Emerging Functional Imaging Biomarkers of Tumour Responses to Radiotherapy
Abstract
:1. Introduction
2. Biological Effects of Radiation
3. Imaging Apoptosis and Necrosis
4. Imaging Changes in Vasculature
4.1. Dynamic Contrast-Enhanced (DCE) CT
4.2. Perfusion MRI
4.3. Ultrasound and Optical Imaging
4.4. PET Imaging of Perfusion
5. Hypoxia Imaging
5.1. PET Imaging of Hypoxia
5.2. MRI Imaging of Hypoxia
6. Imaging Changes in Tissue Structure
6.1. Diffusion-Weighted MRI
6.2. Chemical Exchange Saturation Transfer MRI
7. Imaging Changes in Metabolism
7.1. Imaging Changes in Glycolysis and TCA Cycle Metabolism
7.2. Imaging Proliferation
7.3. PET Imaging of Brain Tumours
8. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | computed tomography |
| DNP | dynamic nuclear polarisation |
| MRI | magnetic resonance imaging |
| MSOT | multispectral optoacoustic tomography |
| OCT | optical coherence tomography |
| PET | positron emission tomography |
| SPECT | single photon emission computed tomography |
References
- World Health Organization (WHO). WHO Handbook for Reporting Results of Cancer Treatment; WHO: Geneva, Switzerland, 1979. [Google Scholar]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulla, S.; Saada, J.; Johnson, G.; Jefferies, S.; Ajithkumar, T. Tumour progression or pseudoprogression? A review of post-treatment radiological appearances of glioblastoma. Clin. Radiol. 2015, 70, 1299–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroobants, S.; Goeminne, J.; Seegers, M.; Dimitrijevic, S.; Dupont, P.; Nuyts, J.; Martens, M.; van den Borne, B.; Cole, P.; Sciot, R.; et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec). Eur. J. Cancer 2003, 39, 2012–2020. [Google Scholar] [CrossRef]
- Barton, M.B.; Jacob, S.; Shafiq, J.; Wong, K.; Thompson, S.R.; Hanna, T.P.; Delaney, G.P. Estimating the demand for radiotherapy from the evidence: A review of changes from 2003 to 2012. Radiother. Oncol. 2014, 112, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Thorwarth, D. Functional imaging for radiotherapy treatment planning: Current status and future directions-a review. Br. J. Radiol. 2015, 88, 20150056. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Subesinghe, M.; Patel, C.; Prestwich, R.; Scarsbrook, A.F. Functional imaging for radiation treatment planning, response assessment, and adaptive therapy in head and neck cancer. Radiographics 2013, 33, 1909–1929. [Google Scholar] [CrossRef] [PubMed]
- Maier, P.; Hartmann, L.; Wenz, F.; Herskind, C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int. J. Mol. Sci. 2016, 17, 102. [Google Scholar] [CrossRef]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef]
- Eriksson, D.; Stigbrand, T. Radiation-induced cell death mechanisms. Tumour Biol. 2010, 31, 363–372. [Google Scholar] [CrossRef]
- Jonathan, E.C.; Bernhard, E.J.; McKenna, W.G. How does radiation kill cells? Curr. Opin. Chem. Biol. 1999, 3, 77–83. [Google Scholar] [CrossRef]
- Lorat, Y.; Timm, S.; Jakob, B.; Taucher-Scholz, G.; Rube, C.E. Clustered double-strand breaks in heterochromatin perturb DNA repair after high linear energy transfer irradiation. Radiother. Oncol. 2016, 121, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Krysko, O.; De Ridder, L.; Cornelissen, M. Phosphatidylserine exposure during early primary necrosis (oncosis) in JB6 cells as evidenced by immunogold labeling technique. Apoptosis 2004, 9, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.A.; Brindle, K.M. Imaging cell death. J. Nucl. Med. 2014, 55, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Challapalli, A.; Smith, G.; Fortt, R.; Aboagye, E.O. Imaging apoptosis with positron emission tomography: ‘bench to bedside’ development of the caspase-3/7 specific radiotracer [18F]ICMT-11. Eur. J. Cancer 2012, 48, 432–440. [Google Scholar] [CrossRef]
- Belhocine, T.Z.; Blankenberg, F.G.; Kartachova, M.S.; Stitt, L.W.; Vanderheyden, J.L.; Hoebers, F.J.; Van de Wiele, C. 99mTc-Annexin A5 quantification of apoptotic tumor response: A systematic review and meta-analysis of clinical imaging trials. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 2083–2097. [Google Scholar] [CrossRef]
- Wang, F.; Fang, W.; Zhao, M.; Wang, Z.; Ji, S.; Li, Y.; Zheng, Y. Imaging paclitaxel (chemotherapy)-induced tumor apoptosis with 99mTc C2A, a domain of synaptotagmin I: A preliminary study. Nucl. Med. Biol. 2008, 35, 359–364. [Google Scholar] [CrossRef]
- Neves, A.A.; Xie, B.; Fawcett, S.; Alam, I.S.; Witney, T.H.; de Backer, M.M.; Summers, J.; Hughes, W.; McGuire, S.; Soloviev, D.; et al. Rapid Imaging of Tumor Cell Death In Vivo Using the C2A Domain of Synaptotagmin-I. J. Nucl. Med. 2017, 58, 881–887. [Google Scholar] [CrossRef]
- Wang, F.; Fang, W.; Zhang, M.R.; Zhao, M.; Liu, B.; Wang, Z.; Hua, Z.; Yang, M.; Kumata, K.; Hatori, A.; et al. Evaluation of chemotherapy response in VX2 rabbit lung cancer with 18F-labeled C2A domain of synaptotagmin I. J. Nucl. Med. 2011, 52, 592–599. [Google Scholar] [CrossRef]
- Krishnan, A.S.; Neves, A.A.; de Backer, M.M.; Hu, D.E.; Davletov, B.; Kettunen, M.I.; Brindle, K.M. Detection of cell death in tumors by using MR imaging and a gadolinium-based targeted contrast agent. Radiology 2008, 246, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Tomaszewski, M.R.; Neves, A.A.; Ros, S.; Hu, D.E.; McGuire, S.; Mullins, S.R.; Tice, D.; Sainson, R.C.A.; Bohndiek, S.E.; et al. Optoacoustic Detection of Early Therapy-Induced Tumor Cell Death Using a Targeted Imaging Agent. Clin. Cancer Res. 2017, 23, 6893–6903. [Google Scholar] [CrossRef] [PubMed]
