Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities
Abstract
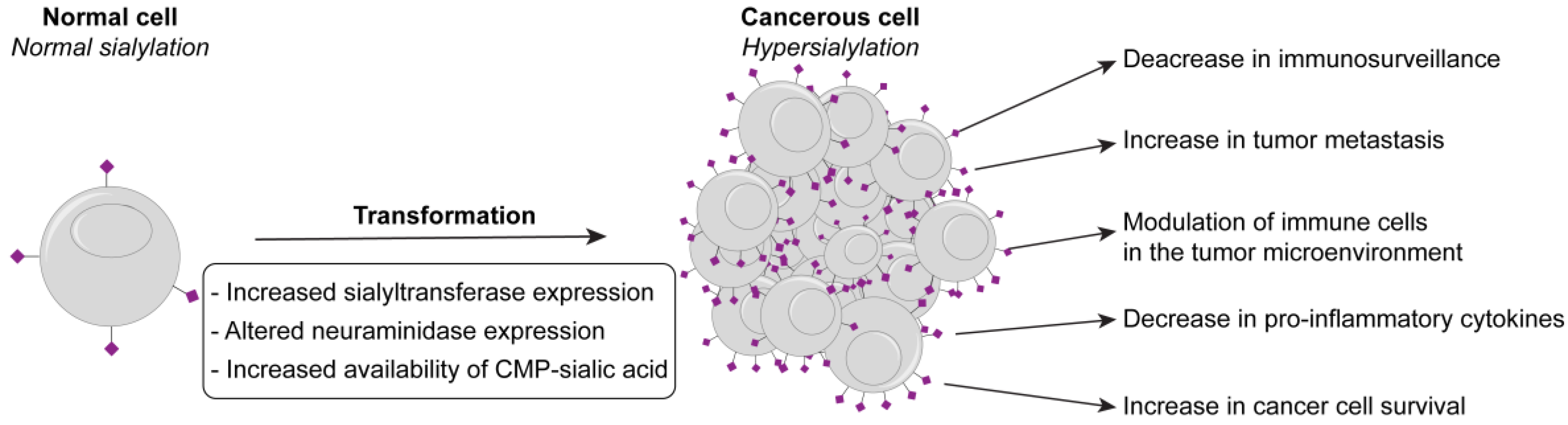
:1. A Growing Link between Hypersialylation, Cancer, and Inflammation
2. Mechanisms Leading to Hypersialylation
2.1. Sialyltransferase Expression
2.1.1. ST6Gal1
2.1.2. ST3Gal1, ST3Gal3, ST3Gal4, and ST3Gal6
2.1.3. ST6GalNAc1 and ST6GalNAc2
2.1.4. ST8Sia2 and ST8Sia4
2.2. Increased Availability of CMP-Sialic Acid
2.3. Neuraminidases
3. How Altered Sialic Acids Modulate Inflammatory Responses by Immune Cells
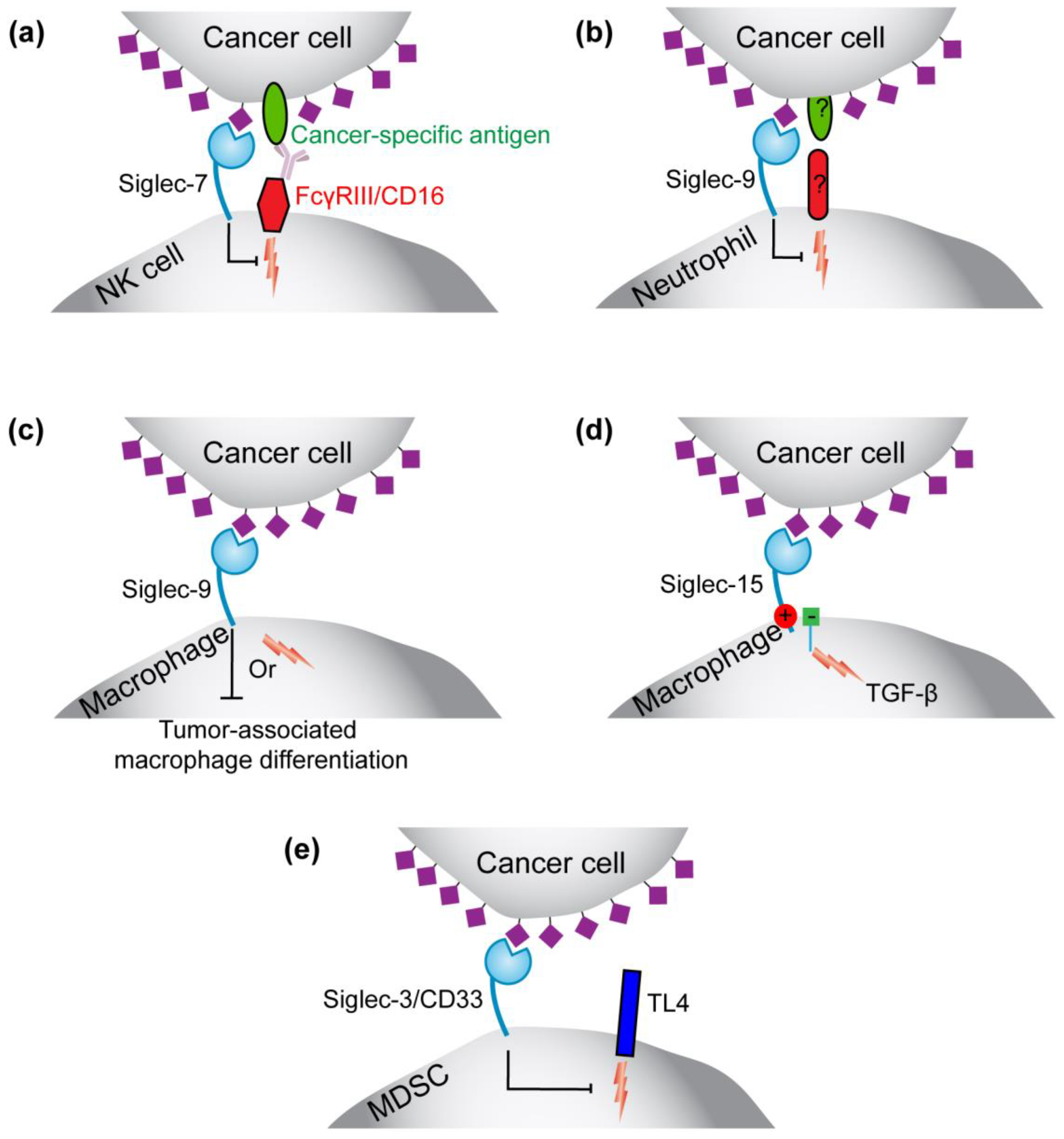
3.1. Siglecs
3.1.1. Roles for Siglec-7 and -9 on NK Cells and Siglec-9 on Neutrophils in Immunosurveillance
3.1.2. Siglec-9 in Regulating the Function or Formation of Tumor-Associated Macrophages
3.1.3. Siglec-15 on Tumor-Associated Macrophages
3.1.4. Siglec-3 (CD33) on Myeloid-Derived Suppressor Cells (MDSCs)
3.2. Selectins
3.3. Neu5Gc
4. Potential Treatment Strategies
4.1. Targeting Neuraminidase to Cancer Cells
4.2. Anti-Siglec and Anti-Selectin Antibodies
4.3. Sialyltransferase Inhibition
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| NSCLC | non-small cell lung carcinoma |
| Siglec | sialic acid-binding immunoglobulin-type lectin |
| NK | natural kill |
| Neu5Ac | N-acetylneuraminic acid |
| Neu5Gc | N-glycolylneuraminic acid |
| CMAH | CMP sialic acid hydroxylase |
| CMP | cytosine monophosphate |
| UDP | uridine diphosphate |
| GlcNAc | N-acetylglucosamine |
| ST | sialyltransferase |
| ITIM | immunotyrosine-based inhibitory motif |
| ITAM | immunotyrosine-based activatory motif |
| Neu | neuraminidase |
| MDSCs | myeloid-derived suppressor cells |
| Gal | galactose |
| GalNAc | N-galactosamine |
| STn | sialyl Tn antigen |
References
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.A.; Minn, A.J. Combination cancer therapy with immune checkpoint blockade: Mechanisms and strategies. Immunity 2018, 48, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Kimbara, S.; Kondo, S. Immune checkpoint and inflammation as therapeutic targets in pancreatic carcinoma. World J. Gastroenterol. 2016, 22, 7440–7452. [Google Scholar] [CrossRef] [PubMed]
- Pearce, O.M.; Laubli, H. Sialic acids in cancer biology and immunity. Glycobiology 2016, 26, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Boligan, K.F.; Mesa, C.; Fernandez, L.E.; von Gunten, S. Cancer intelligence acquired (CIA): Tumor glycosylation and sialylation codes dismantling antitumor defense. Cell. Mol. Life Sci. 2015, 72, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Fraschilla, I.; Pillai, S. Viewing siglecs through the lens of tumor immunology. Immunol. Rev. 2017, 276, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.; Heise, T.; Adema, G.J.; Boltje, T.J. Sialic acid mimetics to target the sialic acid-siglec axis. Trends Biochem. Sci. 2016, 41, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Natoni, A.; Macauley, M.S.; O’Dwyer, M.E. Targeting selectins and their ligands in cancer. Front. Oncol. 2016, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Adams, O.J.; Stanczak, M.A.; von Gunten, S.; Laubli, H. Targeting sialic acid-siglec interactions to reverse immune suppression in cancer. Glycobiology 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, R.E.; van Vliet, S.J.; van Kooyk, Y. Using the glycan toolbox for pathogenic interventions and glycan immunotherapy. Curr. Opin. Biotechnol. 2017, 51, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Fuster, M.M.; Esko, J.D. The sweet and sour of cancer: Glycans as novel therapeutic targets. Nat. Rev. Cancer 2005, 5, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Szabo, R.; Skropeta, D. Advancement of sialyltransferase inhibitors: Therapeutic challenges and opportunities. Med. Res. Rev. 2017, 37, 219–270. [Google Scholar] [CrossRef] [PubMed]
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, Q.; Simillion, C.; von Gunten, S. A cartography of siglecs and sialyltransferases in gynecologic malignancies: Is there a road towards a sweet future? Front. Oncol. 2018, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Swindall, A.F.; Londono-Joshi, A.I.; Schultz, M.J.; Fineberg, N.; Buchsbaum, D.J.; Bellis, S.L. St6Gal-I protein expression is upregulated in human epithelial tumors and correlates with stem cell markers in normal tissues and colon cancer cell lines. Cancer Res. 2013, 73, 2368–2378. [Google Scholar] [CrossRef] [PubMed]
- Gretschel, S.; Haensch, W.; Schlag, P.M.; Kemmner, W. Clinical relevance of sialyltransferases ST6Gal-I and st3gal-iii in gastric cancer. Oncology 2003, 65, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Chandra, S.; Mandal, C. Elevated mRNA level of hsT6Gal I and hST3Gal V positively correlates with the high risk of pediatric acute leukemia. Leuk. Res. 2010, 34, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Recchi, M.A.; Hebbar, M.; Hornez, L.; Harduin-Lepers, A.; Peyrat, J.P.; Delannoy, P. Multiplex reverse transcription polymerase chain reaction assessment of sialyltransferase expression in human breast cancer. Cancer Res. 1998, 58, 4066–4070. [Google Scholar] [PubMed]
- Olio, F.D.; Malagolini, N.; di Stefano, G.; Minni, F.; Marrano, D.; Serafini-Cessi, F. Increased CMP-NeuAc:Gal β1,4glcnac-r α2,6 sialyltransferase activity in human colorectal cancer tissues. Int. J. Cancer 1989, 44, 434–439. [Google Scholar] [CrossRef]
