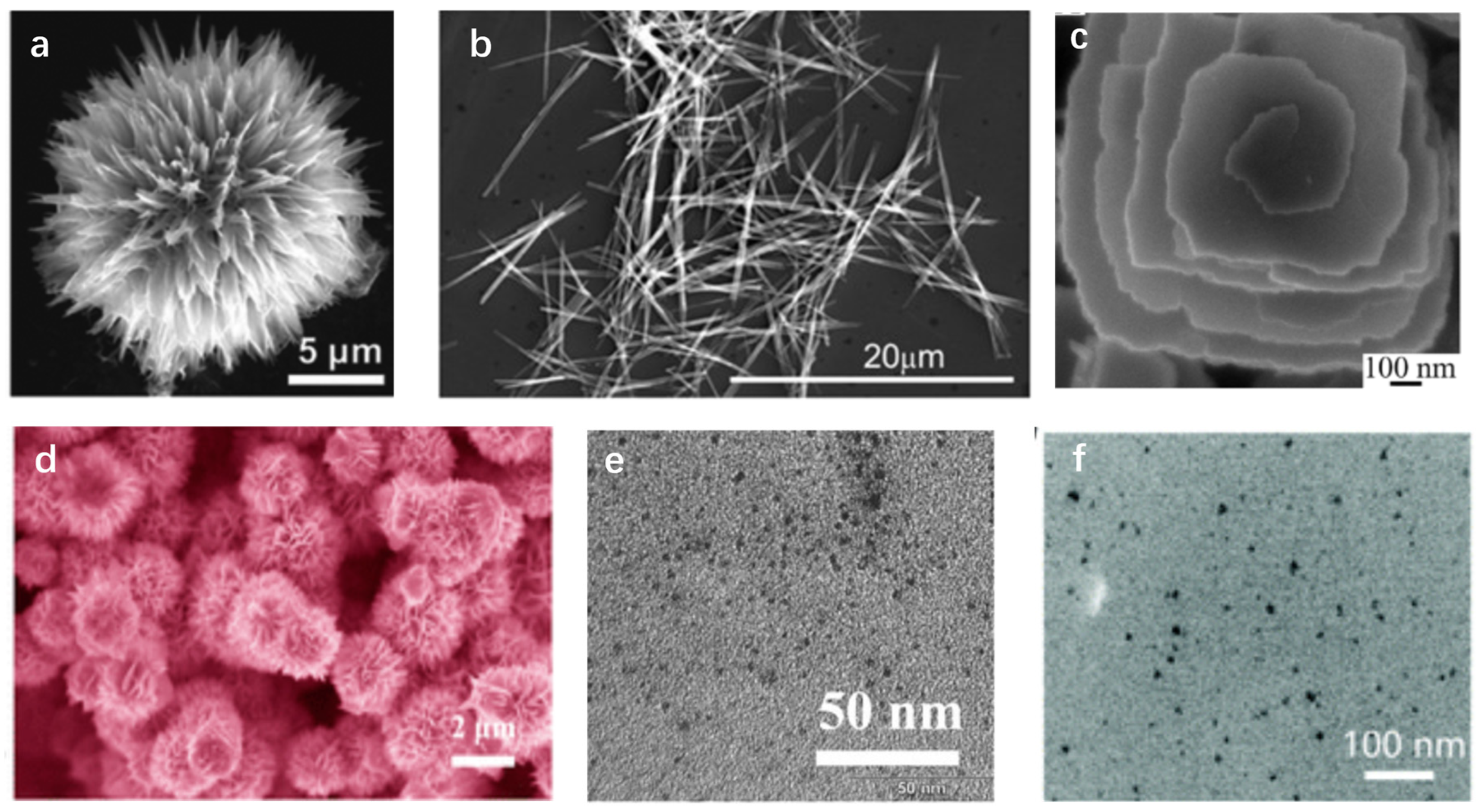
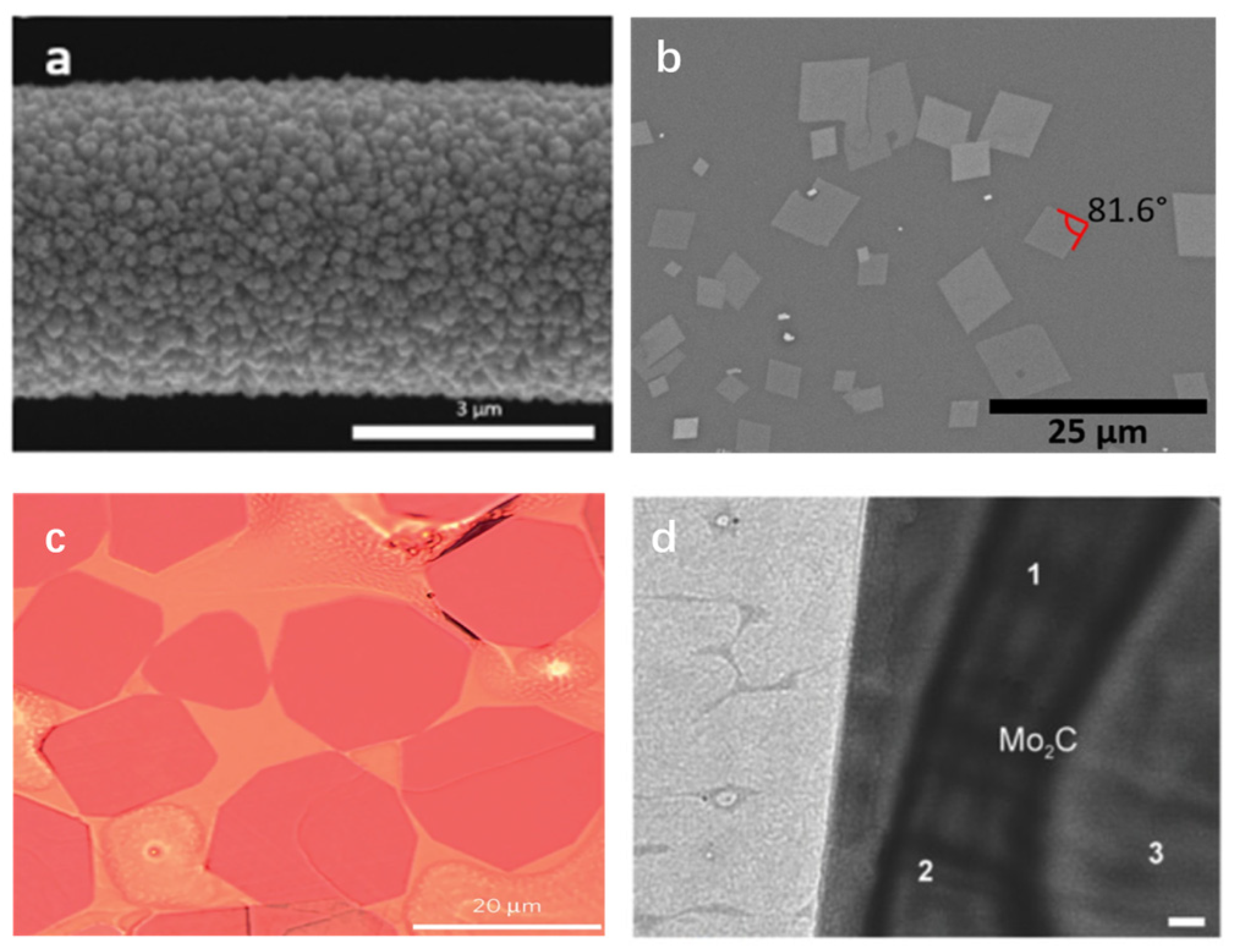
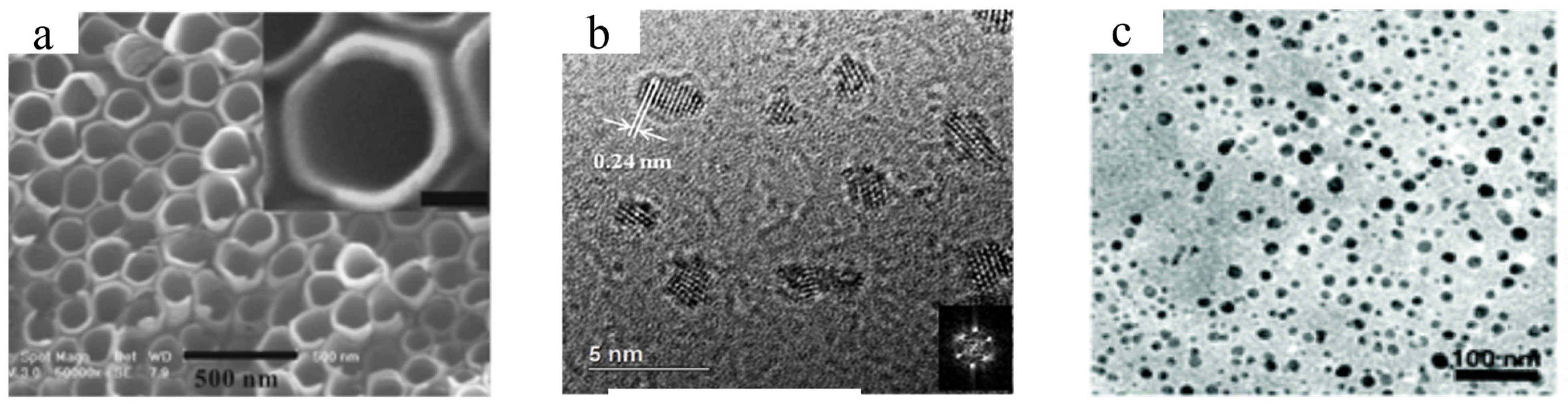

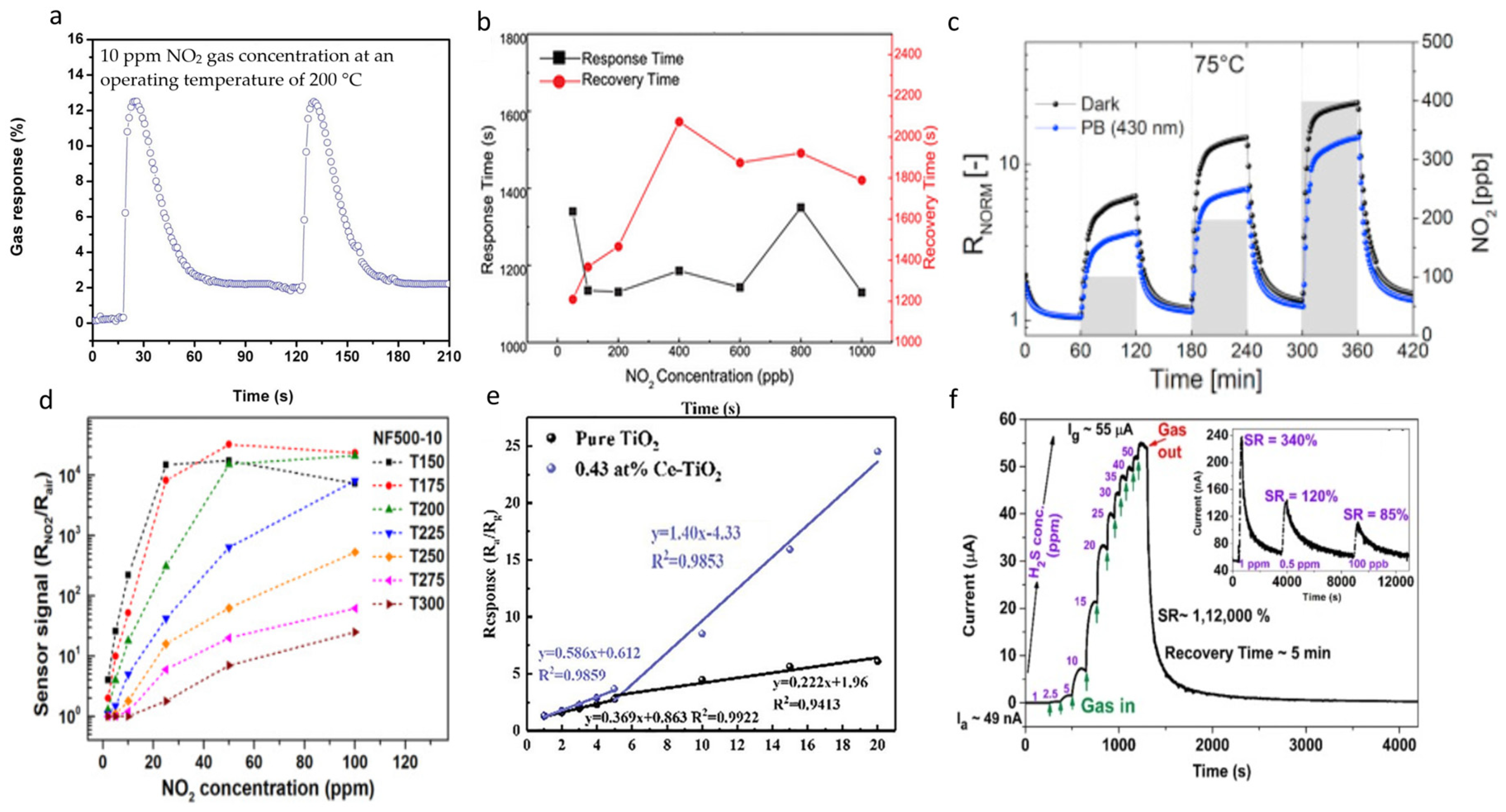
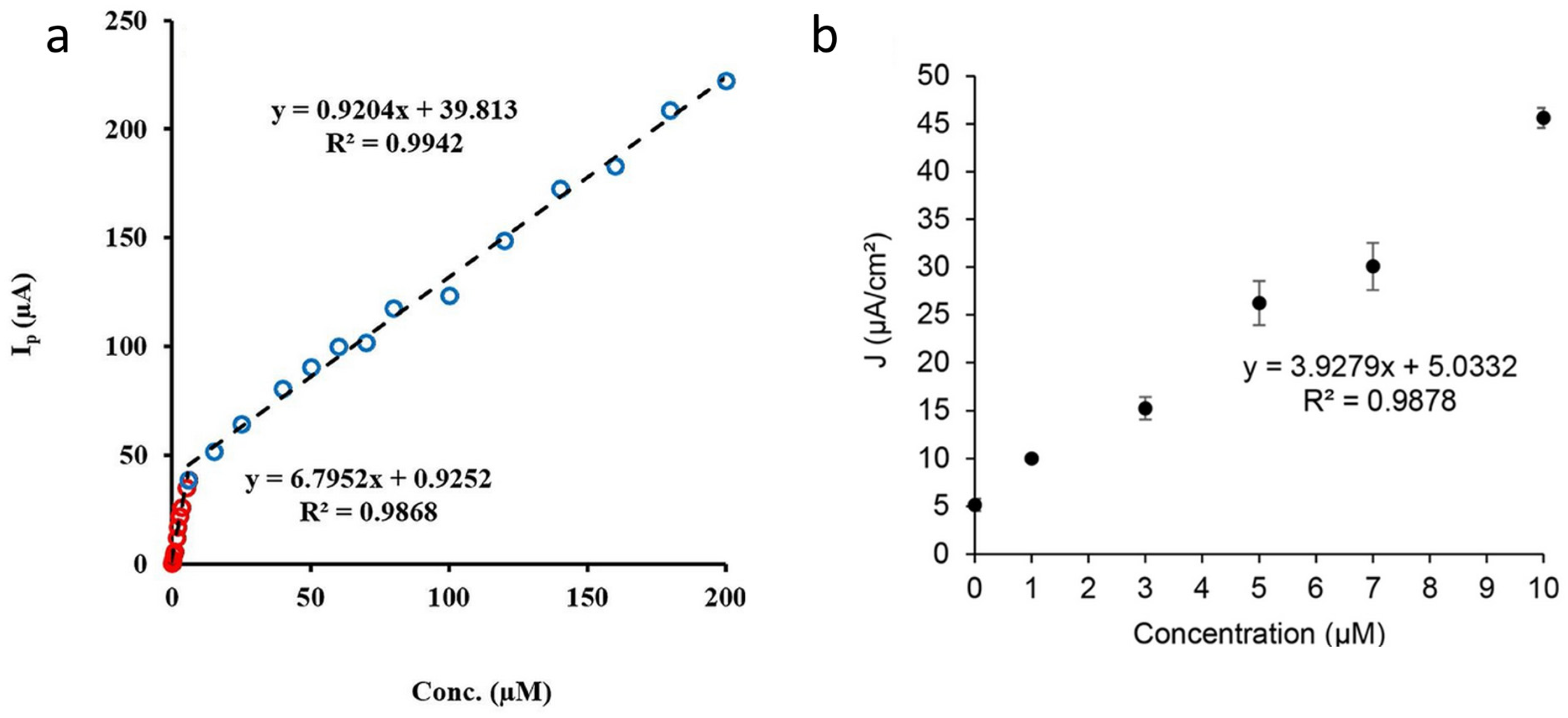
| Synthesis Method | Advantage | Disadvantage |
|---|---|---|
| Chemical Vapor Deposition (CVD) Method | High Purity: Capable of producing materials with high purity levels. Uniformity: Offers high uniformity in film thickness and composition. Precision Control: Allows precise control over film thickness and composition through parameter adjustment. | High Cost: Associated with higher equipment and operational costs. Temperature Constraints: Requires high temperatures, potentially unsuitable for thermally sensitive materials. Complexity: The process is relatively complex and requires meticulous operation. |
