Impact of Planning Method (Conventional versus Virtual) on Time to Therapy Initiation and Resection Margins: A Retrospective Analysis of 104 Immediate Jaw Reconstructions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
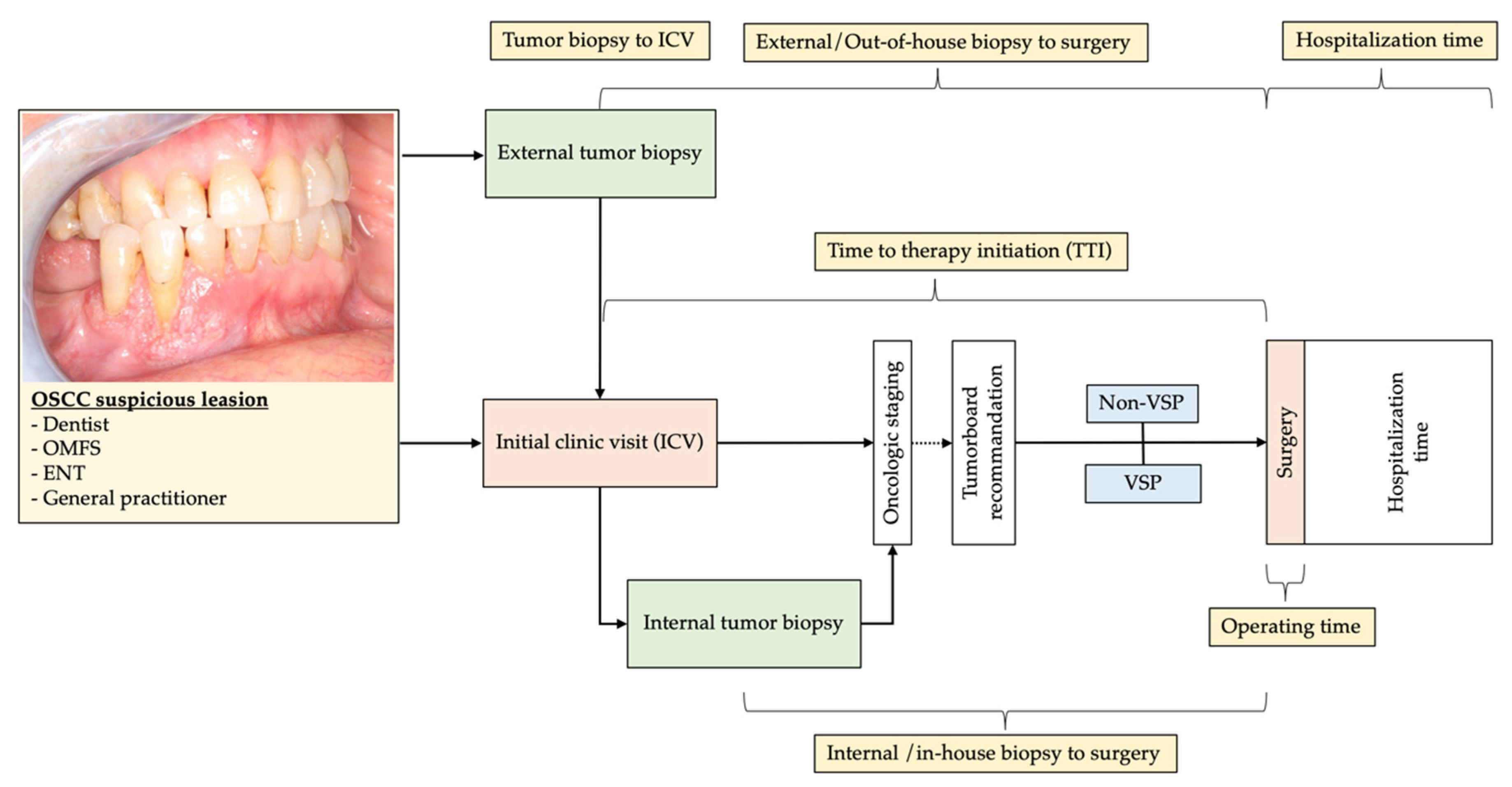
2.1. Study Design and Patient Population
2.2. Study Parameters and Evaluator Calibration
2.3. Inclusion and Exclusion Criteria for Study Subjects
2.4. Statistical Analyses
2.5. Ethics Statement/Confirmation of Patients’ Permission
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markopoulos, A.K. Current aspects on oral squamous cell carcinoma. Open Dent. J. 2012, 6, 126–130. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.M.; Kim, S.W.; Duvvuri, U.; Johnson, J.T.; Myers, E.N.; Ferris, R.L.; Gooding, W.E.; Seethala, R.R.; Chiosea, S.I. Early squamous cell carcinoma of the oral tongue: Comparing margins obtained from the glossectomy specimen to margins from the tumor bed. Oral Oncol. 2013, 49, 1077–1082. [Google Scholar] [CrossRef]
- Hinni, M.L.; Ferlito, A.; Brandwein-Gensler, M.S.; Takes, R.P.; Silver, C.E.; Westra, W.H.; Seethala, R.R.; Rodrigo, J.P.; Corry, J.; Bradford, C.R.; et al. Surgical margins in head and neck cancer: A contemporary review. Head Neck 2013, 35, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.H.; Thompson, L.D.; Brandwein-Gensler, M.S.; Weiss, B.G.; Canis, M.; Purgina, B.; Prabhu, A.V.; Lai, C.; Shuai, Y.; Carroll, W.R.; et al. Early oral tongue squamous cell carcinoma: Sampling of margins from tumor bed and worse local control. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.D.; Oliver, D.A.; Varvares, M.A. Surgical margin determination in head and neck oncology: Current clinical practice. The results of an International American Head and Neck Society Member Survey. Head Neck 2005, 27, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Thomas Robbins, K.; Triantafyllou, A.; Suarez, C.; Lopez, F.; Hunt, J.L.; Strojan, P.; Williams, M.D.; Braakhuis, B.J.M.; de Bree, R.; Hinni, M.L.; et al. Surgical margins in head and neck cancer: Intra- and postoperative considerations. Auris Nasus Larynx 2019, 46, 10–17. [Google Scholar] [CrossRef]
- Muller, S.; Boy, S.C.; Day, T.A.; Magliocca, K.R.; Richardson, M.S.; Sloan, P.; Tilakaratne, W.M.; Zain, R.B.; Thompson, L.D.R. Data set for the reporting of oral cavity carcinomas: Explanations and recommendations of the guidelines from the International Collaboration of Cancer Reporting. Arch. Pathol. Lab. Med. 2019, 143, 439–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Diagnostik und Therapie des Mundhöhlenkarzinoms, Langversion 3.01 (Konsultationsfassung), 2019, AWMF Registernummer: 007/100OL. 2019. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/mundhoehlenkarzinom/ (accessed on 7 February 2021).
- Chen, C.H.; Hsu, M.Y.; Jiang, R.S.; Wu, S.H.; Chen, F.J.; Liu, S.A. Shrinkage of head and neck cancer specimens after formalin fixation. J. Chin. Med. Assoc. 2012, 75, 109–113. [Google Scholar] [CrossRef]
- Looser, K.G.; Shah, J.P.; Strong, E.W. The significance of “positive” margins in surgically resected epidermoid carcinomas. Head Neck Surg. 1978, 1, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, N.F.; Godden, D.R.; Wilson, G.E.; Butterworth, D.M.; Woodwards, R.T. Do frozen sections help achieve adequate surgical margins in the resection of oral carcinoma? Int. J. Oral Maxillofac. Surg. 2003, 32, 152–158. [Google Scholar] [CrossRef]
