Broadband Near-Infrared Emission from Bi/Cr Co-Doped Aluminosilicate Glasses
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials Preparation
2.2. Characterization
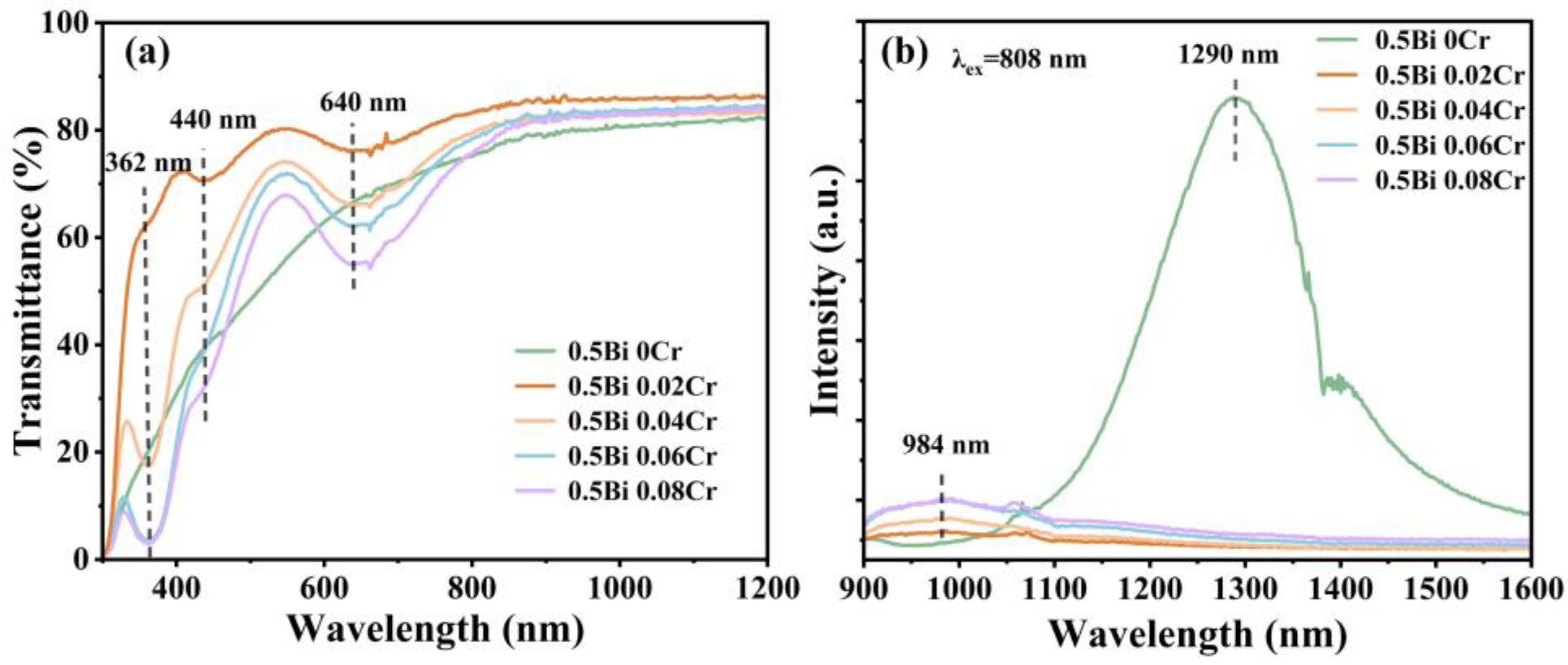
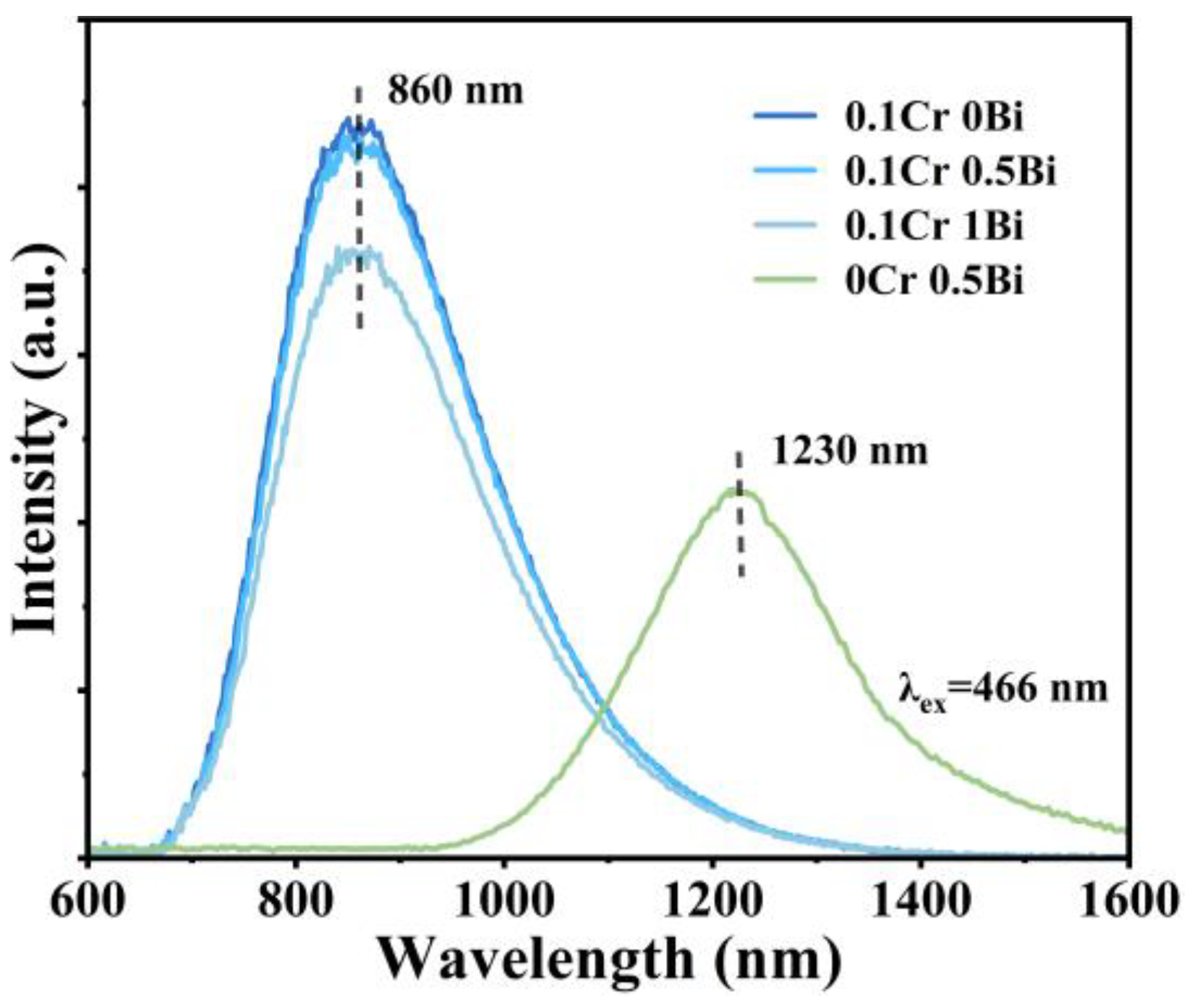
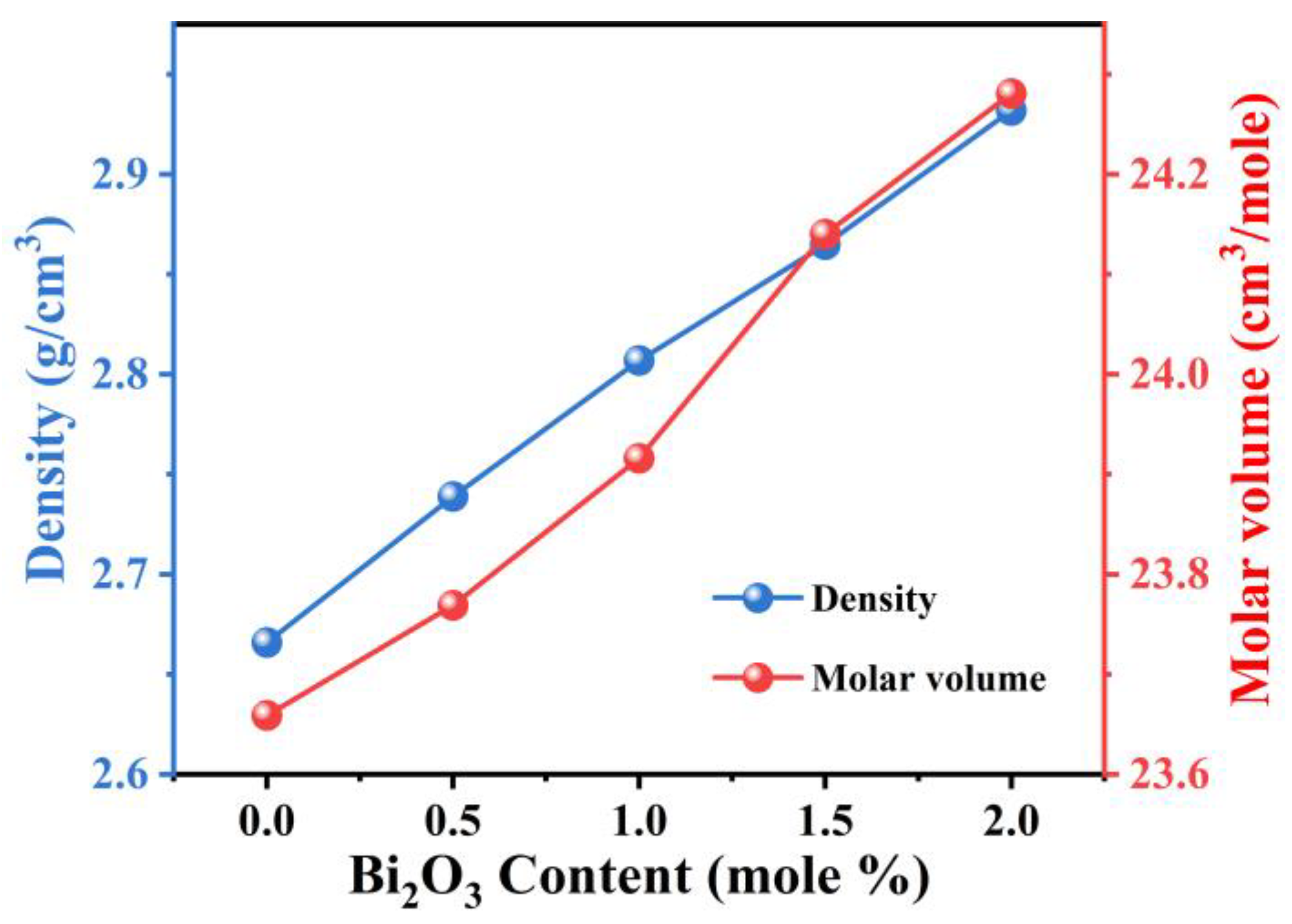
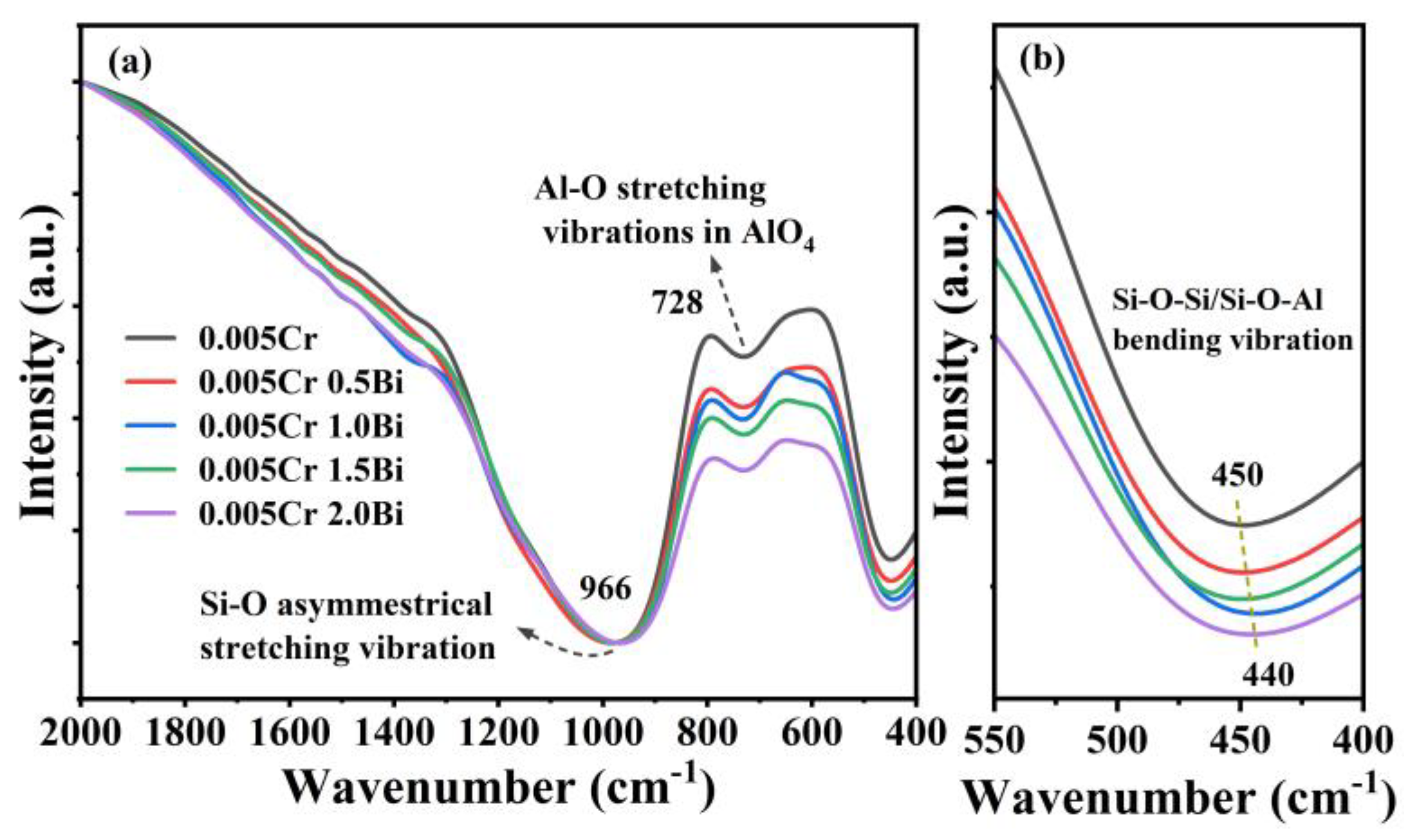
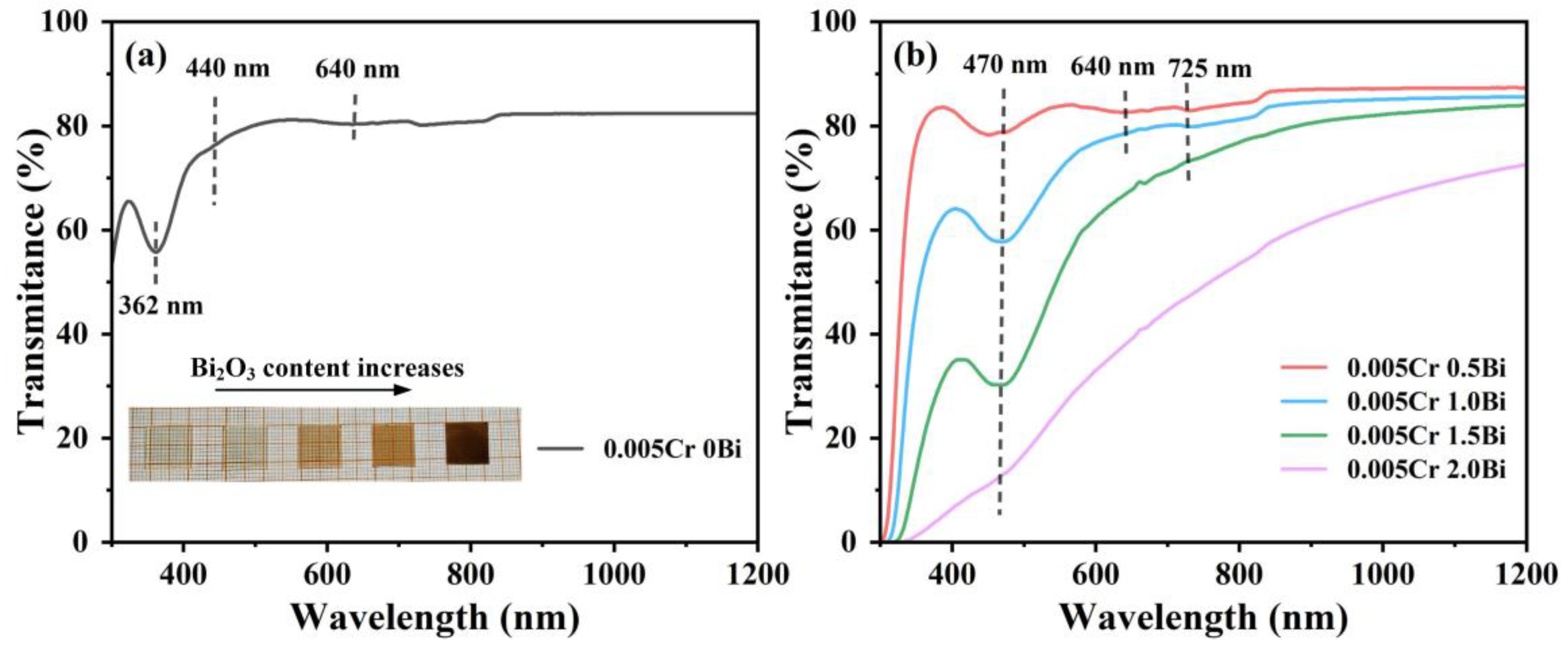
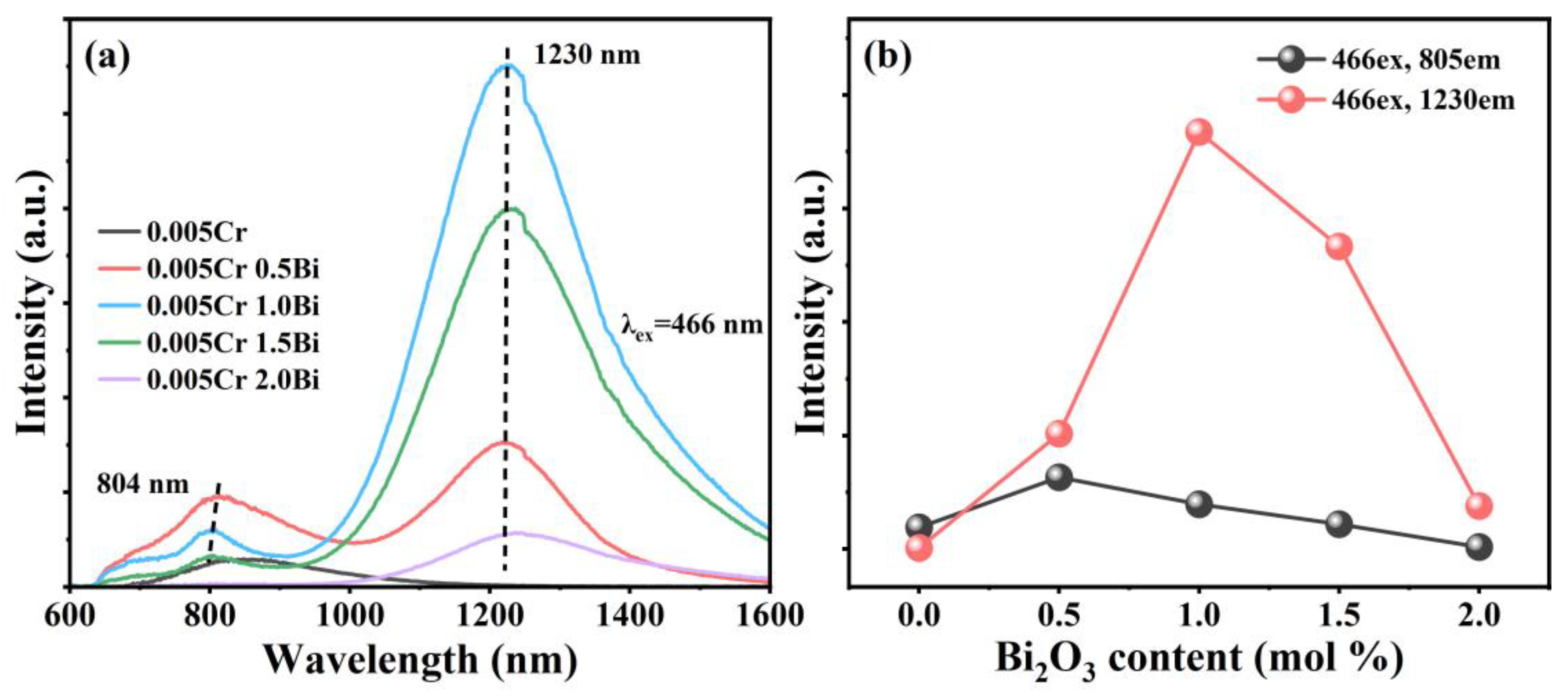
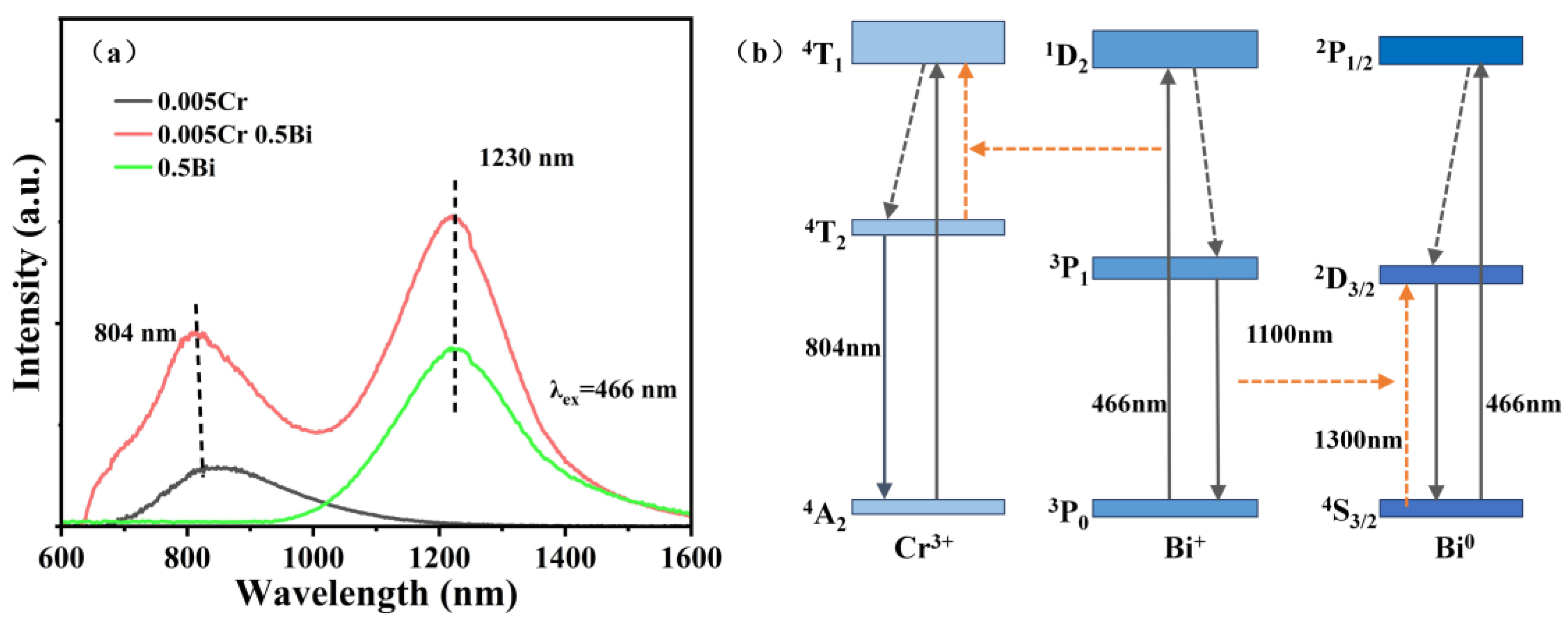
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hilbert, M.; Lopez, P. The world’s technological capacity to store, communicate, and compute information. Science 2011, 332, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Diddams, S.A.; Hollberg, L.; Mbele, V. Molecular fingerprinting with the resolved modes of a femtosecond laser frequency comb. Nature 2007, 445, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Jetschke, S.; Unger, S.; Schwuchow, A.; Leich, M.; Kirchhof, J. Efficient Yb laser fibers with low photodarkening by optimization of the core composition. Opt. Express 2008, 16, 15540–15545. [Google Scholar] [CrossRef]