| Hydrothermal Method | Low-Temperature Synthesis: Generally, operates at lower temperatures. Environmentally Friendly: Utilizes water as a solvent, minimizing environmental impact. Crystallinity: Capable of producing high-quality crystals. | Long Reaction Times: Typically requires longer durations to complete reactions. Size Control Challenges: Control over particle size and shape is relatively difficult. Scalability Limitations: Limited scalability for large-scale production. |
| Controlled Electrodeposition Method | Precise Control: Enables precise control over the thickness and composition of the deposited material. Cost Effectiveness: Generally lower in equipment and operational costs compared to other methods. Low-Temperature Operation: Conducted at room temperature or lower, making it suitable for thermally sensitive materials. Versatility: Applicable to a wide range of materials, including nanomaterials. | Uniformity Issues: Sometimes challenging to ensure uniform deposition layers. Scale Limitations: Challenges in achieving deposition over large areas. Electrochemical Complexity: Involves complex electrochemical processes, requiring skilled operation. |
| Acid Etching Method | High Precision: Allows for accurate control of etch depth and shape. Selectivity: Specific materials can be targeted for etching by choosing appropriate acids. Wide Applicability: Usable on a variety of materials. | Hazardous: Hydrofluoric acid is extremely dangerous, necessitating stringent safety measures. Environmental Impact: environmental pollution. Control Complexity: The etching process can be complex to control, requiring meticulous adjustment. |