- Fridman, E.; Na’ara, S.; Agarwal, J.; Amit, M.; Bachar, G.; Villaret, A.B.; Brandao, J.; Cernea, C.R.; Chaturvedi, P.; Clark, J.; et al. The role of adjuvant treatment in early-stage oral cavity squamous cell carcinoma: An international collaborative study. Cancer 2018, 124, 2948–2955. [Google Scholar] [CrossRef]
- Jayasooriya, P.R.; Pitakotuwage, T.N.; Mendis, B.R.; Lombardi, T. Descriptive study of 896 Oral squamous cell carcinomas from the only University based Oral Pathology Diagnostic Service in Sri Lanka. BMC Oral Health 2016, 16, 1. [Google Scholar] [CrossRef]
- Friedland, P.L.; Bozic, B.; Dewar, J.; Kuan, R.; Meyer, C.; Phillips, M. Impact of multidisciplinary team management in head and neck cancer patients. Br. J. Cancer 2011, 104, 1246–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gou, L.; Yang, W.; Qiao, X.; Ye, L.; Yan, K.; Li, L.; Li, C. Marginal or segmental mandibulectomy: Treatment modality selection for oral cancer: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, D.A. Fibula free flap: A new method of mandible reconstruction. Plast. Reconstr. Surg. 1989, 84, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zlotolow, I.M.; Huryn, J.M.; Piro, J.D.; Lenchewski, E.; Hidalgo, D.A. Osseointegrated implants and functional prosthetic rehabilitation in microvascular fibula free flap reconstructed mandibles. Am. J. Surg. 1992, 164, 677–681. [Google Scholar] [CrossRef]
- Attia, S.; Wiltfang, J.; Streckbein, P.; Wilbrand, J.F.; El Khassawna, T.; Mausbach, K.; Howaldt, H.P.; Schaaf, H. Functional and aesthetic treatment outcomes after immediate jaw reconstruction using a fibula flap and dental implants. J. Craniomaxillofac. Surg. 2019, 47, 786–791. [Google Scholar] [CrossRef]
- Attia, S.; Wiltfang, J.; Pons-Kuhnemann, J.; Wilbrand, J.F.; Streckbein, P.; Kahling, C.; Howaldt, H.P.; Schaaf, H. Survival of dental implants placed in vascularised fibula free flaps after jaw reconstruction. J. Craniomaxillofac. Surg. 2018, 46, 1205–1210. [Google Scholar] [CrossRef]
- Chang, Y.M.; Wei, F.C. Fibula jaw-in-a-day with minimal computer-aided design and manufacturing: Maximizing efficiency, cost-effectiveness, intraoperative flexibility, and quality. Plast. Reconstr. Surg. 2021, 147, 476–479. [Google Scholar] [CrossRef]
- Garrido-Martinez, P.; Pena-Cardelles, J.F.; Pozo-Kreilinger, J.J.; Esparza-Gomez, G.; Montesdeoca-Garcia, N.; Cebrian-Carretero, J.L. Jaw in a day: Osseointegration of the implants in the patient’s leg before reconstructive surgery of a maxilla with ameloblastoma. A 4-year follow-up case report. J. Clin. Exp Dent. 2021, 13, e81–e87. [Google Scholar] [CrossRef] [PubMed]
- Khatib, B.; Cheng, A.; Sim, F.; Bray, B.; Patel, A. Challenges with the jaw in a day technique. J. Oral Maxillofac. Surg. 2020, 78, 1869.e1–1869.e10. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Harrison, P.; Cheng, A.; Bray, B.; Bell, R.B. Fibular reconstruction of the maxilla and mandible with immediate implant-supported prosthetic rehabilitation: Jaw in a day. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 369–386. [Google Scholar] [CrossRef]