- Elvas, F.; Boddaert, J.; Vangestel, C.; Pak, K.; Gray, B.; Kumar-Singh, S.; Staelens, S.; Stroobants, S.; Wyffels, L. 99mTc-Duramycin SPECT Imaging of Early Tumor Response to Targeted Therapy: A Comparison with 18F-FDG PET. J. Nucl. Med. 2017, 58, 665–670. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, K.; Wang, W.; Zhang, X.; Ju, Z.; Qu, B.; Zhang, Z.; Wang, J.; Ling, Z.; Yu, X.; et al. [18F]ML-10 Imaging for Assessment of Apoptosis Response of Intracranial Tumor Early after Radiotherapy by PET/CT. Contrast Media Mol. Imaging 2018, 2018, 9365174. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Ben-Ami, M.; Reshef, A.; Steinmetz, A.; Kundel, Y.; Inbar, E.; Djaldetti, R.; Davidson, T.; Fenig, E.; Ziv, I. Assessment of response of brain metastases to radiotherapy by PET imaging of apoptosis with 18F-ML-10. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Dubash, S.R.; Merchant, S.; Heinzmann, K.; Mauri, F.; Lavdas, I.; Inglese, M.; Kozlowski, K.; Rama, N.; Masrour, N.; Steel, J.F.; et al. Clinical translation of [18F]ICMT-11 for measuring chemotherapy-induced caspase 3/7 activation in breast and lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2285–2299. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Barros, M.; Paris, F.; Cordon-Cardo, C.; Lyden, D.; Rafii, S.; Haimovitz-Friedman, A.; Fuks, Z.; Kolesnick, R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003, 300, 1155–1159. [Google Scholar] [CrossRef]
- Garcia-Barros, M.; Thin, T.H.; Maj, J.; Cordon-Cardo, C.; Haimovitz-Friedman, A.; Fuks, Z.; Kolesnick, R. Impact of stromal sensitivity on radiation response of tumors implanted in SCID hosts revisited. Cancer Res. 2010, 70, 8179–8186. [Google Scholar] [CrossRef]
- Fuks, Z.; Kolesnick, R. Engaging the vascular component of the tumor response. Cancer Cell 2005, 8, 89–91. [Google Scholar] [CrossRef] [Green Version]
- Corre, I.; Guillonneau, M.; Paris, F. Membrane signaling induced by high doses of ionizing radiation in the endothelial compartment. Relevance in radiation toxicity. Int. J. Mol. Sci. 2013, 14, 22678–22696. [Google Scholar] [CrossRef]
- Miyatake, S.; Nonoguchi, N.; Furuse, M.; Yoritsune, E.; Miyata, T.; Kawabata, S.; Kuroiwa, T. Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain. Neurol. Med. Chir. 2015, 55, 50–59. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.; Tofts, P.S.; Miles, K.A.; Parkes, L.M.; Thompson, G.; Jackson, A. Dynamic contrast-enhanced imaging techniques: CT and MRI. Br. J. Radiol. 2011, 84, S112–S120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, C.A.; Lee, K.S.; Kim, E.A.; Han, J.; Kim, H.; Kwon, O.J.; Jeong, Y.J.; Kim, S. Solitary pulmonary nodules: Dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 2004, 233, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Bellomi, M.; Petralia, G.; Sonzogni, A.; Zampino, M.G.; Rocca, A. CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: Initial experience. Radiology 2007, 244, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Sahani, D.V.; Kalva, S.P.; Hamberg, L.M.; Hahn, P.F.; Willett, C.G.; Saini, S.; Mueller, P.R.; Lee, T.Y. Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: Initial observations. Radiology 2005, 234, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Ursino, S.; Faggioni, L.; Guidoccio, F.; Ferrazza, P.; Seccia, V.; Neri, E.; Cernusco, L.N.; Delishaj, D.; Morganti, R.; Volterrani, D.; et al. Role of perfusion CT in the evaluation of functional primary tumour response after radiochemotherapy in head and neck cancer: Preliminary findings. Br. J. Radiol. 2016, 89, 20151070. [Google Scholar] [CrossRef]
- Coolens, C.; Driscoll, B.; Foltz, W.D.; Jaffray, D.A.; Chung, C. Early Detection of Tumor Response Using Volumetric DCE-CT and DCE-MRI in Metastatic Brain Patients Treated with Radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, S7. [Google Scholar] [CrossRef]
- Surlan-Popovic, K.; Bisdas, S.; Rumboldt, Z.; Koh, T.S.; Strojan, P. Changes in perfusion CT of advanced squamous cell carcinoma of the head and neck treated during the course of concomitant chemoradiotherapy. Am. J. Neuroradiol. 2010, 31, 570–575. [Google Scholar] [CrossRef]
- Kino, A.; Shaffer, J.; Maturen, K.E.; Schmiedeskamp, H.; Koong, A.C.; Chang, D.T.; Fleischmann, D.; Kamaya, A. Perfusion CT measurements predict tumor response in rectal carcinoma. Abdom. Radiol. 2017, 42, 1132–1140. [Google Scholar] [CrossRef]
- Banks, T.I.; von Eyben, R.; Hristov, D.; Kidd, E.A. Pilot study of combined FDG-PET and dynamic contrast-enhanced CT of locally advanced cervical carcinoma before and during concurrent chemoradiotherapy suggests association between changes in tumor blood volume and treatment response. Cancer Med. 2018, 7, 3642–3651. [Google Scholar] [CrossRef]
- Zahra, M.A.; Hollingsworth, K.G.; Sala, E.; Lomas, D.J.; Tan, L.T. Dynamic contrast-enhanced MRI as a predictor of tumour response to radiotherapy. Lancet Oncol. 2007, 8, 63–74. [Google Scholar] [CrossRef]
- Padhani, A.R.; Husband, J.E. Dynamic contrast-enhanced MRI studies in oncology with an emphasis on quantification, validation and human studies. Clin. Radiol. 2001, 56, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, F.; Soliman, A.; El-Baz, A.; Abou El-Ghar, M.; El-Diasty, T.; Gimel’farb, G.; Ouseph, R.; Dwyer, A.C. Models and methods for analyzing DCE-MRI: A review. Med. Phys. 2014, 41, 124301. [Google Scholar] [CrossRef] [PubMed]