- Skacel, P.O.; Edwards, A.J.; Harrison, C.T.; Watkins, W.M. Enzymic control of the expression of the x determinant (CD15) in human myeloid cells during maturation: The regulatory role of 6-sialytransferase. Blood 1991, 78, 1452–1460. [Google Scholar] [PubMed]
- Fukushima, K.; Hara-Kuge, S.; Seko, A.; Ikehara, Y.; Yamashita, K. Elevation of alpha2-->6 sialyltransferase and alpha1-->2 fucosyltransferase activities in human choriocarcinoma. Cancer Res. 1998, 58, 4301–4306. [Google Scholar] [PubMed]
- Wang, P.H.; Li, Y.F.; Juang, C.M.; Lee, Y.R.; Chao, H.T.; Tsai, Y.C.; Yuan, C.C. Altered mRNA expression of sialyltransferase in squamous cell carcinomas of the cervix. Gynecol. Oncol. 2001, 83, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Yamamoto, H.; Kersey, D.S.; Colley, K.J.; Leestma, J.E.; Moskal, J.R. The expression of gal beta 1,4GlcNAc alpha 2,6 sialyltransferase and alpha 2,6-linked sialoglycoconjugates in human brain tumors. Acta Neuropathol. 1996, 91, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Y.; Ma, H.; Dong, W.; Zhou, H.; Song, X.; Zhang, J.; Jia, L. Modification of sialylation mediates the invasive properties and chemosensitivity of human hepatocellular carcinoma. Mol. Cell. Proteom. 2014, 13, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Chammas, R.; Bellis, S.L. Sialylation of beta1 integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J. Biol. Chem. 2008, 283, 22177–22185. [Google Scholar] [CrossRef] [PubMed]
- Perdicchio, M.; Ilarregui, J.M.; Verstege, M.I.; Cornelissen, L.A.; Schetters, S.T.; Engels, S.; Ambrosini, M.; Kalay, H.; Veninga, H.; den Haan, J.M.; et al. Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc. Natl. Acad. Sci. USA 2016, 113, 3329–3334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burchell, J.M.; Poulsom, R.; Hanby, A.M.; Whitehouse, C.; Cooper, L.; Clausen, H.B.; Miles, D.; Taylor-Papadimitriou, J. An alpha2,3 sialyltransferase (ST3Gal I) is elevated in primary breast carcinomas. Glycobiology 1999, 9, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Videira, P.A.; Correia, M.; Malagolini, N.; Crespo, H.J.; Ligeiro, D.; Calais, F.M.; Trindade, H.; Dall’Olio, F. St3Gal.I sialyltransferase relevance in bladder cancer tissues and cell lines. BMC Cancer 2009, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Ikehara, Y.; Togayachi, A.; Morozumi, K.; Watanabe, M.; Nakamura, M.; Nishihara, S.; Narimatsu, H. Up-regulation of a set of glycosyltransferase genes in human colorectal cancer. Lab. Investig. 1998, 78, 797–811. [Google Scholar] [PubMed]
- Beatson, R.; Tajadura-Ortega, V.; Achkova, D.; Picco, G.; Tsourouktsoglou, T.D.; Klausing, S.; Hillier, M.; Maher, J.; Noll, T.; Crocker, P.R.; et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat. Immunol. 2016, 17, 1273–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picco, G.; Julien, S.; Brockhausen, I.; Beatson, R.; Antonopoulos, A.; Haslam, S.; Mandel, U.; Dell, A.; Pinder, S.; Taylor-Papadimitriou, J.; et al. Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology 2010, 20, 1241–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Garay, M.; Arteta, B.; Pages, L.; de Llorens, R.; de Bolos, C.; Vidal-Vanaclocha, F.; Peracaula, R. Alpha2,3-sialyltransferase ST3Gal III modulates pancreatic cancer cell motility and adhesion in vitro and enhances its metastatic potential in vivo. PLoS ONE 2010, 5, e12524. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.L.; Zheng, S.S.; Wang, B.S.; Chen, H.L. Correlation of glycosyltransferases mRNA expression in extrahepatic bile duct carcinoma with clinical pathological characteristics. Hepatobiliary Pancreat. Dis. Int. 2004, 3, 292–295. [Google Scholar] [PubMed]
- Wang, P.H.; Li, Y.F.; Juang, C.M.; Lee, Y.R.; Chao, H.T.; Ng, H.T.; Tsai, Y.C.; Yuan, C.C. Expression of sialyltransferase family members in cervix squamous cell carcinoma correlates with lymph node metastasis. Gynecol. Oncol. 2002, 86, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Raclawska, D.S.; Ttofali, F.; Fletcher, A.A.; Harper, D.N.; Bochner, B.S.; Janssen, W.J.; Evans, C.M. Mucins and their sugars. Critical mediators of hyperreactivity and inflammation. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 1), S98–S99. [Google Scholar] [PubMed]
- Gomes, C.; Osorio, H.; Pinto, M.T.; Campos, D.; Oliveira, M.J.; Reis, C.A. Expression of ST3Gal4 leads to Sle(x) expression and induces c-Met activation and an invasive phenotype in gastric carcinoma cells. PLoS ONE 2013, 8, e66737. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Yamashita, S.-I.; Endoh, M.; Yamato, T.; Hoshi, S.; Ohyama, C.; Watanabe, R.; Ito, A.; Satoh, M.; Wada, T.; et al. Clinical significance of ST3Gal IV expression in human renal cell carcinoma. Oncol. Rep. 2002, 6, 1251–1255. [Google Scholar] [CrossRef]