| Ref. | Electrode | Analyte | Sensitivity | LOD | Linear Range |
|---|---|---|---|---|---|
| [111] | Iron oxide | Glucose | 5.31 μA·mM−1·cm−2 | 7 μM | 0.25–8 mM |
| [103] | TiO2/Au | Ascorbic acid | 35,900 μA·mM−1·cm−2 | 1.2 µM | 5–51 µM |
| [112] | TiO2/rGO | Glucose | 35.8 μA·mM−1·cm−2 | 4.8 µM | 0.032–1.67 mM |
| [113] | Fe3O4@Ag/CPE | Olanzapine | 0.50 µA·mM−1 | 0.0018 µM | 0.39–38.4 µM |
| [114] | Fe3O4/GO | Dopamine uric acid | 0.12 µA·mM−1 | 0.053 µM | 0.1–150 µM |
| [102] | rGO/TiO2 | Epithelial cell adhesion molecules | 3.24 µA·ng−1·mL·cm−2 | 0.0065 ng·mL−1 | 0.01–60 ng·mL−1 |
| [104] | Fe3O4/GO | Dopamine | - | 0.48 µM | 1–10 µM |
| [110] | Au–VG/TiO2 | Cancer antigen 125 | 14.82 μA·(log(mU·mL−1))−1 | 0.0001 mU·mL−1 | 0.01–1000 mU·mL−1 |
| [115] | Ti3C2Tx | Glucose | 35.3 μA·mM−1·cm−2 | 0.33 μM | 10–1500 μM |