- Petcharunpaisan, S.; Ramalho, J.; Castillo, M. Arterial spin labeling in neuroimaging. World J. Radiol. 2010, 2, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Mayr, N.A.; Yuh, W.T.; Magnotta, V.A.; Ehrhardt, J.C.; Wheeler, J.A.; Sorosky, J.I.; Davis, C.S.; Wen, B.C.; Martin, D.D.; Pelsang, R.E.; et al. Tumor perfusion studies using fast magnetic resonance imaging technique in advanced cervical cancer: A new noninvasive predictive assay. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 623–633. [Google Scholar] [CrossRef]
- Zahra, M.A.; Tan, L.T.; Priest, A.N.; Graves, M.J.; Arends, M.; Crawford, R.A.; Brenton, J.D.; Lomas, D.J.; Sala, E. Semiquantitative and quantitative dynamic contrast-enhanced magnetic resonance imaging measurements predict radiation response in cervix cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 766–773. [Google Scholar] [CrossRef]
- Yuh, W.T.; Mayr, N.A.; Jarjoura, D.; Wu, D.; Grecula, J.C.; Lo, S.S.; Edwards, S.M.; Magnotta, V.A.; Sammet, S.; Zhang, H.; et al. Predicting control of primary tumor and survival by DCE MRI during early therapy in cervical cancer. Investig. Radiol. 2009, 44, 343–350. [Google Scholar] [CrossRef]
- Dickie, B.R.; Rose, C.J.; Kershaw, L.E.; Withey, S.B.; Carrington, B.M.; Davidson, S.E.; Hutchison, G.; West, C.M.L. The prognostic value of dynamic contrast-enhanced MRI contrast agent transfer constant Ktrans in cervical cancer is explained by plasma flow rather than vessel permeability. Br. J. Cancer 2017, 116, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.I.; Harry, V.N.; Parkin, D.E.; Gilbert, F.J. A combined pharmacokinetic and radiologic assessment of dynamic contrast-enhanced magnetic resonance imaging predicts response to chemoradiation in locally advanced cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 611–617. [Google Scholar] [CrossRef]
- Dijkhoff, R.A.P.; Beets-Tan, R.G.H.; Lambregts, D.M.J.; Beets, G.L.; Maas, M. Value of DCE-MRI for staging and response evaluation in rectal cancer: A systematic review. Eur. J. Radiol. 2017, 95, 155–168. [Google Scholar] [CrossRef]
- King, A.D.; Thoeny, H.C. Functional MRI for the prediction of treatment response in head and neck squamous cell carcinoma: Potential and limitations. Cancer Imaging 2016, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Jakubovic, R.; Sahgal, A.; Soliman, H.; Milwid, R.; Zhang, L.; Eilaghi, A.; Aviv, R.I. Magnetic resonance imaging-based tumour perfusion parameters are biomarkers predicting response after radiation to brain metastases. Clin. Oncol. 2014, 26, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tsien, C.I.; Nagesh, V.; Junck, L.; Ten Haken, R.; Ross, B.D.; Chenevert, T.L.; Lawrence, T.S. Survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, J.; Diao, Q.; Lin, Y.; Zhang, J.; Li, L.; Yang, G.; Fang, X.; Li, X.; Chen, Y.; et al. Assessment of glioma response to radiotherapy using 3D pulsed-continuous arterial spin labeling and 3D segmented volume. Eur. J. Radiol. 2016, 85, 1987–1992. [Google Scholar] [CrossRef] [PubMed]
- Hawighorst, H.; Weikel, W.; Knapstein, P.G.; Knopp, M.V.; Zuna, I.; Schonberg, S.O.; Vaupel, P.; van Kaick, G. Angiogenic activity of cervical carcinoma: Assessment by functional magnetic resonance imaging-based parameters and a histomorphological approach in correlation with disease outcome. Clin. Cancer Res. 1998, 4, 2305–2312. [Google Scholar] [PubMed]
- Bisdas, S.; Naegele, T.; Ritz, R.; Dimostheni, A.; Pfannenberg, C.; Reimold, M.; Koh, T.S.; Ernemann, U. Distinguishing recurrent high-grade gliomas from radiation injury: A pilot study using dynamic contrast-enhanced MR imaging. Acad. Radiol. 2011, 18, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Baradaran, H.; Delgado, D.; Askin, G.; Christos, P.; John Tsiouris, A.; Gupta, A. MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: A systematic review and meta-analysis. Neuro-Oncology 2017, 19, 118–127. [Google Scholar] [CrossRef]
- Ozsunar, Y.; Mullins, M.E.; Kwong, K.; Hochberg, F.H.; Ament, C.; Schaefer, P.W.; Gonzalez, R.G.; Lev, M.H. Glioma recurrence versus radiation necrosis? A pilot comparison of arterial spin-labeled, dynamic susceptibility contrast enhanced MRI, and FDG-PET imaging. Acad. Radiol. 2010, 17, 282–290. [Google Scholar] [CrossRef]
- Ye, J.; Bhagat, S.K.; Li, H.; Luo, X.; Wang, B.; Liu, L.; Yang, G. Differentiation between recurrent gliomas and radiation necrosis using arterial spin labeling perfusion imaging. Exp. Ther. Med. 2016, 11, 2432–2436. [Google Scholar] [CrossRef] [Green Version]
- Razek, A.; El-Serougy, L.; Abdelsalam, M.; Gaballa, G.; Talaat, M. Differentiation of residual/recurrent gliomas from postradiation necrosis with arterial spin labeling and diffusion tensor magnetic resonance imaging-derived metrics. Neuroradiology 2018, 60, 169–177. [Google Scholar] [CrossRef]