- Miyara, M.; Chader, D.; Sage, E.; Sugiyama, D.; Nishikawa, H.; Bouvry, D.; Claer, L.; Hingorani, R.; Balderas, R.; Rohrer, J.; et al. Sialyl Lewis x (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans. Proc. Natl. Acad. Sci. USA 2015, 112, 7225–7230. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Nussbaum, C.; Grewal, P.K.; Marth, J.D.; Sperandio, M. Coordinated roles of ST3Gal-VI and ST3Gal-IV sialyltransferases in the synthesis of selectin ligands. Blood 2012, 120, 1015–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glavey, S.V.; Manier, S.; Natoni, A.; Sacco, A.; Moschetta, M.; Reagan, M.R.; Murillo, L.S.; Sahin, I.; Wu, P.; Mishima, Y.; et al. The sialyltransferase ST3Gal6 influences homing and survival in multiple myeloma. Blood 2014, 124, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, X.; Liang, L.; Pan, X.; Lv, H.; Zhao, Y. Sialyltransferase ST3Gal6 mediates the effect of microRNA-26a on cell growth, migration, and invasion in hepatocellular carcinoma through the protein kinase b/mammalian target of rapamycin pathway. Cancer Sci. 2017, 108, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.S.; Harduin-Lepers, A.; Magalhaes, A.; Machado, E.; Mendes, N.; Costa, L.T.; Matthiesen, R.; Almeida, R.; Costa, J.; Reis, C.A. Differential expression of alpha-2,3-sialyltransferases and alpha-1,3/4-fucosyltransferases regulates the levels of sialyl Lewis a and sialyl Lewis x in gastrointestinal carcinoma cells. Int. J. Biochem. Cell Biol. 2010, 42, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garay, M.; Arteta, B.; Llop, E.; Cobler, L.; Pages, L.; Ortiz, R.; Ferri, M.J.; de Bolos, C.; Figueras, J.; de Llorens, R.; et al. Alpha2,3-sialyltransferase ST3Gal IV promotes migration and metastasis in pancreatic adenocarcinoma cells and tends to be highly expressed in pancreatic adenocarcinoma tissues. Int. J. Biochem. Cell Biol. 2013, 45, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Ivetic, A.; Grigoriadis, A.; QiZe, D.; Burford, B.; Sproviero, D.; Picco, G.; Gillett, C.; Papp, S.L.; Schaffer, L.; et al. Selectin ligand sialyl-Lewis x antigen drives metastasis of hormone-dependent breast cancers. Cancer Res. 2011, 71, 7683–7693. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Adriaenssens, E.; Ottenberg, K.; Furlan, A.; Courtand, G.; Vercoutter-Edouart, A.S.; Hanisch, F.G.; Delannoy, P.; Le Bourhis, X. ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their o-glycosylation pattern and enhances their tumourigenicity. Glycobiology 2006, 16, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Marcos, N.T.; Pinho, S.; Grandela, C.; Cruz, A.; Samyn-Petit, B.; Harduin-Lepers, A.; Almeida, R.; Silva, F.; Morais, V.; Costa, J.; et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004, 64, 7050–7057. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Martin, C.; Cuevas, E.; Gil-Martin, E.; Fernandez-Briera, A. Correlation analysis between tumor-associated antigen sialyl-Tn expression and ST6GalNAc I activity in human colon adenocarcinoma. Oncology 2004, 67, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.; Marcos, N.T.; Ferreira, B.; Carvalho, A.S.; Oliveira, M.J.; Santos-Silva, F.; Harduin-Lepers, A.; Reis, C.A. Biological significance of cancer-associated sialyl-Tn antigen: Modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 2007, 249, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Krzewinski-Recchi, M.-A.; Harduin-Lepers, A.; Gouyer, V.; Huet, G.; Le Bourhis, X.; Delannoy, P. Expression of sialyl-Tn antigen in breast cancer cells transfected with the human CMP-Neu5Ac: GalNAc α2,6-sialyltransferase (ST6GalNAc I) cDNA. Glycoconj. J. 2001, 18, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Luo, S.; Ren, X.; Li, Y.; Hu, J.; Liu, B.; Zhao, L.; Shan, Y.; Zhou, H. miR-182 and miR-135b mediate the tumorigenesis and invasiveness of colorectal cancer cells via targeting ST6GalNAc2 and PI3K/Akt pathway. Dig. Dis. Sci. 2017, 62, 3447–3459. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, E.M.; Geddes-Sweeney, J.E.; Cedeno-Laurent, F.; Walley, K.C.; Barthel, S.R.; Opperman, M.J.; Liang, J.; Lin, J.Y.; Schatton, T.; Laga, A.C.; et al. Melanoma cell galectin-1 ligands functionally correlate with malignant potential. J. Investig. Dermatol. 2015, 135, 1849–1862. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Suzuki, M.; Nakayama, J.; Suzuki, A.; Angata, K.; Chen, S.; Sakai, K.; Hagihara, K.; Yamaguchi, Y.; Fukuda, M. Polysialic acid facilitates tumor invasion by glioma cells. Glycobiology 2005, 15, 887–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Dong, W.; Zhou, H.; Li, H.; Wang, N.; Miao, X.; Jia, L. Alpha-2,8-sialyltransferase is involved in the development of multidrug resistance via PI3K/Akt pathway in human chronic myeloid leukemia. IUBMB Life 2015, 67, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dong, W.; Su, Z.; Zhao, L.; Miao, Y.; Li, N.; Zhou, H.; Jia, L. Functional roles of sialylation in breast cancer progression through miR-26a/26b targeting ST8Sia4. Cell Death Dis. 2016, 7, e2561. [Google Scholar] [CrossRef] [PubMed]
- Lehoux, S.; Groux-Degroote, S.; Cazet, A.; Dhaenens, C.M.; Maurage, C.A.; Caillet-Boudin, M.L.; Delannoy, P.; Krzewinski-Recchi, M.A. Transcriptional regulation of the human ST6Gal2 gene in cerebral cortex and neuronal cells. Glycoconj. J. 2010, 27, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Gu, J. Significance of beta-galactoside alpha2,6 sialyltranferase 1 in cancers. Molecules 2015, 20, 7509–7527. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Galvan, M.; He, J.; Baum, L.G. The st6gal i sialyltransferase selectively modifies n-glycans on CD45 to negatively regulate galectin-1-induced CD45 clustering, phosphatase modulation, and T cell death. J. Biol. Chem. 2003, 278, 7469–7475. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta (BBA) Gen. Subj. 2002, 1572, 232–254. [Google Scholar] [CrossRef]