- Yan, D.; Zhu, J.; Wu, H.; Yang, Z.; Qiu, J.; Song, Z.; Yu, X.; Yang, Y.; Zhou, D.; Yin, Z.; et al. Energy transfer and photoluminescence modification in Yb–Er–Tm triply doped Y2Ti2O7 upconversion inverse opal. J. Mater. Chem. 2012, 22, 18558–18563. [Google Scholar] [CrossRef]
- Tanabe, S. Rare-earth-doped glasses for fiber amplifiers. C. R. Chim. 2002, 5, 815–824. [Google Scholar] [CrossRef]
- Xu, Y.; Qi, J.; Ren, J.; Chen, G.; Huang, F.; Li, Y.; Lu, S.; Dai, S. Luminescence and energy transfer in Er3+/Nd3+ ion-codoped Ge–In–S–CsBr chalcohalide glasses. Mater. Res. Bull. 2013, 48, 4733–4737. [Google Scholar] [CrossRef]
- Yasushi, F.; Masahiro, N. Infrared Luminescence from Bismuth-doped Silica Glass. Jpn. Soc. Appl. Phys. 2001, 40, 279–281. [Google Scholar] [CrossRef]
- Qiu, J. Bi-Doped Glass for Photonic Devices. Int. J. Appl. Glass Sci. 2015, 6, 275–286. [Google Scholar] [CrossRef]
- Bufetov, I.A.; Melkumov, M.A.; Firstov, S.V.; Riumkin, K.E.; Shubin, A.V.; Khopin, V.F.; Guryanov, A.N.; Dianov, E.M. Bi-Doped Optical Fibers and Fiber Lasers. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 111–125. [Google Scholar] [CrossRef]
- Dianov, E.M.; Dvoyrin, V.V.; Mashinsky, V.M.; Umnikov, A.A.; Yashkov, M.V.; Gur’yanov, A.N. CW bismuth fibre laser. Quantum Electron. 2005, 35, 1083–1084. [Google Scholar] [CrossRef]