- Nyberg, E.; Honce, J.; Kleinschmidt-DeMasters, B.K.; Shukri, B.; Kreidler, S.; Nagae, L. Arterial spin labeling: Pathologically proven superiority over conventional MRI for detection of high-grade glioma progression after treatment. Neuroradiol. J. 2016, 29, 377–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Kim, S.H.; Kang, B.J.; Kim, Y.J. Contrast-Enhanced Ultrasound for Early Prediction of Response of Breast Cancer to Neoadjuvant Chemotherapy. Ultraschall Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, S.H.; Song, B.J.; Kang, B.J.; Yim, K.I.; Lee, A.; Nam, Y. Early Prediction of Response to Neoadjuvant Chemotherapy Using Dynamic Contrast-Enhanced MRI and Ultrasound in Breast Cancer. Korean J. Radiol. 2018, 19, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Lassau, N.; Koscielny, S.; Albiges, L.; Chami, L.; Benatsou, B.; Chebil, M.; Roche, A.; Escudier, B.J. Metastatic renal cell carcinoma treated with sunitinib: Early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clin. Cancer Res. 2010, 16, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Kasoji, S.K.; Rivera, J.N.; Gessner, R.C.; Chang, S.X.; Dayton, P.A. Early Assessment of Tumor Response to Radiation Therapy using High-Resolution Quantitative Microvascular Ultrasound Imaging. Theranostics 2018, 8, 156–168. [Google Scholar] [CrossRef] [Green Version]
- Arteaga-Marrero, N.; Mainou-Gomez, J.F.; Brekke Rygh, C.; Lutay, N.; Roehrich, D.; Reed, R.K.; Olsen, D.R. Radiation treatment monitoring with DCE-US in CWR22 prostate tumor xenografts. Acta Radiol. 2018. [Google Scholar] [CrossRef]
- Abdollahi, A.; Griggs, D.W.; Zieher, H.; Roth, A.; Lipson, K.E.; Saffrich, R.; Grone, H.J.; Hallahan, D.E.; Reisfeld, R.A.; Debus, J.; et al. Inhibition of αvβ3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin. Cancer Res. 2005, 11, 6270–6279. [Google Scholar] [CrossRef]
- Zysk, A.M.; Nguyen, F.T.; Oldenburg, A.L.; Marks, D.L.; Boppart, S.A. Optical coherence tomography: A review of clinical development from bench to bedside. J. Biomed. Opt. 2007, 12, 051403. [Google Scholar] [CrossRef]
- Demidov, V.; Maeda, A.; Sugita, M.; Madge, V.; Sadanand, S.; Flueraru, C.; Vitkin, I.A. Preclinical longitudinal imaging of tumor microvascular radiobiological response with functional optical coherence tomography. Sci. Rep. 2018, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Maeda, A.; Leung, M.K.; Conroy, L.; Chen, Y.; Bu, J.; Lindsay, P.E.; Mintzberg, S.; Virtanen, C.; Tsao, J.; Winegarden, N.A.; et al. In vivo optical imaging of tumor and microvascular response to ionizing radiation. PLoS ONE 2012, 7, e42133. [Google Scholar] [CrossRef]
- McNally, L.R.; Mezera, M.; Morgan, D.E.; Frederick, P.J.; Yang, E.S.; Eltoum, I.E.; Grizzle, W.E. Current and Emerging Clinical Applications of Multispectral Optoacoustic Tomography (MSOT) in Oncology. Clin. Cancer Res. 2016, 22, 3432–3439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomaszewski, M.R.; Gehrung, M.; Joseph, J.; Quiros-Gonzalez, I.; Disselhorst, J.A.; Bohndiek, S.E. Oxygen-Enhanced and Dynamic Contrast-Enhanced Optoacoustic Tomography Provide Surrogate Biomarkers of Tumor Vascular Function, Hypoxia, and Necrosis. Cancer Res. 2018, 78, 5980–5991. [Google Scholar] [CrossRef] [PubMed]
- Rich, L.J.; Seshadri, M. Photoacoustic monitoring of tumor and normal tissue response to radiation. Sci. Rep. 2016, 6, 21237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zackrisson, S.; van de Ven, S.; Gambhir, S.S. Light in and sound out: Emerging translational strategies for photoacoustic imaging. Cancer Res. 2014, 74, 979–1004. [Google Scholar] [CrossRef] [PubMed]
- Lodge, M.A.; Jacene, H.A.; Pili, R.; Wahl, R.L. Reproducibility of tumor blood flow quantification with 15O-water PET. J. Nucl. Med. 2008, 49, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- De Langen, A.J.; Lubberink, M.; Boellaard, R.; Spreeuwenberg, M.D.; Smit, E.F.; Hoekstra, O.S.; Lammertsma, A.A. Reproducibility of tumor perfusion measurements using 15O-labeled water and PET. J. Nucl. Med. 2008, 49, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Lehtio, K.; Eskola, O.; Viljanen, T.; Oikonen, V.; Gronroos, T.; Sillanmaki, L.; Grenman, R.; Minn, H. Imaging perfusion and hypoxia with PET to predict radiotherapy response in head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 971–982. [Google Scholar] [CrossRef]
- Rockwell, S.; Dobrucki, I.T.; Kim, E.Y.; Marrison, S.T.; Vu, V.T. Hypoxia and radiation therapy: Past history, ongoing research and future promise. Curr. Mol. Med. 2009, 9, 442–458. [Google Scholar] [CrossRef]
- Harris, A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef]
- Masaki, Y.; Shimizu, Y.; Yoshioka, T.; Nishijima, K.I.; Zhao, S.; Higashino, K.; Numata, Y.; Tamaki, N.; Kuge, Y. FMISO accumulation in tumor is dependent on glutathione conjugation capacity in addition to hypoxic state. Ann. Nucl. Med. 2017, 31, 596–604. [Google Scholar] [CrossRef] [Green Version]
- Lock, S.; Perrin, R.; Seidlitz, A.; Bandurska-Luque, A.; Zschaeck, S.; Zophel, K.; Krause, M.; Steinbach, J.; Kotzerke, J.; Zips, D.; et al. Residual tumour hypoxia in head-and-neck cancer patients undergoing primary radiochemotherapy, final results of a prospective trial on repeat FMISO-PET imaging. Radiother. Oncol. 2017, 124, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Welz, S.; Monnich, D.; Pfannenberg, C.; Nikolaou, K.; Reimold, M.; La Fougere, C.; Reischl, G.; Mauz, P.S.; Paulsen, F.; Alber, M.; et al. Prognostic value of dynamic hypoxia PET in head and neck cancer: Results from a planned interim analysis of a randomized phase II hypoxia-image guided dose escalation trial. Radiother. Oncol. 2017, 124, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Wada, M.; Anderson, N.J.; Lim Joon, D.; Lee, S.T.; Gong, S.J.; Gunawardana, D.H.; Sachinidis, J.; O’Keefe, G.; Gan, H.K.; et al. Hypoxia-targeted radiotherapy dose painting for head and neck cancer using 18F-FMISO PET: A biological modeling study. Acta Oncol. 2013, 52, 1723–1729. [Google Scholar] [CrossRef]