- Blidner, A.G.; Mendez-Huergo, S.P.; Cagnoni, A.J.; Rabinovich, G.A. Re-wiring regulatory cell networks in immunity by galectin-glycan interactions. FEBS Lett. 2015, 589, 3407–3418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sproviero, D.; Julien, S.; Burford, B.; Taylor-Papadimitriou, J.; Burchell, J.M. Cyclooxygenase-2 enzyme induces the expression of the alpha-2,3-sialyltransferase-3 (ST3Gal-I) in breast cancer. J. Biol. Chem. 2012, 287, 44490–44497. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Sakuma, K.; Kawamura, Y.I.; Izawa, M.; Ohmori, K.; Mitsuki, M.; Yamaji, T.; Hashimoto, Y.; Suzuki, A.; Saito, Y.; et al. Colonic epithelial cells express specific ligands for mucosal macrophage immunosuppressive receptors Siglec-7 and -9. J. Immunol. 2012, 188, 4690–4700. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Videira, P.A.; Delannoy, P. Sialyl-Tn in cancer: (How) did we miss the target? Biomolecules 2012, 2, 435–466. [Google Scholar] [CrossRef] [PubMed]
- Munkley, J. The role of sialyl-tn in cancer. Int. J. Mol. Sci. 2016, 17, 275. [Google Scholar] [CrossRef] [PubMed]
- Carrascal, M.A.; Severino, P.F.; Guadalupe Cabral, M.; Silva, M.; Ferreira, J.A.; Calais, F.; Quinto, H.; Pen, C.; Ligeiro, D.; Santos, L.L.; et al. Sialyl Tn-expressing bladder cancer cells induce a tolerogenic phenotype in innate and adaptive immune cells. Mol. Oncol. 2014, 8, 753–765. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Videira, P.A.; Lima, L.; Pereira, S.; Silva, M.; Carrascal, M.; Severino, P.F.; Fernandes, E.; Almeida, A.; Costa, C.; et al. Overexpression of tumour-associated carbohydrate antigen sialyl-tn in advanced bladder tumours. Mol. Oncol. 2013, 7, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Earl, L.A.; Bi, S.; Baum, L.G. N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death. J. Biol. Chem. 2010, 285, 2232–2244. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.G.; Seilhamer, J.J.; Pang, M.; Levine, W.B.; Beynon, D.; Berliner, J.A. Synthesis of an endogeneous lectin, galectin-1, by human endothelial-cells is up-regulated by endothelial-cell activation. Glycoconj. J. 1995, 12, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Koopman, L.A.; Kopcow, H.D.; Rybalov, B.; Boyson, J.E.; Orange, J.S.; Schatz, F.; Masch, R.; Lockwood, C.J.; Schachter, A.D.; Park, P.J.; et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J. Exp. Med. 2003, 198, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Postel, R.; Brandwijk, R.J.; Dings, R.P.; Nesmelova, I.; Satijn, S.; Verhofstad, N.; Nakabeppu, Y.; Baum, L.G.; Bakkers, J.; et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl. Acad. Sci. USA 2006, 103, 15975–15980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angata, K.; Suzuki, M.; McAuliffe, J.; Ding, Y.; Hindsgaul, O.; Fukuda, M. Differential biosynthesis of polysialic acid on neural cell adhesion molecule (ncam) and oligosaccharide acceptors by three distinct alpha 2,8-sialyltransferases, ST8Sia IV (PST), ST8Sia II (STX), and ST8Sia III. J. Biol. Chem. 2000, 275, 18594–18601. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, H.; Becker, C.; Mürau, M.; Gerardy-Schahn, R.; Rahmann, H. Heterogeneous expression of the polysialyltransferases ST8Sia II and ST8Sia IV during postnatal rat brain development. J. Neurochem. 2002, 71, 2339–2348. [Google Scholar] [CrossRef]
- Ong, E.; Nakayama, J.; Angata, K.; Reyes, L.; Katsuyama, T.; Arai, Y.; Fukuda, M. Developmental regulation of polysialic acid synthesis in mouse directed by two polysialyltransferases, PST and STX. Glycobiology 1998, 8, 415–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, L.; Zhou, X.; Yang, J.; Jiang, Y.; Yang, H. Effects of the regulation of polysialyltransferase ST8Siaii on the invasiveness and metastasis of small cell lung cancer cells. Oncol. Rep. 2017, 37, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Almaraz, R.T.; Tian, Y.; Bhattarcharya, R.; Tan, E.; Chen, S.H.; Dallas, M.R.; Chen, L.; Zhang, Z.; Zhang, H.; Konstantopoulos, K.; et al. Metabolic flux increases glycoprotein sialylation: Implications for cell adhesion and cancer metastasis. Mol. Cell. Proteom. 2012, 11, M112.017558. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.S.; Partridge, E.A.; Grigorian, A.; Silvescu, C.I.; Reinhold, V.N.; Demetriou, M.; Dennis, J.W. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 2007, 129, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Badr, H.A.; AlSadek, D.M.; Mathew, M.P.; Li, C.Z.; Djansugurova, L.B.; Yarema, K.J.; Ahmed, H. Nutrient-deprived cancer cells preferentially use sialic acid to maintain cell surface glycosylation. Biomaterials 2015, 70, 23–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohnz, R.A.; Roberts, L.S.; DeTomaso, D.; Bideyan, L.; Yan, P.; Bandyopadhyay, S.; Goga, A.; Yosef, N.; Nomura, D.K. Protein sialylation regulates a gene expression signature that promotes breast cancer cell pathogenicity. ACS Chem. Biol. 2016, 11, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Saeui, C.T.; Nairn, A.V.; Galizzi, M.; Douville, C.; Gowda, P.; Park, M.; Dharmarha, V.; Shah, S.R.; Clarke, A.; Austin, M.; et al. Integration of genetic and metabolic features related to sialic acid metabolism distinguishes human breast cell subtypes. PLoS ONE 2018, 13, e0195812. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, A.M.; Abdulkhalek, S.; Cheng, T.S.; Alghamdi, F.; Jayanth, P.; O’Shea, L.K.; Geen, O.; Arvizu, L.A.; Szewczuk, M.R. A novel epidermal growth factor receptor-signaling platform and its targeted translation in pancreatic cancer. Cell Signal. 2013, 25, 2587–2603. [Google Scholar] [CrossRef] [PubMed]