- Sukato, D.C.; Hammer, D.; Wang, W.; Shokri, T.; Williams, F.; Ducic, Y. Experience with “Jaw in a Day” technique. J. Craniofac. Surg. 2020, 31, 1212–1217. [Google Scholar] [CrossRef]
- Han, H.H.; Kim, H.Y.; Lee, J.Y. The pros and cons of computer-aided surgery for segmental mandibular reconstruction after oncological surgery. Arch. Craniofac. Surg. 2017, 18, 149–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alassaf, M.H.; Li, W.; Joshi, A.S.; Hahn, J.K. Computer-based planning system for mandibular reconstruction. Stud. Health Technol. Inf. 2014, 196, 6–10. [Google Scholar]
- Ciocca, L.; De Crescenzio, F.; Fantini, M.; Scotti, R. CAD/CAM and rapid prototyped scaffold construction for bone regenerative medicine and surgical transfer of virtual planning: A pilot study. Comput. Med. Imaging Graph. 2009, 33, 58–62. [Google Scholar] [CrossRef]
- Ciocca, L.; Mazzoni, S.; Fantini, M.; Persiani, F.; Marchetti, C.; Scotti, R. CAD/CAM guided secondary mandibular reconstruction of a discontinuity defect after ablative cancer surgery. J. Craniomaxillofac. Surg. 2012, 40, e511–e515. [Google Scholar] [CrossRef]
- Ciocca, L.; Marchetti, C.; Mazzoni, S.; Baldissara, P.; Gatto, M.R.; Cipriani, R.; Scotti, R.; Tarsitano, A. Accuracy of fibular sectioning and insertion into a rapid-prototyped bone plate, for mandibular reconstruction using CAD-CAM technology. J. Craniomaxillofac. Surg. 2015, 43, 28–33. [Google Scholar] [CrossRef]
- Toto, J.M.; Chang, E.I.; Agag, R.; Devarajan, K.; Patel, S.A.; Topham, N.S. Improved operative efficiency of free fibula flap mandible reconstruction with patient-specific, computer-guided preoperative planning. Head Neck 2015, 37, 1660–1664. [Google Scholar] [CrossRef] [PubMed]
- Culie, D.; Dassonville, O.; Poissonnet, G.; Riss, J.C.; Fernandez, J.; Bozec, A. Virtual planning and guided surgery in fibular free-flap mandibular reconstruction: A 29-case series. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 175–178. [Google Scholar] [CrossRef]
- Hanken, H.; Schablowsky, C.; Smeets, R.; Heiland, M.; Sehner, S.; Riecke, B.; Nourwali, I.; Vorwig, O.; Grobe, A.; Al-Dam, A. Virtual planning of complex head and neck reconstruction results in satisfactory match between real outcomes and virtual models. Clin. Oral Investig. 2015, 19, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Wilde, F.; Hanken, H.; Probst, F.; Schramm, A.; Heiland, M.; Cornelius, C.P. Multicenter study on the use of patient-specific CAD/CAM reconstruction plates for mandibular reconstruction. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 2035–2051. [Google Scholar] [CrossRef]
- Mazzola, F.; Smithers, F.; Cheng, K.; Mukherjee, P.; Hubert Low, T.H.; Ch’ng, S.; Palme, C.E.; Clark, J.R. Time and cost-analysis of virtual surgical planning for head and neck reconstruction: A matched pair analysis. Oral Oncol. 2020, 100, 104491. [Google Scholar] [CrossRef] [PubMed]
- Bosc, R.; Hersant, B.; Carloni, R.; Niddam, J.; Bouhassira, J.; De Kermadec, H.; Bequignon, E.; Wojcik, T.; Julieron, M.; Meningaud, J.P. Mandibular reconstruction after cancer: An in-house approach to manufacturing cutting guides. Int. J. Oral Maxillofac. Surg. 2017, 46, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Mottini, M.; Seyed Jafari, S.M.; Shafighi, M.; Schaller, B. New approach for virtual surgical planning and mandibular reconstruction using a fibula free flap. Oral Oncol. 2016, 59, e6–e9. [Google Scholar] [CrossRef]
