Anticancer Activities of 9-chloro-6-(piperazin-1-yl)-11H-indeno[1,2-c] quinolin-11-one (SJ10) in Glioblastoma Multiforme (GBM) Chemoradioresistant Cell Cycle-Related Oncogenic Signatures
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Dataset Collection
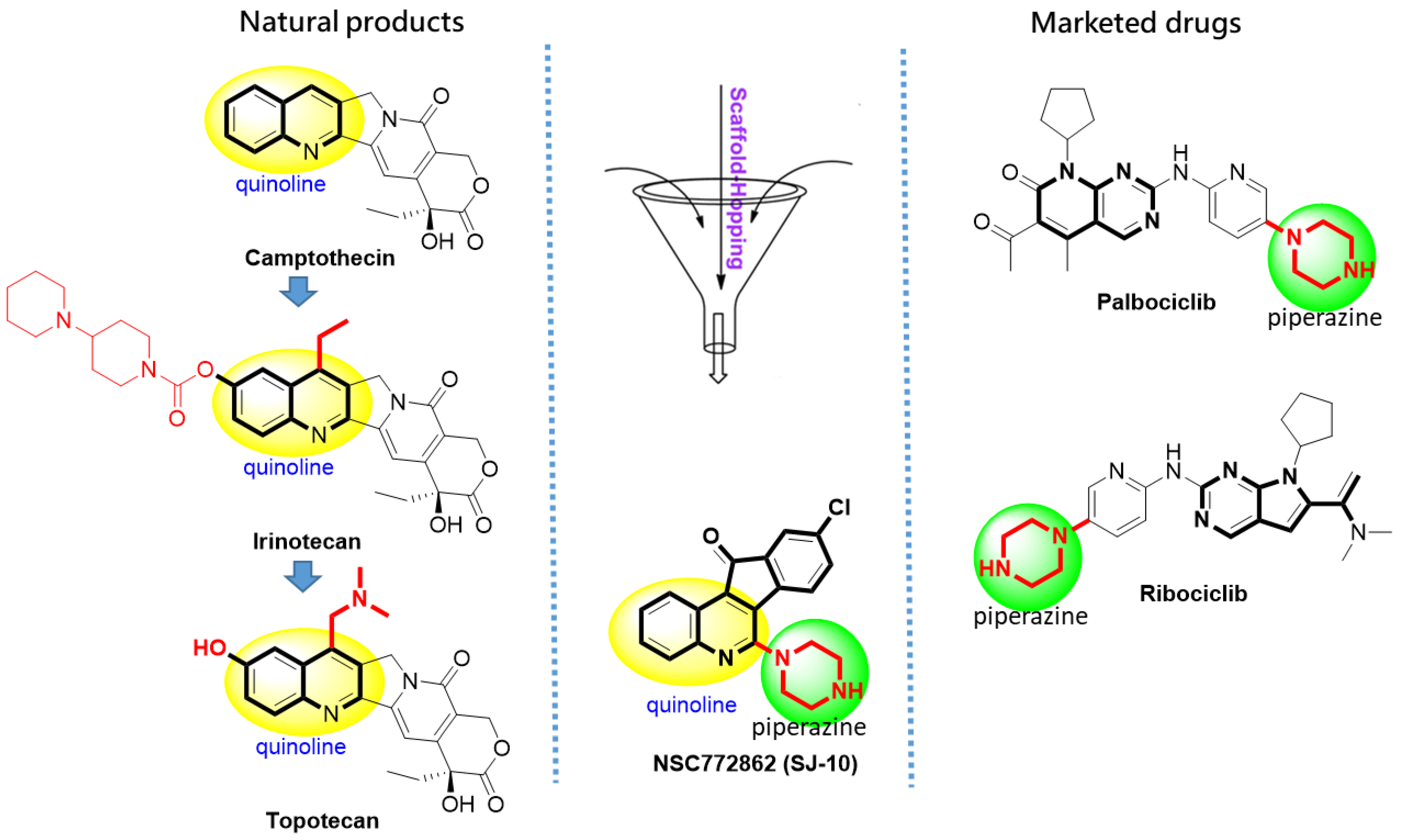
2.2. Identifying Molecular Targets and Therapeutic Classes of SJ10
2.3. DEG Identification by the Tumor Immune Estimation Resource (TIMER)
2.4. Validation of DEGs in GBM
2.5. Protein-Protein Interaction (PPI) Network Construction and Functional Enrichment Analysis