- Li, X.; Cao, J.; Peng, M. The origin of the heterogeneous distribution of bismuth in aluminosilicate laser glasses. J. Am. Ceram. Soc. 2018, 101, 2921–2929. [Google Scholar] [CrossRef]
- Meng, X.G.; Qiu, J.R.; Peng, M.Y.; Chen, D.P.; Zhao, Q.Z.; Jiang, X.W.; Zhu, C.S. Infrared broadband emission of bismuth-doped barium-aluminum-borate glasses. Opt. Express 2005, 13, 1635–1642. [Google Scholar] [CrossRef]
- Cao, J.; Peng, J.; Wang, L.; Luo, H.; Wang, X.; Xiong, P.; Wang, Y.; Peng, M. Broadband NIR emission from multiple Bi centers in nitridated borogermanate glassesviatailoring local glass structure. J. Mater. Chem. C 2019, 7, 2076–2084. [Google Scholar] [CrossRef]
- Cao, J.; Xu, S.; Zhang, Q.; Yang, Z.; Peng, M. Ultrabroad Photoemission from an Amorphous Solid by Topochemical Reduction. Adv. Opt. Mater. 2018, 6, 1801059. [Google Scholar] [CrossRef]
- Cao, J.; Li, L.; Wang, L.; Li, X.; Zhang, Z.; Xu, S.; Peng, M. Creating and stabilizing Bi NIR-emitting centers in low Bi content materials by topo-chemical reduction and tailoring of the local glass structure. J. Mater. Chem. C 2018, 6, 5384–5390. [Google Scholar] [CrossRef]