- Smithers, F.A.E.; Cheng, K.; Jayaram, R.; Mukherjee, P.; Clark, J.R. Maxillofacial reconstruction using in-house virtual surgical planning. ANZ J. Surg. 2018, 88, 907–912. [Google Scholar] [CrossRef]
- Xiao, R.; Ward, M.C.; Yang, K.; Adelstein, D.J.; Koyfman, S.A.; Prendes, B.L.; Burkey, B.B. Increased pathologic upstaging with rising time to treatment initiation for head and neck cancer: A mechanism for increased mortality. Cancer 2018, 124, 1400–1414. [Google Scholar] [CrossRef]
- Barry, C.P.; MacDhabheid, C.; Tobin, K.; Stassen, L.F.; Lennon, P.; Toner, M.; O’Regan, E.; Clark, J.R. ‘Out of house’ virtual surgical planning for mandible reconstruction after cancer resection: Is it oncologically safe? Int. J. Oral Maxillofac. Surg. 2020. [Google Scholar] [CrossRef]
- Liu, X.J.; Gui, L.; Mao, C.; Peng, X.; Yu, G.Y. Applying computer techniques in maxillofacial reconstruction using a fibula flap: A messenger and an evaluation method. J. Craniofac. Surg. 2009, 20, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.S.; Barry, C.; Ho, M.; Shaw, R. A new classification for mandibular defects after oncological resection. Lancet Oncol. 2016, 17, e23–e30. [Google Scholar] [CrossRef]
- Brown, J.S.; Shaw, R.J. Reconstruction of the maxilla and midface: Introducing a new classification. Lancet Oncol. 2010, 11, 1001–1008. [Google Scholar] [CrossRef]
- Freeman, G.H.; Halton, J.H. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika 1951, 38, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Holzle, F.; Ristow, O.; Rau, A.; Mucke, T.; Loeffelbein, D.J.; Mitchell, D.A.; Wolff, K.D.; Kesting, M.R. Evaluation of the vessels of the lower leg before microsurgical fibular transfer. Part I: Anatomical variations in the arteries of the lower leg. Br. J. Oral Maxillofac. Surg. 2011, 49, 270–274. [Google Scholar] [CrossRef]
- Battaglia, S.; Maiolo, V.; Savastio, G.; Zompatori, M.; Contedini, F.; Antoniazzi, E.; Cipriani, R.; Marchetti, C.; Tarsitano, A. Osteomyocutaneous fibular flap harvesting: Computer-assisted planning of perforator vessels using Computed Tomographic Angiography scan and cutting guide. J. Craniomaxillofac. Surg. 2017, 45, 1681–1686. [Google Scholar] [CrossRef]
- Ribuffo, D.; Atzeni, M.; Saba, L.; Guerra, M.; Mallarini, G.; Proto, E.B.; Grinsell, D.; Ashton, M.W.; Rozen, W.M. Clinical study of peroneal artery perforators with computed tomographic angiography: Implications for fibular flap harvest. Surg. Radiol. Anat. 2010, 32, 329–334. [Google Scholar] [CrossRef]
- Abou-Foul, A.K.; Borumandi, F. Anatomical variants of lower limb vasculature and implications for free fibula flap: Systematic review and critical analysis. Microsurgery 2016, 36, 165–172. [Google Scholar] [CrossRef]
- Ettinger, K.S.; Alexander, A.E.; Arce, K. Computed Tomographic Angiography Perforator Localization for Virtual Surgical Planning of Osteocutaneous Fibular Free Flaps in Head and Neck Reconstruction. J. Oral Maxillofac. Surg. 2018, 76, 2220–2230. [Google Scholar] [CrossRef] [Green Version]