| Lactate | 11.4 μA·mM−1·cm−2 | 0.67 μM | 10–22,000 μM | ||
| [81] | f–Ti3C2 | CEA | 37.9 µA·ng−1·mL·cm−2 | 18 fg/mL | 100 fg/mL−2 μg/mL |
| [78] | MoS2/Ti3C2 | miRNA-182 | - | 0.43 fM | 1 fM to 0.1 nM |
| [79] | GSH-MQDs | miRNA-221 | - | 10 fM | 10 fM to 10 nM |
| Ref. | Electrode | Sensitivity | Detection Limit | Linear Range |
|---|---|---|---|---|
| [118] | Fe3O4/Graphene | 0.037 µA·μM−1·cm−2 | 4.79 μM | 10 to 110 μM |
| [119] | Ni–Fe3O4@s-rGO | 6.012 µA·μM−1 | 0.2 μM | 1 to 1000 μM |
| [120] | TiO2 nano-particles | N/A | 0.061 mM | 0.1 to 50 mM |
| [121] | TiO2@NS-rGO | 0.188 µA·μM−1 | 0.019 µM | 2 to 1000 μM |
| [86] | WC/CoNP | 6.696 μA·μM−1·cm−2 | 6.3 nM | 50 nM to 1.0 mM |
| [87] | p-Mo2C/NC | 0.577 μA·μM−1·cm−2 | 0.22 μM | 0.05 to 4.5 mM |
| [88] | Cu-Mo2C/Mo3P/C | 0.653 μA·μM−1·cm−2 | 37 nM | 0.55 μM to 2.06 mM |
| [89] | MX/CS/PB/GCE | N/A | 4 nM | 50 nM to 667 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashhadian, A.; Jian, R.; Tian, S.; Wu, S.; Xiong, G. An Overview of Electrochemical Sensors Based on Transition Metal Carbides and Oxides: Synthesis and Applications. Micromachines 2024, 15, 42. https://doi.org/10.3390/mi15010042
Mashhadian A, Jian R, Tian S, Wu S, Xiong G. An Overview of Electrochemical Sensors Based on Transition Metal Carbides and Oxides: Synthesis and Applications. Micromachines. 2024; 15(1):42. https://doi.org/10.3390/mi15010042
Chicago/Turabian StyleMashhadian, Amirarsalan, Ruda Jian, Siyu Tian, Shiwen Wu, and Guoping Xiong. 2024. "An Overview of Electrochemical Sensors Based on Transition Metal Carbides and Oxides: Synthesis and Applications" Micromachines 15, no. 1: 42. https://doi.org/10.3390/mi15010042