- Wei, Y.; Dang, P.; Dai, Z.; Li, G.; Lin, J. Advances in Near-Infrared Luminescent Materials without Cr3+: Crystal Structure Design, Luminescence Properties, and Applications. Chem. Mater. 2021, 33, 5496–5526. [Google Scholar] [CrossRef]
- Zhong, J.; Zhuo, Y.; Du, F.; Zhang, H.; Zhao, W.; Brgoch, J. Efficient and Tunable Luminescence in Ga2−xInxO3: Cr3+ for Near-Infrared Imaging. ACS Appl. Mater. Interfaces 2021, 13, 31835–31842. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, L.; Xu, Y.; Yin, S.; You, H. Effect of R+ (R = Li, Na and K) codoping on the luminescence enhancement of broadband NIR BaZrGe3O9: Cr3+ phosphors for NIR LED. J. Lumin. 2021, 236, 118084. [Google Scholar] [CrossRef]
- Mao, N.; Liu, S.; Song, Z.; Yu, Y.; Liu, Q. A broadband near-infrared phosphor Ca3Y2Ge3O12: Cr3+ with garnet structure. J. Alloys Compd. 2021, 863, 158699. [Google Scholar] [CrossRef]
- Liu, C.; Xia, Z.; Chen, M.; Molokeev, M.S.; Liu, Q. Near-infrared luminescence and color tunable chromophores based on Cr3+-doped mullite-type Bi2(Ga,Al)4O9 solid solutions. Inorg. Chem. 2015, 54, 1876–1882. [Google Scholar] [CrossRef]