- Kelly, A.M.; Cronin, P.; Hussain, H.K.; Londy, F.J.; Chepeha, D.B.; Carlos, R.C. Preoperative MR angiography in free fibula flap transfer for head and neck cancer: Clinical application and influence on surgical decision making. AJR Am. J. Roentgenol. 2007, 188, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosson, G.D.; Singh, N.K. Devascularizing complications of free fibula harvest: Peronea arteria magna. J. Reconstr Microsurg. 2005, 21, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Cariati, P.; Farhat, M.C.; Dyalram, D.; Ferrari, S.; Lubek, J.E. The deep circumflex iliac artery free flap in maxillofacial reconstruction: A comparative institutional analysis. Oral Maxillofac. Surg. 2021, 1–6. [Google Scholar] [CrossRef]
- Jehn, P.; Spalthoff, S.; Korn, P.; Zeller, A.N.; Dittmann, J.; Zimmerer, R.; Tavassol, F.; Gellrich, N.C. Patient-specific implant modification for alloplastic bridging of mandibular segmental defects in head and neck surgery. J. Craniomaxillofac. Surg. 2020, 48, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T.; Galloway, T.J.; Handorf, E.A.; Egleston, B.L.; Wang, L.S.; Mehra, R.; Flieder, D.B.; Ridge, J.A. Survival impact of increasing time to treatment initiation for patients with head and neck cancer in the United States. J. Clin. Oncol. 2016, 34, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Kim, S.; Tighiouart, M.; Mita, A.; Scher, K.S.; Epstein, J.B.; Laury, A.; Prasad, R.; Ali, N.; Patio, C.; et al. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer 2018, 124, 3154–3162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeGraaff, L.H.; Platek, A.J.; Iovoli, A.J.; Wooten, K.E.; Arshad, H.; Gupta, V.; McSpadden, R.P.; Kuriakose, M.A.; Hicks, W.L., Jr.; Platek, M.E.; et al. The effect of time between diagnosis and initiation of treatment on outcomes in patients with head and neck squamous cell carcinoma. Oral Oncol. 2019, 96, 148–152. [Google Scholar] [CrossRef]
- Abler, A.; Roser, M.; Weingart, D. On the indications for and morbidity of segmental resection of the mandible for squamous cell carcinoma in the lower oral cavity. Mund Kiefer Gesichtschir. 2005, 9, 137–142. [Google Scholar] [CrossRef]
- Kansy, K.; Mueller, A.A.; Mucke, T.; Kopp, J.B.; Koersgen, F.; Wolff, K.D.; Zeilhofer, H.F.; Holzle, F.; Pradel, W.; Schneider, M.; et al. Microsurgical reconstruction of the head and neck-current concepts of maxillofacial surgery in Europe. J. Craniomaxillofac. Surg. 2014, 42, 1610–1613. [Google Scholar] [CrossRef]
- Rogers, S.N.; Devine, J.; Lowe, D.; Shokar, P.; Brown, J.S.; Vaugman, E.D. Longitudinal health-related quality of life after mandibular resection for oral cancer: A comparison between rim and segment. Head Neck 2004, 26, 54–62. [Google Scholar] [CrossRef]
- Cornelius, C.P.; Smolka, W.; Giessler, G.A.; Wilde, F.; Probst, F.A. Patient-specific reconstruction plates are the missing link in computer-assisted mandibular reconstruction: A showcase for technical description. J. Craniomaxillofac. Surg. 2015, 43, 624–629. [Google Scholar] [CrossRef]
- Weitz, J.; Bauer, F.J.; Hapfelmeier, A.; Rohleder, N.H.; Wolff, K.D.; Kesting, M.R. Accuracy of mandibular reconstruction by three-dimensional guided vascularised fibular free flap after segmental mandibulectomy. Br. J. Oral Maxillofac. Surg. 2016, 54, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Wilde, F.; Cornelius, C.P.; Schramm, A. Computer-assisted mandibular reconstruction using a patient-specific reconstruction plate fabricated with computer-aided design and manufacturing techniques. Craniomaxillofac. Trauma Reconstr. 2014, 7, 158–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rustemeyer, J.; Busch, A.; Sari-Rieger, A. Application of computer-aided designed/computer-aided manufactured techniques in reconstructing maxillofacial bony structures. Oral Maxillofac. Surg. 2014, 18, 471–476. [Google Scholar] [CrossRef]