- Koseki, K.; Wada, T.; Hosono, M.; Hata, K.; Yamaguchi, K.; Nitta, K.; Miyagi, T. Human cytosolic sialidase NEU2-low general tissue expression but involvement in PC-3 prostate cancer cell survival. Biochem. Biophys. Res. Commun. 2012, 428, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Tringali, C.; Lupo, B.; Anastasia, L.; Papini, N.; Monti, E.; Bresciani, R.; Tettamanti, G.; Venerando, B. Expression of sialidase Neu2 in leukemic K562 cells induces apoptosis by impairing Bcr-Abl/Src kinases signaling. J. Biol. Chem. 2007, 282, 14364–14372. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Shao, Y.; Liang, Z.; Shao, C.; Wang, B.; Guo, B.; Li, N.; Zhao, X.; Li, Y.; et al. Effects of a human plasma membrane-associated sialidase siRNA on prostate cancer invasion. Biochem. Biophys. Res. Commun. 2011, 416, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Tringali, C.; Silvestri, I.; Testa, F.; Baldassari, P.; Anastasia, L.; Mortarini, R.; Anichini, A.; Lopez-Requena, A.; Tettamanti, G.; Venerando, B. Molecular subtyping of metastatic melanoma based on cell ganglioside metabolism profiles. BMC Cancer 2014, 14, 560. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Kambe, M.; Tajima, O.; Moriya, S.; Sawaki, H.; Hotta, H.; Kondo, Y.; Narimatsu, H.; Miyagi, T.; Furukawa, K.; et al. Membrane sialidase NEU3 is highly expressed in human melanoma cells promoting cell growth with minimal changes in the composition of gangliosides. Cancer Sci. 2011, 102, 2139–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, H.; Tamada, Y.; Miyagi, T.; Suzuki, A.; Taira, M.; Suzuki, N.; Susumu, N.; Irimura, T.; Aoki, D. Expression of NEU3 (plasma membrane-associated sialidase) in clear cell adenocarcinoma of the ovary: Its relationship with T factor of pTNM classification. Oncol. Res. Featur. Preclin. Clin. Cancer Therap. 2006, 16, 289–297. [Google Scholar] [CrossRef]
- Bonardi, D.; Papini, N.; Pasini, M.; Dileo, L.; Orizio, F.; Monti, E.; Caimi, L.; Venerando, B.; Bresciani, R. Sialidase NEU3 dynamically associates to different membrane domains specifically modifying their ganglioside pattern and triggering akt phosphorylation. PLoS ONE 2014, 9, e99405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquina, G.; Waki, H.; Fernandez, L.E.; Kon, K.; Carr, A.; Valiente, O.; Perez, R.; Ando, S. Gangliosides expressed in human breast cancer. Cancer Res. 1996, 56, 5165–5171. [Google Scholar] [PubMed]
- Ravindranath, M.H.; Tsuchida, T.; Morton, D.L.; Irie, R.F. Ganglioside GM3-GD3 ratio as an index for the management of melanoma. Cancer 1991, 67, 3029–3035. [Google Scholar] [CrossRef]
- Raz, A.; Goldman, R.; Yuli, I.; Inbar, M. Isolation of plasma membrane fragments and vesicles from ascites fluid of lymphoma-bearing mice and their possible role in the escape mechanism of tumors from host immune rejection. Cancer Immunol. Immunother. 1978, 4, 53–59. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, J.; Mi, W.; Yang, J.; Han, F.; Lu, X.; Yu, W. Silencing of gm3 synthase suppresses lung metastasis of murine breast cancer cells. Breast Cancer Res. 2008, 10, R1. [Google Scholar] [CrossRef] [PubMed]
- Ladisch, S.; Becker, H.; Ulsh, L. Immunosuppression by human gangliosides: I. Relationship of carbohydrate structure to the inhibition of T cell responses. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1992, 1125, 180–188. [Google Scholar] [CrossRef]
- Shiozaki, K.; Yamaguchi, K.; Takahashi, K.; Moriya, S.; Miyagi, T. Regulation of sialyl Lewis antigen expression in colon cancer cells by sialidase NEU4. J. Biol. Chem. 2011, 286, 21052–21061. [Google Scholar] [CrossRef] [PubMed]
- Fedarko, N.S.; Fohr, B.; Robey, P.G.; Young, M.F.; Fisher, L.W. Factor H binding to bone sialoprotein and osteopontin enables tumor cell evasion of complement-mediated attack. J. Biol. Chem. 2000, 275, 16666–16672. [Google Scholar] [CrossRef] [PubMed]
- Blaum, B.S.; Hannan, J.P.; Herbert, A.P.; Kavanagh, D.; Uhrin, D.; Stehle, T. Structural basis for sialic acid-mediated self-recognition by complement factor h. Nat. Chem. Biol. 2015, 11, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Perdicchio, M.; Cornelissen, L.A.; Streng-Ouwehand, I.; Engels, S.; Verstege, M.I.; Boon, L.; Geerts, D.; van Kooyk, Y.; Unger, W.W. Tumor sialylation impedes T cell mediated anti-tumor responses while promoting tumor associated-regulatory T cells. Oncotarget 2016, 7, 8771–8782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bull, C.; Boltje, T.J.; Balneger, N.; Weischer, S.M.; Wassink, M.; van Gemst, J.J.; Bloemendal, V.; Boon, L.; van der Vlag, J.; Heise, T.; et al. Sialic acid blockade suppresses tumor growth by enhancing T cell-mediated tumor immunity. Cancer Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Swindall, A.F.; Bellis, S.L. Sialylation of the fas death receptor by ST6Gal-I provides protection against FAS-mediated apoptosis in colon carcinoma cells. J. Biol. Chem. 2011, 286, 22982–22990. [Google Scholar] [CrossRef] [PubMed]