- Vera, P.; Thureau, S.; Chaumet-Riffaud, P.; Modzelewski, R.; Bohn, P.; Vermandel, M.; Hapdey, S.; Pallardy, A.; Mahe, M.A.; Lacombe, M.; et al. Phase II Study of a Radiotherapy Total Dose Increase in Hypoxic Lesions Identified by 18F-Misonidazole PET/CT in Patients with Non-Small Cell Lung Carcinoma (RTEP5 Study). J. Nucl. Med. 2017, 58, 1045–1053. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Kolb, H.C.; Walsh, J.C.; Zhang, J.; Guan, Y. 18F-HX4 hypoxia imaging with PET/CT in head and neck cancer: A comparison with 18F-FMISO. Nucl. Med. Commun. 2012, 33, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Saga, T.; Inubushi, M.; Koizumi, M.; Yoshikawa, K.; Zhang, M.R.; Obata, T.; Tanimoto, K.; Harada, R.; Uno, T.; Fujibayashi, Y. Prognostic value of PET/CT with 18F-fluoroazomycin arabinoside for patients with head and neck squamous cell carcinomas receiving chemoradiotherapy. Ann. Nucl. Med. 2016, 30, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Servagi-Vernat, S.; Differding, S.; Sterpin, E.; Hanin, F.X.; Labar, D.; Bol, A.; Lee, J.A.; Gregoire, V. Hypoxia-guided adaptive radiation dose escalation in head and neck carcinoma: A planning study. Acta Oncol. 2015, 54, 1008–1016. [Google Scholar] [CrossRef]
- Bollineni, V.R.; Koole, M.J.; Pruim, J.; Brouwer, C.L.; Wiegman, E.M.; Groen, H.J.; Vlasman, R.; Halmos, G.B.; Oosting, S.F.; Langendijk, J.A.; et al. Dynamics of tumor hypoxia assessed by 18F-FAZA PET/CT in head and neck and lung cancer patients during chemoradiation: Possible implications for radiotherapy treatment planning strategies. Radiother. Oncol. 2014, 113, 198–203. [Google Scholar] [CrossRef]
- Servagi-Vernat, S.; Differding, S.; Hanin, F.X.; Labar, D.; Bol, A.; Lee, J.A.; Gregoire, V. A prospective clinical study of 18F-FAZA PET-CT hypoxia imaging in head and neck squamous cell carcinoma before and during radiation therapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1544–1552. [Google Scholar] [CrossRef]
- Trinkaus, M.E.; Blum, R.; Rischin, D.; Callahan, J.; Bressel, M.; Segard, T.; Roselt, P.; Eu, P.; Binns, D.; MacManus, M.P.; et al. Imaging of hypoxia with 18F-FAZA PET in patients with locally advanced non-small cell lung cancer treated with definitive chemoradiotherapy. J. Med. Imaging Radiat. Oncol. 2013, 57, 475–481. [Google Scholar] [CrossRef]
- Lewin, J.; Khamly, K.K.; Young, R.J.; Mitchell, C.; Hicks, R.J.; Toner, G.C.; Ngan, S.Y.; Chander, S.; Powell, G.J.; Herschtal, A.; et al. A phase Ib/II translational study of sunitinib with neoadjuvant radiotherapy in soft-tissue sarcoma. Br. J. Cancer 2014, 111, 2254–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horsman, M.R.; Mortensen, L.S.; Petersen, J.B.; Busk, M.; Overgaard, J. Imaging hypoxia to improve radiotherapy outcome. Nat. Rev. Clin. Oncol. 2012, 9, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Nyflot, M.J.; Kruser, T.J.; Traynor, A.M.; Khuntia, D.; Yang, D.T.; Hartig, G.K.; McCulloch, T.M.; Wiederholt, P.A.; Gentry, L.R.; Hoang, T.; et al. Phase 1 trial of bevacizumab with concurrent chemoradiation therapy for squamous cell carcinoma of the head and neck with exploratory functional imaging of tumor hypoxia, proliferation, and perfusion. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, Y.; Shizukuishi, K.; Koike, I.; Horiuchi, C.; Watanuki, K.; Hata, M.; Omura, M.; Odagiri, K.; Tohnai, I.; Inoue, T.; et al. Assessment of tumor hypoxia by 62Cu-ATSM PET/CT as a predictor of response in head and neck cancer: A pilot study. Ann. Nucl. Med. 2011, 25, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Dietz, D.W.; Dehdashti, F.; Grigsby, P.W.; Malyapa, R.S.; Myerson, R.J.; Picus, J.; Ritter, J.; Lewis, J.S.; Welch, M.J.; Siegel, B.A. Tumor hypoxia detected by positron emission tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing Neoadjuvant chemoradiotherapy for rectal carcinoma: A pilot study. Dis. Colon Rectum 2008, 51, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Grassi, I.; Nanni, C.; Cicoria, G.; Blasi, C.; Bunkheila, F.; Lopci, E.; Colletti, P.M.; Rubello, D.; Fanti, S. Usefulness of 64Cu-ATSM in head and neck cancer: A preliminary prospective study. Clin. Nucl. Med. 2014, 39, e59–e63. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.; Robinson, S.; Waterton, J. Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI. Br. J. Radiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.; Boult, J.K.; Jamin, Y.; Babur, M.; Finegan, K.G.; Williams, K.J.; Little, R.A.; Jackson, A.; Parker, G.J.; Reynolds, A.R.; et al. Oxygen-Enhanced MRI Accurately Identifies, Quantifies, and Maps Tumor Hypoxia in Preclinical Cancer Models. Cancer Res. 2016, 76, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, M.W.; Birer, S.R. Oxygen-Enhanced MRI Is a Major Advance in Tumor Hypoxia Imaging. Cancer Res. 2016, 76, 769–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, L.M.; Howe, F.A.; Griffiths, J.R.; Robinson, S.P. Tumor R2* is a prognostic indicator of acute radiotherapeutic response in rodent tumors. J. Magn. Reson. Imaging 2004, 19, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Rumley, C.N.; Lee, M.T.; Holloway, L.; Rai, R.; Min, M.; Forstner, D.; Fowler, A.; Liney, G. Multiparametric magnetic resonance imaging in mucosal primary head and neck cancer: A prospective imaging biomarker study. BMC Cancer 2017, 17, 475. [Google Scholar] [CrossRef] [PubMed]