- Deek, N.F.; Wei, F.C. Computer-assisted surgery for segmental mandibular reconstruction with the osteoseptocutaneous fibula flap: Can we instigate ideological and technological reforms? Plast. Reconstr. Surg. 2016, 137, 963–970. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, H.Q.; Fan, S.; Zhang, D.M.; Huang, Z.Q.; Chen, W.L.; Ye, J.T.; Li, J.S. Mandibular reconstruction with the vascularized fibula flap: Comparison of virtual planning surgery and conventional surgery. Int. J. Oral Maxillofac. Surg. 2016, 45, 1400–1405. [Google Scholar] [CrossRef]
- Bell, R.B. Computer planning and intraoperative navigation in cranio-maxillofacial surgery. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 135–156. [Google Scholar] [CrossRef]
- Antony, A.K.; Chen, W.F.; Kolokythas, A.; Weimer, K.A.; Cohen, M.N. Use of virtual surgery and stereolithography-guided osteotomy for mandibular reconstruction with the free fibula. Plast. Reconstr. Surg. 2011, 128, 1080–1084. [Google Scholar] [CrossRef]
- Modabber, A.; Legros, C.; Rana, M.; Gerressen, M.; Riediger, D.; Ghassemi, A. Evaluation of computer-assisted jaw reconstruction with free vascularized fibular flap compared to conventional surgery: A clinical pilot study. Int. J. Med. Robot. 2012, 8, 215–220. [Google Scholar] [CrossRef]
- Roser, S.M.; Ramachandra, S.; Blair, H.; Grist, W.; Carlson, G.W.; Christensen, A.M.; Weimer, K.A.; Steed, M.B. The accuracy of virtual surgical planning in free fibula mandibular reconstruction: Comparison of planned and final results. J. Oral Maxillofac. Surg. 2010, 68, 2824–2832. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, L.; Mazzoni, S.; Fantini, M.; Persiani, F.; Baldissara, P.; Marchetti, C.; Scotti, R. A CAD/CAM-prototyped anatomical condylar prosthesis connected to a custom-made bone plate to support a fibula free flap. Med. Biol. Eng. Comput. 2012, 50, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, H.; Wahab-Gothe, T.; Kerkmann, H.; Streckbein, P.; Obert, M.; Pons-Kuehnemann, J.; Ahrens, M.; Howaldt, H.P.; Attia, S. Comparison between flat-panel volume computed tomography and histologic assessments of bone invasion of maxillofacial tumors: Utility of an instantaneous radiologic diagnostic tool. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 191–198. [Google Scholar] [CrossRef]
- Slootweg, P.J.; Hordijk, G.J.; Schade, Y.; van Es, R.J.; Koole, R. Treatment failure and margin status in head and neck cancer. A critical view on the potential value of molecular pathology. Oral Oncol. 2002, 38, 500–503. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Murali, R.; Gao, K.; Elliott, M.S.; Clark, J.R. The prognostic and staging implications of bone invasion in oral squamous cell carcinoma. Cancer 2011, 117, 4460–4467. [Google Scholar] [CrossRef]