- Denker, B.; Galagan, B.; Osiko, V.; Sverchkov, S.; Dianov, E. Luminescent properties of Bi-doped boro-alumino-phosphate glasses. Appl. Phys. B 2006, 87, 135–137. [Google Scholar] [CrossRef]
- Qiu, Y.Q.; Kang, J.; Li, C.X.; Dong, X.Y.; Zhao, C.L. Broadband near-infrared luminescence in bismuth borate glasses. Laser Phys. 2009, 20, 487–492. [Google Scholar] [CrossRef]
- Jiang, Z.; Dai, N.; Yang, L.; Peng, J.; Li, H.; Li, J.; Liu, W. Effects of Al2O3 composition on the near-infrared emission in Bi-doped and Yb–Bi-codoped silicate glasses for broadband optical amplification. J. Non-Cryst. Solids 2014, 383, 196–199. [Google Scholar] [CrossRef]
- Wan, R.; Song, Z.; Li, Y.; Liu, Q.; Zhou, Y.; Qiu, J.; Yang, Z.; Yin, Z.; Wang, Q.; Zhou, D. Influence of alkali metal ions on thermal stability of Bi-activated NIR-emitting alkali-aluminoborosilicate glasses. Chin. Opt. Lett. 2014, 12, 111601. [Google Scholar]
- Zhang, N.; Sharafudeen, K.N.; Dong, G.; Peng, M.; Qiu, J. Mixed Network Effect of Broadband Near-Infrared Emission in Bi-Doped B2O3-GeO2 Glasses. J. Am. Ceram. Soc. 2012, 95, 3842–3846. [Google Scholar] [CrossRef]