- Elkashef, S.M.; Allison, S.J.; Sadiq, M.; Basheer, H.A.; Ribeiro Morais, G.; Loadman, P.M.; Pors, K.; Falconer, R.A. Polysialic acid sustains cancer cell survival and migratory capacity in a hypoxic environment. Sci. Rep. 2016, 6, 33026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dharmadhikari, G.; Stolz, K.; Hauke, M.; Morgan, N.G.; Varki, A.; de Koning, E.; Kelm, S.; Maedler, K. Siglec-7 restores beta-cell function and survival and reduces inflammation in pancreatic islets from patients with diabetes. Sci. Rep. 2017, 7, 45319. [Google Scholar] [CrossRef] [PubMed]
- Gicheva, N.; Macauley, M.S.; Arlian, B.M.; Paulson, J.C.; Kawasaki, N. Siglec-F is a novel intestinal M cell marker. Biochem. Biophys. Res. Commun. 2016, 479, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.R.; Fong, J.J.; Carlin, A.F.; Busch, T.D.; Linden, R.; Angata, T.; Areschoug, T.; Parast, M.; Varki, N.; Murray, J.; et al. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group b streptococcus. J. Exp. Med. 2014, 211, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Rumer, K.K.; Uyenishi, J.; Hoffman, M.C.; Fisher, B.M.; Winn, V.D. Siglec-6 expression is increased in placentas from pregnancies complicated by preterm preeclampsia. Reprod. Sci. 2013, 20, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Wicki, S.; Graeter, S.; Timcheva, T.M.; Keller, C.W.; Quast, I.; Leontyev, D.; Djoumerska-Alexieva, I.K.; Kasermann, F.; Jakob, S.M.; et al. IVIG regulates the survival of human but not mouse neutrophils. Sci. Rep. 2017, 7, 1296. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D.J.; O’Sullivan, J.A.; Nix, D.B.; Cao, Y.; Tiemeyer, M.; Bochner, B.S. Siglec-8 is an activating receptor on human eosinophils mediating integrin-dependent adhesion, ROS generation and apoptosis. Glycobiology 2017, 27, 1172–1173. [Google Scholar]
- Crocker, P.R.; McMillan, S.J.; Richards, H.E. CD33-related siglecs as potential modulators of inflammatory responses. Ann. N. Y. Acad. Sci. 2012, 1253, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, G.; Avril, T.; Lock, K.; Furukawa, K.; Bovin, N.; Crocker, P.R. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via Siglec-7-dependent and -independent mechanisms. Eur. J. Immunol. 2003, 33, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Hudak, J.E.; Canham, S.M.; Bertozzi, C.R. Glycocalyx engineering reveals a siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 2014, 10, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Jandus, C.; Boligan, K.F.; Chijioke, O.; Liu, H.; Dahlhaus, M.; Demoulins, T.; Schneider, C.; Wehrli, M.; Hunger, R.E.; Baerlocher, G.M.; et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J. Clin. Investig. 2014, 124, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Querol, E.; Rosales, C. Neutrophils in cancer: Two sides of the same coin. J. Immunol. Res. 2015, 2015, 983698. [Google Scholar] [CrossRef] [PubMed]
- Laubli, H.; Pearce, O.M.; Schwarz, F.; Siddiqui, S.S.; Deng, L.; Stanczak, M.A.; Deng, L.; Verhagen, A.; Secrest, P.; Lusk, C.; et al. Engagement of myelomonocytic siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 14211–14216. [Google Scholar] [CrossRef] [PubMed]
- Angata, T.; Tabuchi, Y.; Nakamura, K.; Nakamura, M. Siglec-15: An immune system siglec conserved throughout vertebrate evolution. Glycobiology 2007, 17, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, R.; Ohtsubo, K.; Takamatsu, S.; Taniguchi, N.; Angata, T. The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-beta secretion from monocytes/macrophages through the DAP12-Syk pathway. Glycobiology 2013, 23, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Condamine, T.; Dominguez, G.A.; Youn, J.I.; Kossenkov, A.V.; Mony, S.; Alicea-Torres, K.; Tcyganov, E.; Hashimoto, A.; Nefedova, Y.; Lin, C.; et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci. Immunol. 2016, 1, aaf8943. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Eksioglu, E.A.; Zhou, J.; Zhang, L.; Djeu, J.; Fortenbery, N.; Epling-Burnette, P.; Van Bijnen, S.; Dolstra, H.; Cannon, J.; et al. Induction of myelodysplasia by myeloid-derived suppressor cells. J. Clin. Investig. 2013, 123, 4595–4611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.D.; Willment, J.A.; Whitehead, L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 2018, 18, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, M.; Gleissner, C.A.; Ley, K. Glycosylation in immune cell trafficking. Immunol. Rev. 2009, 230, 97–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borsig, L. Selectins in cancer immunity. Glycobiology 2017. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.G.; Balmana, M.; Macedo, J.A.; Pocas, J.; Fernandes, A.; de-Freitas-Junior, J.C.M.; Pinho, S.S.; Gomes, J.; Magalhaes, A.; Gomes, C.; et al. Glycosylation in cancer: Selected roles in tumour progression, immune modulation and metastasis. Cell. Immunol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Trinchera, M.; Aronica, A.; Dall’Olio, F. Selectin ligands sialyl-Lewis a and sialyl-Lewis x in gastrointestinal cancers. Biology 2017, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Aoki, M.; Kannagi, R. Transcription factors c-Myc and CDX2 mediate E-selectin ligand expression in colon cancer cells undergoing EGF/bFGF-induced epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. USA 2012, 109, 7776–7781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, M.J.; Fogg, K.C.; Patel, H.A.; Krause, H.B.; Mancha, A.S.; Patankar, M.S.; Weisman, P.S.; Barroilhet, L.; Kreeger, P.K. Alternatively activated macrophages upregulate mesothelial expression of P-selectin to enhance adhesion of ovarian cancer cells. Cancer Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Carrascal, M.A.; Silva, M.; Ramalho, J.S.; Pen, C.; Martins, M.; Pascoal, C.; Amaral, C.; Serrano, I.; Oliveira, M.J.; Sackstein, R.; et al. Inhibition of fucosylation in human invasive ductal carcinoma reduces E-selectin ligand expression, cell proliferation, and ERK1/2 and p38 mapk activation. Mol. Oncol. 2018, 12, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.A.; Beckmann, N.; Adams, C.; Hessler, G.; Kramer, M.; Gulbins, E.; Carpinteiro, A. Melanoma cell metastasis via P-selectin-mediated activation of acid sphingomyelinase in platelets. Clin. Exp. Metastasis 2017, 34, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Samraj, A.N.; Pearce, O.M.; Laubli, H.; Crittenden, A.N.; Bergfeld, A.K.; Banda, K.; Gregg, C.J.; Bingman, A.E.; Secrest, P.; Diaz, S.L.; et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc. Natl. Acad. Sci. USA 2015, 112, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Varki, N.M.; Varki, A. Diversity in cell surface sialic acid presentations: Implications for biology and disease. Lab. Investig. 2007, 87, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.H.; Takematsu, H.; Diaz, S.; Iber, J.; Nickerson, E.; Wright, K.L.; Muchmore, E.A.; Nelson, D.L.; Warren, S.T.; Varki, A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc. Natl. Acad. Sci. USA 1998, 95, 11751–11756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardor, M.; Nguyen, D.H.; Diaz, S.; Varki, A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J. Biol. Chem. 2005, 280, 4228–4237. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Woods, E.C.; Vukojicic, P.; Bertozzi, C.R. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2016, 113, 10304–10309. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.T.; Wrede, C.; Niemann, J.; Brooks, J.; Schwarzer, D.; Kuhnel, F.; Gerardy-Schahn, R. Targeting polysialic acid-abundant cancers using oncolytic adenoviruses with fibers fused to active bacteriophage borne endosialidase. Biomaterials 2018, 158, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Carlin, A.F.; Uchiyama, S.; Chang, Y.C.; Lewis, A.L.; Nizet, V.; Varki, A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 2009, 113, 3333–3336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spence, S.; Greene, M.K.; Fay, F.; Hams, E.; Saunders, S.P.; Hamid, U.; Fitzgerald, M.; Beck, J.; Bains, B.K.; Smyth, P.; et al. Targeting siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci. Transl. Med. 2015, 7, 303ra140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, S.; Zhu, X.; You, N.; Zhang, W.; Zheng, F.; Cai, B.; Zhou, T.; Wang, Y.; Sun, Q.; Yang, Z.; et al. The fab fragment of a human anti-Siglec-9 monoclonal antibody suppresses LPS-induced inflammatory responses in human macrophages. Front. Immunol. 2016, 7, 649. [Google Scholar] [CrossRef] [PubMed]
- Barthel, S.R.; Gavino, J.D.; Descheny, L.; Dimitroff, C.J. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin. Ther. Targets 2007, 11, 1473–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolo, R.; Herbrich, S.; Rao, A.; Zweidler-McKay, P.; Kannan, S.; Gopalakrishnan, V. Targeting p-selectin blocks neuroblastoma growth. Oncotarget 2017, 8, 86657–86670. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Garcia, I.; Aznar, E.; Arnaiz, B.; Martinez-Bisbal, M.C.; Liz-Marzan, L.M.; Penades, S.; Martinez-Manez, R. Lectin-gated and glycan functionalized mesoporous silica nanocontainers for targeting cancer cells overexpressing lewis x antigen. Nanoscale 2017, 10, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Ferber, S.; Tiram, G.; Sousa-Herves, A.; Eldar-Boock, A.; Krivitsky, A.; Scomparin, A.; Yeini, E.; Ofek, P.; Ben-Shushan, D.; Vossen, L.I.; et al. Co-targeting the tumor endothelium and P-selectin-expressing glioblastoma cells leads to a remarkable therapeutic outcome. Elife 2017, 6, e25281. [Google Scholar] [CrossRef] [PubMed]