- Rodby, K.A.; Turin, S.; Jacobs, R.J.; Cruz, J.F.; Hassid, V.J.; Kolokythas, A.; Antony, A.K. Advances in oncologic head and neck reconstruction: Systematic review and future considerations of virtual surgical planning and computer aided design/computer aided modeling. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Yanamoto, S.; Yamada, S.; Takahashi, H.; Yoshitomi, I.; Kawasaki, G.; Ikeda, H.; Minamizato, T.; Shiraishi, T.; Fujita, S.; Ikeda, T.; et al. Clinicopathological risk factors for local recurrence in oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2012, 41, 1195–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanoni, D.K.; Migliacci, J.C.; Xu, B.; Katabi, N.; Montero, P.H.; Ganly, I.; Shah, J.P.; Wong, R.J.; Ghossein, R.A.; Patel, S.G. A Proposal to redefine close surgical margins in squamous cell carcinoma of the oral tongue. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Lai, C.H.; Fang, C.C.; Yang, Y.H.; Chen, P.C.; Lee, C.P.; Chen, M.F. Identification of high-risk subgroups of patients with oral cavity cancer in need of postoperative adjuvant radiotherapy or chemo-radiotherapy. Medicine 2016, 95, e3770. [Google Scholar] [CrossRef]
- Magliocca, K.R. Surgical margins: The perspective of pathology. Oral Maxillofac. Surg. Clin. N. Am. 2017, 29, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.W.; Koljenovic, S.; Hardillo, J.A.; Ten Hove, I.; Meeuwis, C.A.; Sewnaik, A.; Dronkers, E.A.; Bakker Schut, T.C.; Langeveld, T.P.; Molenaar, J.; et al. Resection margins in oral cancer surgery: Room for improvement. Head Neck 2016, 38, E2197–E2203. [Google Scholar] [CrossRef] [PubMed]




| N = 104 | VSP FFF (%) 41 (39.4%) | Non-VSP FFF (%) 63 (60.6%) | p |
|---|---|---|---|
| Age (years), mean ± SD | 62.0 ± 10.0 | 59.2 ± 9.2 | 0.146 |
| Follow-up (months), mean ± SD | 20.5 ± 16.4 | 64.9 ± 52.1 | 0.502 |
| Sex, n (%) | |||
| Male | 26 (63.4%) | 44 (68.5%) | |
| Female | 15 (36.6%) | 19 (31.5%) | 0.318 |
| Tumor site, n (%) | |||
| Gingiva of alveolar crest | 6 (14.6%) | 24 (34.8%) | |
| Mouth floor | 12 (29.3%) | 23 (33.3%) | |
| Retromolar region | 18 (43.9%) | 10 (14.5%) | |
| Maxilla | 5 (12.2%) | 5 (7.2%) | |
| Planum buccale | - | 1 (1.4%) | |
| UICC tumor stage, n (%) | |||
| Stage I | 5 (12.2%) | 5 (7.2%) | |
| Stage II | 1 (2.4%) | 10 (14.5%) | |
| Stage III | 6 (14.6%) | 6 (8.7%) | |
| Stage IV | 29 (70.7%) | 42 (60.9%) | |
| Tumor diameter (mm), mean ± SD | 37.0 ± 16.4 | 33.5 ± 16.0 | 0.283 |
| Node-positive, n (%) | 20 (48.8%) | 31 (49.2%) | 0.562 |
| Extracapsular spread, n (%) | 6 (14.6%) | - | |
| Bone invasion, n (%) | 23 (56.1%) | 32 (50.8%) | 0.372 |
| Bone erosion, n (%) | 6 (14.6%) | 12 (19.0%) | 0.559 |
| Bone margin, n (%) | |||
| Clear | 39 (95.1%) | 61 (96.8%) | |
| Close | - | - | |
| Involved | 2 (4.9%) | 2 (3.2%) | 0.516 |
| Soft tissue margin, n (%) | |||
| Clear | 26 (63.4%) | 46 (73.0%) | |
| Close | 10 (24.4%) | 13 (20.6%) | |
| Involved | 5 (12.2%) | 4 (6.3%) | 0.470 |
| Oncologic therapy, n (%) | |||
| S | 13 (31.7%) | 33 (52.4%) | |
| S + RT | 13 (31.7%) | 12 (19.0%) | |
| S + RCT | 15 (36.6%) | 18 (28.6%) | 0.102 |
| N = 104 | VSP FFF (%) 41 (39.4%) | Non-VSP FFF (%) 63 (60.6%) | p |
|---|---|---|---|
| Maxilla (Brown class), n (%) | |||
| II | 3 (7.3%) | 5 (7.9%) | - |
| III | 1 (2.4%) | - | - |
| IV | 1 (2.4%) | - | - |
| Mandible (Brown class), n (%) | |||
| I | 9 (22.0%) | 19 (30.2%) | - |
| II | 10 (24.4%) | 18 (28.6%) | - |
| III | 14 (34.1%) | 20 (31.7%) | - |
| IV | 3 (7.3%) | 1 (1.6%) | - |