- White, D.A.; Zhang, Z.; Li, L.; Gerberich, J.; Stojadinovic, S.; Peschke, P.; Mason, R.P. Developing oxygen-enhanced magnetic resonance imaging as a prognostic biomarker of radiation response. Cancer Lett. 2016, 380, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beeman, S.C.; Shui, Y.B.; Perez-Torres, C.J.; Engelbach, J.A.; Ackerman, J.J.; Garbow, J.R. O2-sensitive MRI distinguishes brain tumor versus radiation necrosis in murine models. Magn. Reson. Med. 2016, 75, 2442–2447. [Google Scholar] [CrossRef] [PubMed]
- Panagiotaki, E.; Walker-Samuel, S.; Siow, B.; Johnson, S.P.; Rajkumar, V.; Pedley, R.B.; Lythgoe, M.F.; Alexander, D.C. Noninvasive quantification of solid tumor microstructure using VERDICT MRI. Cancer Res. 2014, 74, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Steven, A.J.; Zhuo, J.; Melhem, E.R. Diffusion kurtosis imaging: An emerging technique for evaluating the microstructural environment of the brain. Am. J. Roentgenol. 2014, 202, W26–W33. [Google Scholar] [CrossRef]
- Soares, J.M.; Marques, P.; Alves, V.; Sousa, N. A hitchhiker’s guide to diffusion tensor imaging. Front. Neurosci. 2013, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Schilling, F.; Ros, S.; Hu, D.E.; D’Santos, P.; McGuire, S.; Mair, R.; Wright, A.J.; Mannion, E.; Franklin, R.J.; Neves, A.A.; et al. MRI measurements of reporter-mediated increases in transmembrane water exchange enable detection of a gene reporter. Nat. Biotechnol. 2017, 35, 75–80. [Google Scholar] [CrossRef]
- Lampinen, B.; Szczepankiewicz, F.; van Westen, D.; Englund, E.; Sundgren, P.C.; Lätt, J.; Ståhlberg, F.; Nilsson, M. Optimal experimental design for filter exchange imaging: Apparent exchange rate measurements in the healthy brain and in intracranial tumors. Magn. Reson. Med. 2017, 77, 1104–1114. [Google Scholar] [CrossRef]
- Sehy, J.V.; Ackerman, J.J.; Neil, J.J. Evidence that both fast and slow water ADC components arise from intracellular space. Magn. Reson. Med. 2002, 48, 765–770. [Google Scholar] [CrossRef] [Green Version]
- Surov, A.; Meyer, H.J.; Wienke, A. Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: A meta-analysis. Oncotarget 2017, 8, 59492–59499. [Google Scholar] [CrossRef]
- Afaq, A.; Andreou, A.; Koh, D.M. Diffusion-weighted magnetic resonance imaging for tumour response assessment: Why, when and how? Cancer Imaging 2010, 10, S179–S188. [Google Scholar] [CrossRef] [PubMed]
- Chenevert, T.L.; Stegman, L.D.; Taylor, J.M.; Robertson, P.L.; Greenberg, H.S.; Rehemtulla, A.; Ross, B.D. Diffusion magnetic resonance imaging: An early surrogate marker of therapeutic efficacy in brain tumors. J. Natl. Cancer Inst. 2000, 92, 2029–2036. [Google Scholar] [CrossRef]
- Mahmood, F.; Johannesen, H.H.; Geertsen, P.; Hansen, R.H. Repeated diffusion MRI reveals earliest time point for stratification of radiotherapy response in brain metastases. Phys. Med. Biol. 2017, 62, 2990–3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Loevner, L.; Quon, H.; Sherman, E.; Weinstein, G.; Kilger, A.; Poptani, H. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin. Cancer Res. 2009, 15, 986–994. [Google Scholar] [CrossRef]
- Schreuder, S.M.; Lensing, R.; Stoker, J.; Bipat, S. Monitoring treatment response in patients undergoing chemoradiotherapy for locally advanced uterine cervical cancer by additional diffusion-weighted imaging: A systematic review. J. Magn. Reson. Imaging 2015, 42, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.; Deroose, C.M.; Vandecaveye, V.; Haustermans, K. The role of diffusion-weighted MRI and 18F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: A systematic review. Radiother. Oncol. 2014, 113, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.; Debucquoy, A.; Deroose, C.M.; Vandecaveye, V.; Cutsem, E.V.; Wolthuis, A.; D’Hoore, A.; Sagaert, X.; Zhou, M.; Gevaert, O.; et al. Quantitative imaging outperforms molecular markers when predicting response to chemoradiotherapy for rectal cancer. Radiother. Oncol. 2017, 124, 104–109. [Google Scholar] [CrossRef]
- Chu, H.H.; Choi, S.H.; Ryoo, I.; Kim, S.C.; Yeom, J.A.; Shin, H.; Jung, S.C.; Lee, A.L.; Yoon, T.J.; Kim, T.M.; et al. Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: Comparison study of standard and high-b-value diffusion-weighted imaging. Radiology 2013, 269, 831–840. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.E.; Jo, Y.; Shim, W.H.; Nam, S.J.; Kim, J.H.; Yoo, R.E.; Choi, S.H.; Kim, H.S. Incorporating diffusion- and perfusion-weighted MRI into a radiomics model improves diagnostic performance for pseudoprogression in glioblastoma patients. Neuro-Oncology 2018. [Google Scholar] [CrossRef]
- Mannelli, L.; Kim, S.; Hajdu, C.H.; Babb, J.S.; Clark, T.W.; Taouli, B. Assessment of tumor necrosis of hepatocellular carcinoma after chemoembolization: Diffusion-weighted and contrast-enhanced MRI with histopathologic correlation of the explanted liver. Am. J. Roentgenol. 2009, 193, 1044–1052. [Google Scholar] [CrossRef]
- Hein, P.A.; Eskey, C.J.; Dunn, J.F.; Hug, E.B. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: Tumor recurrence versus radiation injury. Am. J. Neuroradiol. 2004, 25, 201–209. [Google Scholar] [PubMed]
- Asao, C.; Korogi, Y.; Kitajima, M.; Hirai, T.; Baba, Y.; Makino, K.; Kochi, M.; Morishita, S.; Yamashita, Y. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. Am. J. Neuroradiol. 2005, 26, 1455–1460. [Google Scholar] [PubMed]