- Natoni, A.; Smith, T.A.G.; Keane, N.; McEllistrim, C.; Connolly, C.; Jha, A.; Andrulis, M.; Ellert, E.; Raab, M.S.; Glavey, S.V.; et al. E-selectin ligands recognised by HECA452 induce drug resistance in myeloma, which is overcome by the e-selectin antagonist, GMI-1271. Leukemia 2017, 31, 2642–2651. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Kamal, M.; Kang, S.A.; Zhang, R.; Lokesh, G.L.; Thiviyanathan, V.; Hasan, N.; Woo, S.; Zhao, D.; Leslie, M.; et al. E-selectin targeting pegylated-thioaptamer prevents breast cancer metastases. Mol. Ther. Nucleic Acids 2016, 5, e399. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.E.; Thomas, P. Inhibition of CMP-sialic acid transport in human liver and colorectal cancer cell lines by a sialic acid nucleoside conjugate (KI-8110). Biochem. Biophys. Res. Commun. 1993, 190, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Rillahan, C.D.; Antonopoulos, A.; Lefort, C.T.; Sonon, R.; Azadi, P.; Ley, K.; Dell, A.; Haslam, S.M.; Paulson, J.C. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 2012, 8, 661–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macauley, M.S.; Arlian, B.M.; Rillahan, C.D.; Pang, P.C.; Bortell, N.; Marcondes, M.C.; Haslam, S.M.; Dell, A.; Paulson, J.C. Systemic blockade of sialylation in mice with a global inhibitor of sialyltransferases. J. Biol. Chem. 2014, 289, 35149–35158. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.; Boltje, T.J.; Wassink, M.; de Graaf, A.M.; van Delft, F.L.; den Brok, M.H.; Adema, G.J. Targeting aberrant sialylation in cancer cells using a fluorinated sialic acid analog impairs adhesion, migration, and in vivo tumor growth. Mol. Cancer Ther. 2013, 12, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.; Boltje, T.J.; van Dinther, E.A.; Peters, T.; de Graaf, A.M.; Leusen, J.H.; Kreutz, M.; Figdor, C.G.; den Brok, M.H.; Adema, G.J. Targeted delivery of a sialic acid-blocking glycomimetic to cancer cells inhibits metastatic spread. ACS Nano 2015, 9, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Bednar, K.J.; Shanina, E.; Ballet, R.; Connors, E.P.; Duan, S.; Juan, J.; Arlian, B.M.; Kulis, M.D.; Butcher, E.C.; Fung-Leung, W.P.; et al. Human CD22 inhibits murine b cell receptor activation in a human CD22 transgenic mouse model. J. Immunol. 2017, 199, 3116–3128. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.A.; Wei, Y.; Carroll, D.J.; Moreno-Vinasco, L.; Cao, Y.; Zhang, F.; Lee, J.J.; Zhu, Z.; Bochner, B.S. Characterization of a novel mouse strain expressing human Siglec-8 only on eosinophils. J. Leukoc. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Landig, C.S.; Siddiqui, S.; Secundino, I.; Olson, J.; Varki, N.; Nizet, V.; Varki, A. Paired siglec receptors generate opposite inflammatory responses to a human-specific pathogen. EMBO J. 2017, 36, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Yu, K.R.; Kenderian, S.S.; Ruella, M.; Chen, S.; Shin, T.H.; Aljanahi, A.A.; Schreeder, D.; Klichinsky, M.; Shestova, O.; et al. Genetic inactivation of CD33 in hematopoietic stem cells to enable car t cell immunotherapy for acute myeloid leukemia. Cell 2018, 173, 1439–1453.e1419. [Google Scholar] [CrossRef] [PubMed]

| Sialyltransferase | Cancer-Specific Glycosylation Change | Cancer Types | Gene Expression Changes | Link to Inflammation |
|---|---|---|---|---|
| ST6Gal1 | Increase in α2-6 sialosides | Epithelial [17], gastric [18], leukemia [19], breast [20], colorectal [21], acute myeloid leukemia [22], choriocarcinoma [23], cervix [24], brain [25], liver [26] | Elevated | Evading TNFα induced apoptosis [27]; Promoting naïve CD4+ T cell differentiation into regulatory T-cells [28] |
| ST3Gal1 | Increased α2-3 sialylated Core 1 O-glycans | Breast [29], bladder [30], colon [31] | Elevated | Modulated macrophage differentiation [32]; Masking of tumor from surrounding immune cells [33] |
| ST3Gal3 | Increased sialyl-Lewisa, sialyl-Lewisa | Pancreas [34], gastric [18], bile duct [35], cervix [36] | Elevated | Induction of apoptosis on eosinophils [37] |
| ST3Gal4 | Increased sialyl-Lewisa, sialyl-Lewisa | Gastric [38], renal [39] | Elevated | Targeting of regulatory T-cells to suppress tissue-localized inflammation [40]; Promoting metastasis through creating Selectin ligands [41] |
| ST3Gal6 | Increased sialyl-Lewisa, sialyl-Lewisa | Multiple myeloma [42], liver [43], gastrointestinal [44], adenocarcinoma [45], breast [46] | Elevated | |
| ST6GalNAc1 | Increase sialyl Tn | Breast [47], colon [48], adenocarcinoma [49], gastric [50] | Elevated | Induction of low levels of Th1-inducing cytokines [47,51]; Blocking of dendritic cell (DC) maturation [47] |
| ST6GalNAc2 | Increased extended Core 2 O-glycans | Colerectal [52], melanoma [53] | Decreased | Increase in Galectin-1 ligands to suppress immune response [53] |
| ST8Sia2 | Increased polysialic acid | Liver [26], astrocytoma [54] | Elevated | Modulation of PI3K/Akt pathway to negatively regulate pro-inflammatory cytokines [55] |
| ST8Sia4 | Increased polysialic acid | Chronic myeloid leukemia [55], breast [56], astrocytoma [54] | Elevated |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, E.; Macauley, M.S. Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities. Cancers 2018, 10, 207. https://doi.org/10.3390/cancers10060207
Rodrigues E, Macauley MS. Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities. Cancers. 2018; 10(6):207. https://doi.org/10.3390/cancers10060207
Chicago/Turabian StyleRodrigues, Emily, and Matthew S. Macauley. 2018. "Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities" Cancers 10, no. 6: 207. https://doi.org/10.3390/cancers10060207
APA StyleRodrigues, E., & Macauley, M. S. (2018). Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities. Cancers, 10(6), 207. https://doi.org/10.3390/cancers10060207