| Reconstruction length (cm), mean ± SD | 9.02 ± 2.80 | 7.23 ± 1.83 | 0.002 |
| Number of segments, n (%) | |||
| 1 | 10 (24.4%) | 30 (47.6%) | |
| 2 | 20 (48.8%) | 20 (31.7%) | |
| 3 | 11 (26.8%) | 13 (20.6%) | 0.014 |
| N =104 | VSP FFF n = 41 (39.4%) | Non-VSP FFF n = 63 (60.6%) | p |
|---|---|---|---|
| Tumor biopsy, n (%) | |||
| Internal/In-house | 27 (65.9%) | 40 (63.5%) | - |
| External/Out-of-house | 14 (34.1%) | 23 (36.5%) | 0.806 |
| ICV to surgery (days), mean ± SD (median) | |||
| Internal/In-house | 53.5 ± 26.8 (46.5) | 36.8 ± 21.1 (33.0) | 0.006 |
| External/Out-of-house | 35.8 ± 15.5 (35.0) | 33.9 ± 13.4 (31.0) | 0.696 |
| Biopsy to surgery (days), mean ± SD (median) | |||
| Internal/In-house | 47.7 ± 25.2 (43.0) | 34.5 ± 20.6 (34.5) | 0.022 |
| External/Out-of-house | 44.7 ± 15.4 (46.0) | 44.7 ± 14.1 (46.0) | 0.995 |
| ICV to (days), mean ± SD (median) | |||
| Internal/In-house | 5.6 ± 9.0 (1.0) | 2.3 ± 4.0 (1.0) | 0.045 |
| External/Out-of-house * | −9.6 ± 6.2 (−7.0) | −8.0 ± 5.6 (−7.0) | 0.424 |
| CT recipient site | 10.1 ± 9.5 (8.0) | 13.4 ± 12.8 (10.0) | 0.160 |
| CTA/MRA donor site | 14.6 ± 11.6 (14.0) | 15.7 ± 11.8 (13.0) | 0.641 |
| Kickoff VSP | 21.3 ± 15.6 (8.0) | - | - |
| Approval VSP | 34.4 ± 17.8 (34.5) | - | - |
| Shipping VSP/PSI | 38.6 ± 18.5 (35.5) | - | - |
| Surgery | 47.2 ± 24.5 (42.0) | 35.7 ± 18.6 (31.0) | 0.008 |
| Oncologic board ** | 14.9 ± 8.9 (14.0) | - | - |
| Entire VSP turnaround time | 16.9 ± 8.5 (15.0) | - | - |
| CT recipient site–surgery (days), mean ± SD (median) | 34.8 ± 17.6 (32.0) | 25.09 ± 17.2 (22.0) | 0.008 |
| Operating time (minutes), mean ± SD | 508.2 ± 88.8 | 562.6 ± 98.5 | 0.005 |
| Hospitalization time (days), mean ± SD | 22.5 ± 12.0 | 20.33 ± 9.0 | 0.285 |
| Microanastomosis revision, n (%) | 4 (9.8%) | 5 (7.9%) | 0.504 |
| Flap success, n (%) | 35 (85.4%) | 57 (90.6%) | 0.310 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knitschke, M.; Bäcker, C.; Schmermund, D.; Böttger, S.; Streckbein, P.; Howaldt, H.-P.; Attia, S. Impact of Planning Method (Conventional versus Virtual) on Time to Therapy Initiation and Resection Margins: A Retrospective Analysis of 104 Immediate Jaw Reconstructions. Cancers 2021, 13, 3013. https://doi.org/10.3390/cancers13123013
Knitschke M, Bäcker C, Schmermund D, Böttger S, Streckbein P, Howaldt H-P, Attia S. Impact of Planning Method (Conventional versus Virtual) on Time to Therapy Initiation and Resection Margins: A Retrospective Analysis of 104 Immediate Jaw Reconstructions. Cancers. 2021; 13(12):3013. https://doi.org/10.3390/cancers13123013
Chicago/Turabian StyleKnitschke, Michael, Christina Bäcker, Daniel Schmermund, Sebastian Böttger, Philipp Streckbein, Hans-Peter Howaldt, and Sameh Attia. 2021. "Impact of Planning Method (Conventional versus Virtual) on Time to Therapy Initiation and Resection Margins: A Retrospective Analysis of 104 Immediate Jaw Reconstructions" Cancers 13, no. 12: 3013. https://doi.org/10.3390/cancers13123013
APA StyleKnitschke, M., Bäcker, C., Schmermund, D., Böttger, S., Streckbein, P., Howaldt, H. -P., & Attia, S. (2021). Impact of Planning Method (Conventional versus Virtual) on Time to Therapy Initiation and Resection Margins: A Retrospective Analysis of 104 Immediate Jaw Reconstructions. Cancers, 13(12), 3013. https://doi.org/10.3390/cancers13123013