- Zeng, Q.S.; Li, C.F.; Liu, H.; Zhen, J.H.; Feng, D.C. Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Brindle, K. New approaches for imaging tumour responses to treatment. Nat. Rev. Cancer 2008, 8, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Van Zijl, P.C.; Yadav, N.N. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn. Reson. Med. 2011, 65, 927–948. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhu, H.; Lim, M.; Blair, L.; Quinones-Hinojosa, A.; Messina, S.A.; Eberhart, C.G.; Pomper, M.G.; Laterra, J.; Barker, P.B.; et al. Three-dimensional amide proton transfer MR imaging of gliomas: Initial experience and comparison with gadolinium enhancement. J. Magn. Reson. Imaging 2013, 38, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Mehrabian, H.; Desmond, K.L.; Soliman, H.; Sahgal, A.; Stanisz, G.J. Differentiation between Radiation Necrosis and Tumor Progression Using Chemical Exchange Saturation Transfer. Clin. Cancer Res. 2017, 23, 3667–3675. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Negelein, E. Über den stoffwechsel der carcinomzelle. Biochem. Z. 1924, 152, 319–344. [Google Scholar] [CrossRef]
- Hensley, C.T.; Faubert, B.; Yuan, Q.; Lev-Cohain, N.; Jin, E.; Kim, J.; Jiang, L.; Ko, B.; Skelton, R.; Loudat, L.; et al. Metabolic Heterogeneity in Human Lung Tumors. Cell 2016, 164, 681–694. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl. 1), 122S–150S. [Google Scholar] [CrossRef]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.A.; Pruim, J.; Price, P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
- Slevin, F.; Subesinghe, M.; Ramasamy, S.; Sen, M.; Scarsbrook, A.F.; Prestwich, R.J. Assessment of outcomes with delayed 18F-FDG PET-CT response assessment in head and neck squamous cell carcinoma. Br. J. Radiol. 2015, 88, 20140592. [Google Scholar] [CrossRef]
- Prestwich, R.J.; Subesinghe, M.; Gilbert, A.; Chowdhury, F.U.; Sen, M.; Scarsbrook, A.F. Delayed response assessment with FDG-PET-CT following (chemo) radiotherapy for locally advanced head and neck squamous cell carcinoma. Clin. Radiol. 2012, 67, 966–975. [Google Scholar] [CrossRef]
- Hicks, R.J.; Mac Manus, M.P.; Matthews, J.P.; Hogg, A.; Binns, D.; Rischin, D.; Ball, D.L.; Peters, L.J. Early FDG-PET imaging after radical radiotherapy for non-small-cell lung cancer: Inflammatory changes in normal tissues correlate with tumor response and do not confound therapeutic response evaluation. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, M.; Appold, S.; Schreiber, A.; Abramyuk, A.; Abolmaali, N.; Kotzerke, J.; Baumann, M.; Zophel, K. Serial FDG-PET on patients with head and neck cancer: Implications for radiation therapy. Int. J. Radiat. Biol. 2009, 85, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Kubota, R.; Yamada, S.; Kubota, K.; Ishiwata, K.; Tamahashi, N.; Ido, T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: High accumulation in macrophages and granulation tissues studied by microautoradiography. J. Nucl. Med. 1992, 33, 1972–1980. [Google Scholar] [PubMed]
- Ardenkjaer-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef]
- Hesketh, R.L.; Brindle, K.M. Magnetic resonance imaging of cancer metabolism with hyperpolarized 13C-labeled cell metabolites. Curr. Opin. Chem. Biol. 2018, 45, 187–194. [Google Scholar] [CrossRef]
- Gutte, H.; Hansen, A.E.; Henriksen, S.T.; Johannesen, H.H.; Ardenkjaer-Larsen, J.; Vignaud, A.; Hansen, A.E.; Borresen, B.; Klausen, T.L.; Wittekind, A.M.; et al. Simultaneous hyperpolarized 13C-pyruvate MRI and 18F-FDG-PET in cancer (hyperPET): Feasibility of a new imaging concept using a clinical PET/MRI scanner. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 38–45. [Google Scholar]
- Aggarwal, R.; Vigneron, D.B.; Kurhanewicz, J. Hyperpolarized 1-[13C]-Pyruvate Magnetic Resonance Imaging Detects an Early Metabolic Response to Androgen Ablation Therapy in Prostate Cancer. Eur. Urol. 2017, 72, 1028–1029. [Google Scholar] [CrossRef]
- Day, S.E.; Kettunen, M.I.; Cherukuri, M.K.; Mitchell, J.B.; Lizak, M.J.; Morris, H.D.; Matsumoto, S.; Koretsky, A.P.; Brindle, K.M. Detecting response of rat C6 glioma tumors to radiotherapy using hyperpolarized [1-13C]pyruvate and 13C magnetic resonance spectroscopic imaging. Magn. Reson. Med. 2011, 65, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, F.A.; Kettunen, M.I.; Hu, D.E.; Jensen, P.R.; Zandt, R.I.; Karlsson, M.; Gisselsson, A.; Nelson, S.K.; Witney, T.H.; Bohndiek, S.E.; et al. Production of hyperpolarized [1,4-13C2]malate from [1,4-13C2]fumarate is a marker of cell necrosis and treatment response in tumors. Proc. Natl. Acad. Sci. USA 2009, 106, 19801–19806. [Google Scholar] [CrossRef] [PubMed]
- Duwel, S.; Durst, M.; Gringeri, C.V.; Kosanke, Y.; Gross, C.; Janich, M.A.; Haase, A.; Glaser, S.J.; Schwaiger, M.; Schulte, R.F.; et al. Multiparametric human hepatocellular carcinoma characterization and therapy response evaluation by hyperpolarized 13C MRSI. NMR Biomed. 2016, 29, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Bollineni, V.R.; Kramer, G.M.; Jansma, E.P.; Liu, Y.; Oyen, W.J. A systematic review on [18F]FLT-PET uptake as a measure of treatment response in cancer patients. Eur. J. Cancer 2016, 55, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Sanghera, B.; Wong, W.L.; Sonoda, L.I.; Beynon, G.; Makris, A.; Woolf, D.; Ardeshna, K. FLT PET-CT in evaluation of treatment response. Indian J. Nucl. Med. 2014, 29, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Kishino, T.; Hoshikawa, H.; Nishiyama, Y.; Yamamoto, Y.; Mori, N. Usefulness of 3′-deoxy-3′-18F-fluorothymidine PET for predicting early response to chemoradiotherapy in head and neck cancer. J. Nucl. Med. 2012, 53, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, F.; Grigsby, P.W.; Myerson, R.J.; Nalbantoglu, I.; Ma, C.; Siegel, B.A. Positron emission tomography with [18F]-3′-deoxy-3′fluorothymidine (FLT) as a predictor of outcome in patients with locally advanced resectable rectal cancer: A pilot study. Mol. Imaging Biol. 2013, 15, 106–113. [Google Scholar] [CrossRef]