- Xu, Z.-S.; Xu, C.; Guo, Q.-B.; Liu, X.-F.; Qiu, J.-R. Effect of TeO2 on Near-Infrared Emission from Bismuth Centers in Borogermanate Glasses. J. Am. Ceram. Soc. 2016, 99, 760–764. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, B.; Li, J.; Liang, H.; Luo, Z.; Lu, A.; Zhang, L.; You, W.; Han, L.; Xia, L. Ultra-broadband NIR luminescence in chromium doped magnesium aluminosilicate glass as future light sources for spectroscopy applications. J. Non-Cryst. Solids 2022, 578, 121362. [Google Scholar] [CrossRef]
- Yusub, S.; Rao, D.K. The role of chromium ions on dielectric and spectroscopic properties of LiO-PbO-BO-PO glasses. J. Non-Cryst. Solids 2014, 398, 1–9. [Google Scholar] [CrossRef]
- Othman, A.M.; Abd El-Fattah, Z.M.; Farouk, M.; Moneep, A.M.; Hassan, M.A. Optical spectroscopy of chromium doped bismuth-lithium borate glasses. J. Non-Cryst. Solids 2021, 558, 120665. [Google Scholar] [CrossRef]
- Minh Hau, T.; Wang, R.; Yu, X.; Zhou, D.; Song, Z.; Yang, Z.; He, X.; Qiu, J. Near-infrared broadband luminescence and energy transfer in Bi–Tm–Er co-doped lanthanum aluminosilicate glasses. J. Phys. Chem. Solids 2012, 73, 1182–1186. [Google Scholar] [CrossRef]