- Shah, R.; Vattoth, S.; Jacob, R.; Manzil, F.F.; O’Malley, J.P.; Borghei, P.; Patel, B.N.; Cure, J.K. Radiation necrosis in the brain: Imaging features and differentiation from tumor recurrence. Radiographics 2012, 32, 1343–1359. [Google Scholar] [CrossRef]
- Leung, K. L-[methyl-11C[Methionine. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information: Bethesda, MD, USA, 2005. [Google Scholar]
- Zhao, C.; Zhang, Y.; Wang, J. A meta-analysis on the diagnostic performance of 18F-FDG and 11C-methionine PET for differentiating brain tumors. Am. J. Neuroradiol. 2014, 35, 1058–1065. [Google Scholar] [CrossRef]
- Sharma, R.; D’Souza, M.; Jaimini, A.; Hazari, P.P.; Saw, S.; Pandey, S.; Singh, D.; Solanki, Y.; Kumar, N.; Mishra, A.K.; et al. A comparison study of 11C-methionine and 18F-fluorodeoxyglucose positron emission tomography-computed tomography scans in evaluation of patients with recurrent brain tumors. Indian J. Nucl. Med. 2016, 31, 93–102. [Google Scholar] [CrossRef]
- Langen, K.J.; Hamacher, K.; Weckesser, M.; Floeth, F.; Stoffels, G.; Bauer, D.; Coenen, H.H.; Pauleit, D. O-(2-[18F]fluoroethyl)-L-tyrosine: Uptake mechanisms and clinical applications. Nucl. Med. Biol. 2006, 33, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Dunet, V.; Rossier, C.; Buck, A.; Stupp, R.; Prior, J.O. Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: A systematic review and Metaanalysis. J. Nucl. Med. 2012, 53, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Langen, K.J.; Holy, R.; Pinkawa, M.; Stoffels, G.; Nolte, K.W.; Kaiser, H.J.; Filss, C.P.; Fink, G.R.; Coenen, H.H.; et al. Assessment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J. Nucl. Med. 2012, 53, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Asano, Y.; Shinoda, J.; Nomura, Y.; Yonezawa, S.; Miwa, K.; Yano, H.; Iwama, T. Comparison of 11C-methionine, 11C-choline, and 18F-fluorodeoxyglucose-PET for distinguishing glioma recurrence from radiation necrosis. Neurol. Med. Chir. 2014, 54, 280–289. [Google Scholar] [CrossRef]
- Calabria, F.F.; Barbarisi, M.; Gangemi, V.; Grillea, G.; Cascini, G.L. Molecular imaging of brain tumors with radiolabeled choline PET. Neurosurg. Rev. 2018, 41, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Cicone, F.; Minniti, G.; Romano, A.; Papa, A.; Scaringi, C.; Tavanti, F.; Bozzao, A.; Maurizi Enrici, R.; Scopinaro, F. Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Lapela, M.; Eigtved, A.; Jyrkkio, S.; Grenman, R.; Kurki, T.; Lindholm, P.; Nuutinen, J.; Sutinen, E.; Solin, O.; Bjornskov, I.; et al. Experience in qualitative and quantitative FDG PET in follow-up of patients with suspected recurrence from head and neck cancer. Eur. J. Cancer 2000, 36, 858–867. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Aboagye, E.O.; Adams, J.E.; Aerts, H.J.; Barrington, S.F.; Beer, A.J.; Boellaard, R.; Bohndiek, S.E.; Brady, M.; Brown, G.; et al. Imaging biomarker roadmap for cancer studies. Nat. Rev. Clin. Oncol. 2017, 14, 169–186. [Google Scholar] [CrossRef]


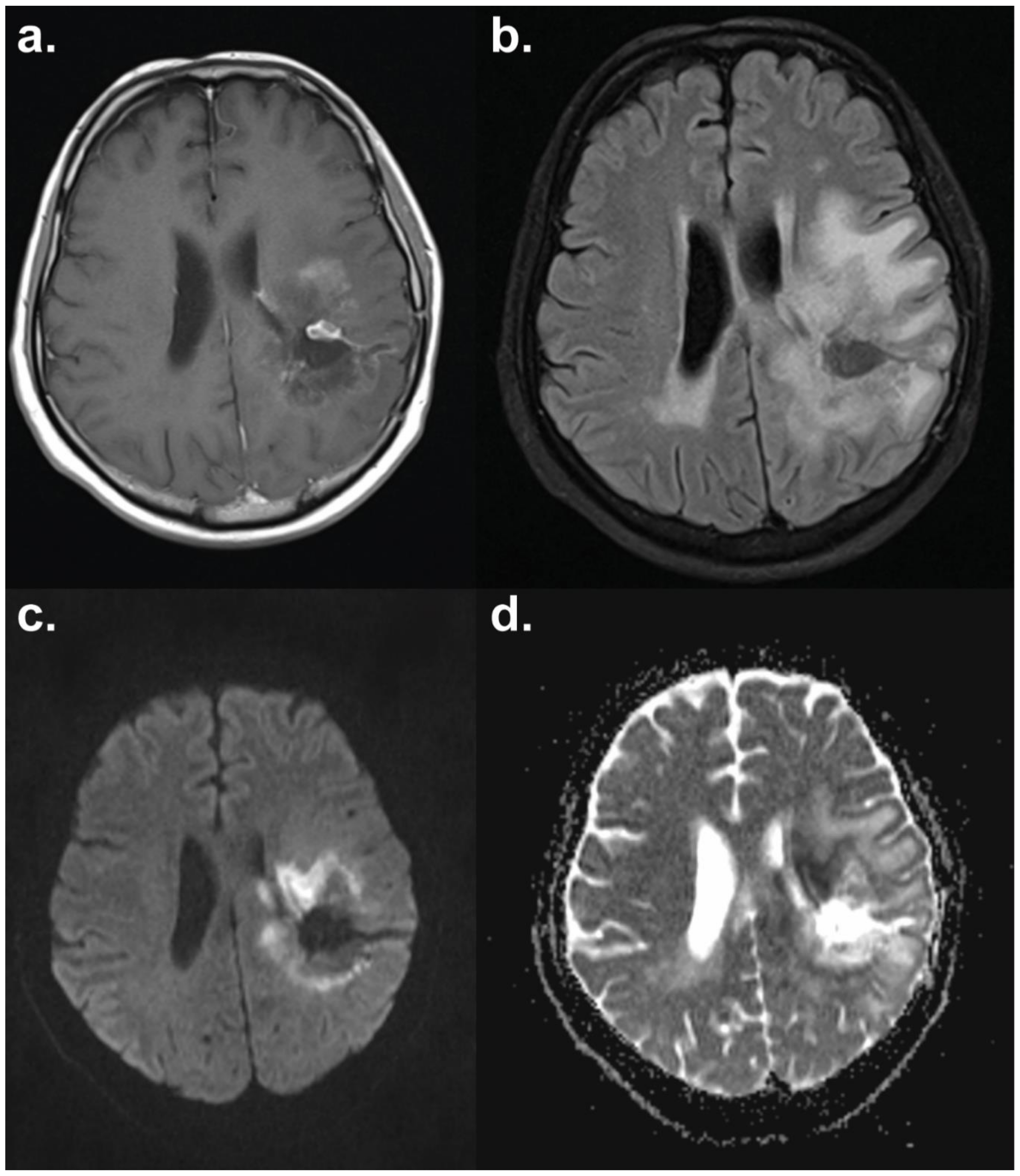



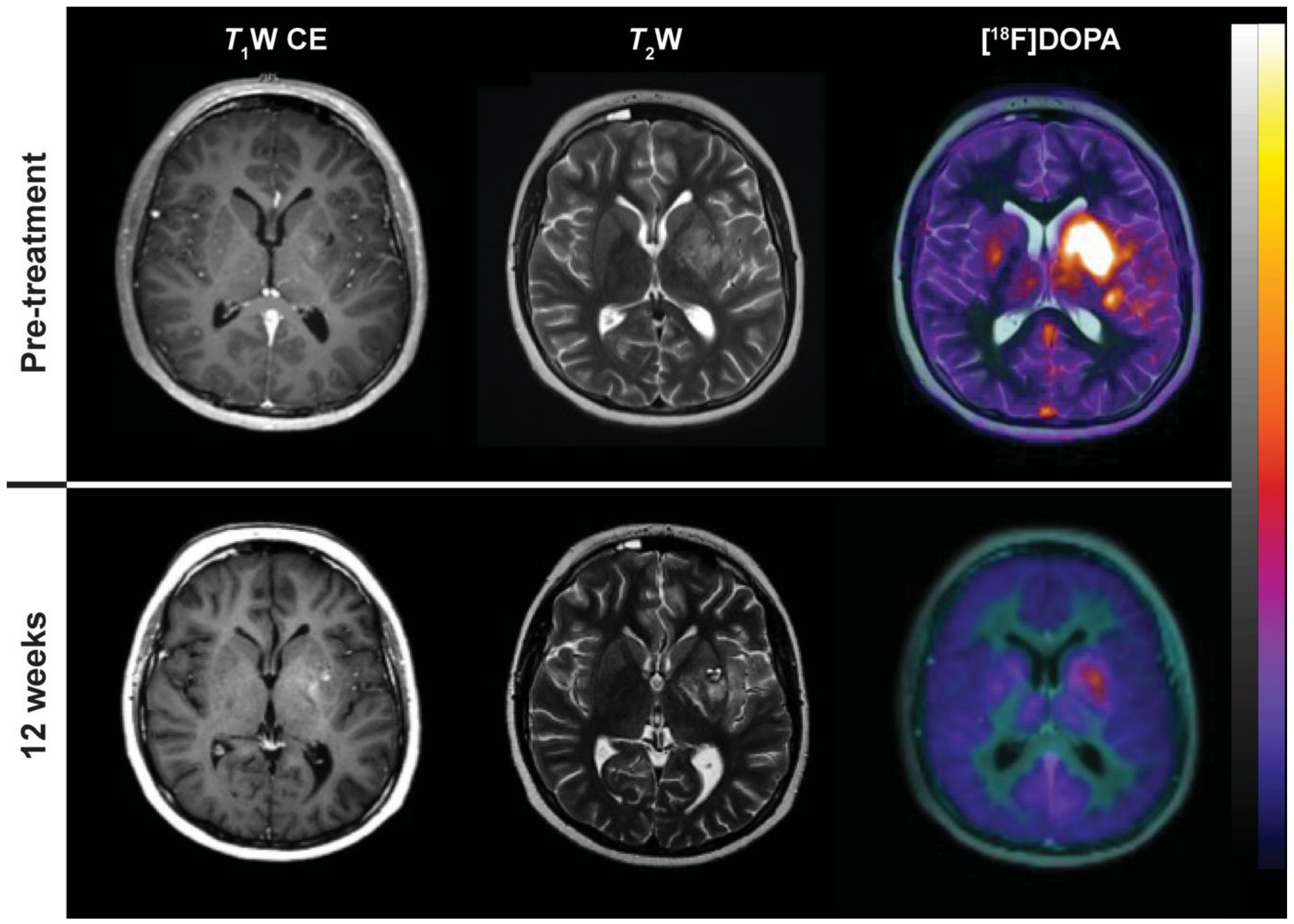
| WHO | RECIST 1.0 | RECIST 1.1 | |
|---|---|---|---|
| Measurement | Sum of maximal perpendicular diameters of all measured lesions | Sum of long axis of up to 10 target lesions | Sum of long axis of up to 5 target lesions (short axis for lymph nodes) |
| Complete response | Disappearance of all known disease | Disappearance of all target lesions | Disappearance of all target lesions |
| Partial response | ≥50% decrease in lesion size and no new lesions | ≥30% decrease in sum of target lesion diameters | ≥30% decrease in sum of target lesion diameters |
| No change/ Stable disease | Neither partial response or progressive disease | Neither partial response or progressive disease | Neither partial response or progressive disease |
| Progressive disease | ≥25% increase in lesion size or ≥1 new lesion | ≥20% increase in the sum of target lesions (no minimum size increase) | ≥20% increase in the sum of target lesions (≥5 mm absolute increase) |
| Functional imaging | None | None | 18F-FDG-PET can be used to complement CT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, A.; Davis, L.M.; Wilkinson, S.K.; Hesketh, R.L. Emerging Functional Imaging Biomarkers of Tumour Responses to Radiotherapy. Cancers 2019, 11, 131. https://doi.org/10.3390/cancers11020131
Campbell A, Davis LM, Wilkinson SK, Hesketh RL. Emerging Functional Imaging Biomarkers of Tumour Responses to Radiotherapy. Cancers. 2019; 11(2):131. https://doi.org/10.3390/cancers11020131
Chicago/Turabian StyleCampbell, Alan, Laura M. Davis, Sophie K. Wilkinson, and Richard L. Hesketh. 2019. "Emerging Functional Imaging Biomarkers of Tumour Responses to Radiotherapy" Cancers 11, no. 2: 131. https://doi.org/10.3390/cancers11020131
APA StyleCampbell, A., Davis, L. M., Wilkinson, S. K., & Hesketh, R. L. (2019). Emerging Functional Imaging Biomarkers of Tumour Responses to Radiotherapy. Cancers, 11(2), 131. https://doi.org/10.3390/cancers11020131