- E, F.; Zhang, D.; Ye, R.; Hua, Y.; Xu, S.; Huang, F. Structural engineering of germanosilicate glass network for enhanced Bi: NIR luminescence. Opt. Mater. 2019, 95, 109222. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Y.; Luo, H.; Lin, L.; Zhou, S. Simultaneous control of the chemical state and local environment of Cr dopant in glass for extended emission. J. Am. Ceram. Soc. 2017, 101, 159–166. [Google Scholar] [CrossRef]
- Mohan, S.; Thind, K.S.; Sharma, G.; Gerward, L. Spectroscopic investigations of Nd3+ doped flouro- and chloro-borate glasses. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2008, 70, 1173–1179. [Google Scholar] [CrossRef]
- Saad, N.; Haouari, M.; Bulou, A.; Hadi Kassiba, A.; Ben Ouada, H. Structural and optical properties of Cr3+ embedded in a P2O5–B2O3–ZnO–BaF2–AlF3 fluoroborophosphate glasses. Mater. Chem. Phys. 2018, 212, 461–470. [Google Scholar] [CrossRef]
- Ahmad, F. Study the effect of alkali/alkaline earth addition on the environment of borochromate glasses by means of spectroscopic analysis. J. Alloys Compd. 2014, 586, 605–610. [Google Scholar] [CrossRef]
- Li, X.; Cao, J.; Wang, L.; Peng, M. Predictable tendency of Bi NIR emission in Bi-doped magnesium aluminosilicate laser glasses. J. Am. Ceram. Soc. 2017, 101, 1159–1168. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.; Wang, L.; Zhang, Z.; Xu, S.; Peng, M. New strategy to enhance the broadband near-infrared emission of bismuth-doped laser glasses. J. Am. Ceram. Soc. 2018, 101, 2297–2304. [Google Scholar] [CrossRef]
- Li, X.; Peng, M.; Cao, J.; Yang, Z.; Xu, S. Distribution and stabilization of bismuth NIR centers in Bi-doped aluminosilicate laser glasses by managing glass network structure. J. Mater. Chem. C 2018, 6, 7814–7821. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Chen, W.; Xiong, P.; Jiang, B.; Zhou, S.; Ma, Z.; Peng, M. Regulating the Bi NIR luminescence behaviours in fluorine and nitrogen co-doped germanate glasses. Mater. Adv. 2021, 2, 4743–4751. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Zhang, M. Broadband Near-Infrared Emission from Bi/Cr Co-Doped Aluminosilicate Glasses. Micromachines 2024, 15, 1093. https://doi.org/10.3390/mi15091093
Song S, Zhang M. Broadband Near-Infrared Emission from Bi/Cr Co-Doped Aluminosilicate Glasses. Micromachines. 2024; 15(9):1093. https://doi.org/10.3390/mi15091093
Chicago/Turabian StyleSong, Shiwen, and Min Zhang. 2024. "Broadband Near-Infrared Emission from Bi/Cr Co-Doped Aluminosilicate Glasses" Micromachines 15, no. 9: 1093. https://doi.org/10.3390/mi15091093