2.6. Predictions of Patient Clinical Outcomes with Radiomics Signature Construction
2.7. Receiver Operating Characteristic (ROC) Curves and Kaplan-Meier (KM) Analyses Were Used to Validate the Prognostic Values of the CCNB1, CDC42, MAPK7, and CD44 Oncogenic Signatures in GBM Samples
2.8. Evaluation of Drug Likeness, Pharmacokinetics (PKs), and Medicinal Chemistry of SJ10
2.9. In Vitro Anticancer Screening of SJ10 against NC1-60 CNS Cells
2.10. Molecular Docking Analysis
2.11. Statistical Analysis
3. Results
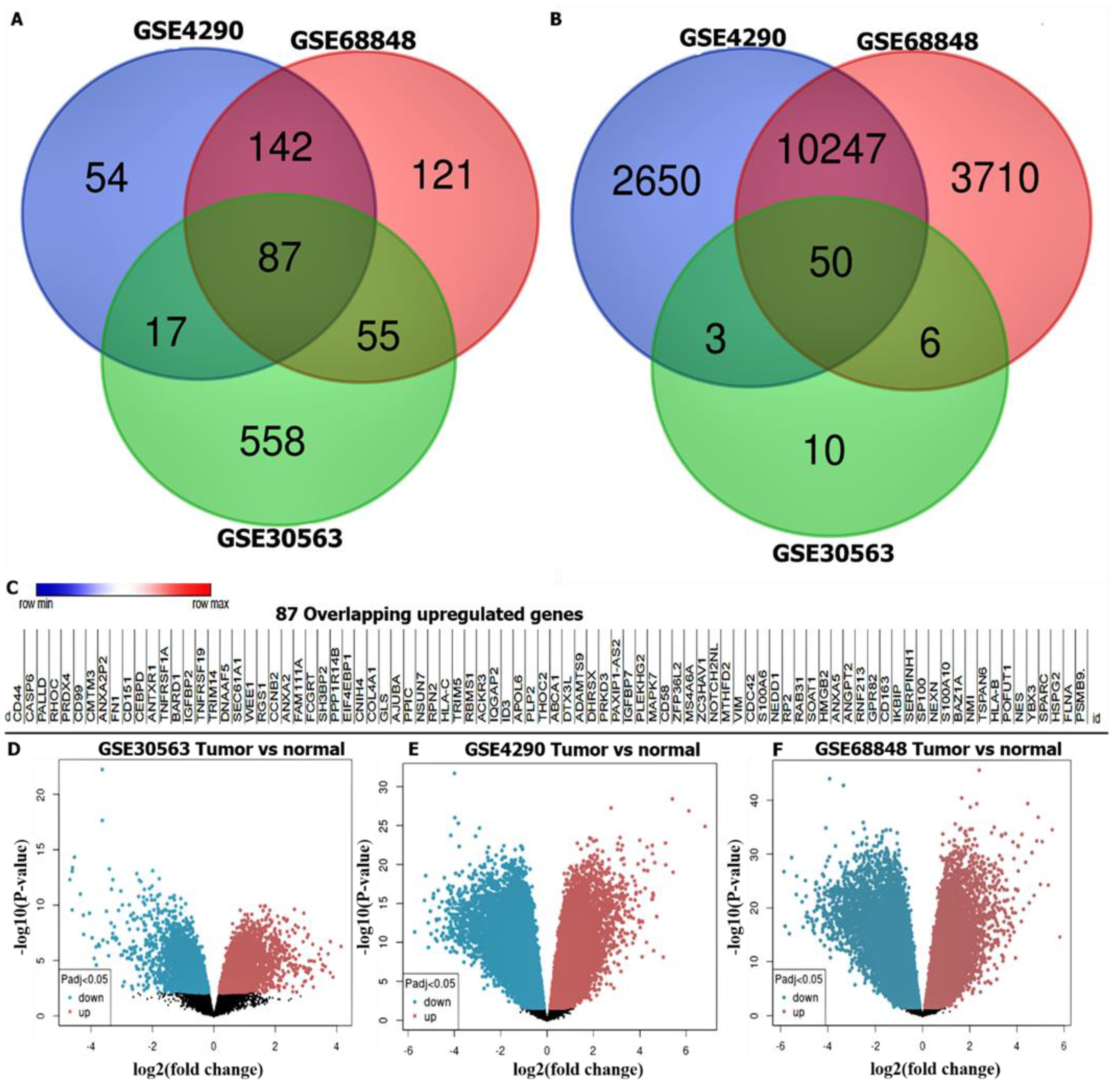
3.1. Identification of DEGs in GBM
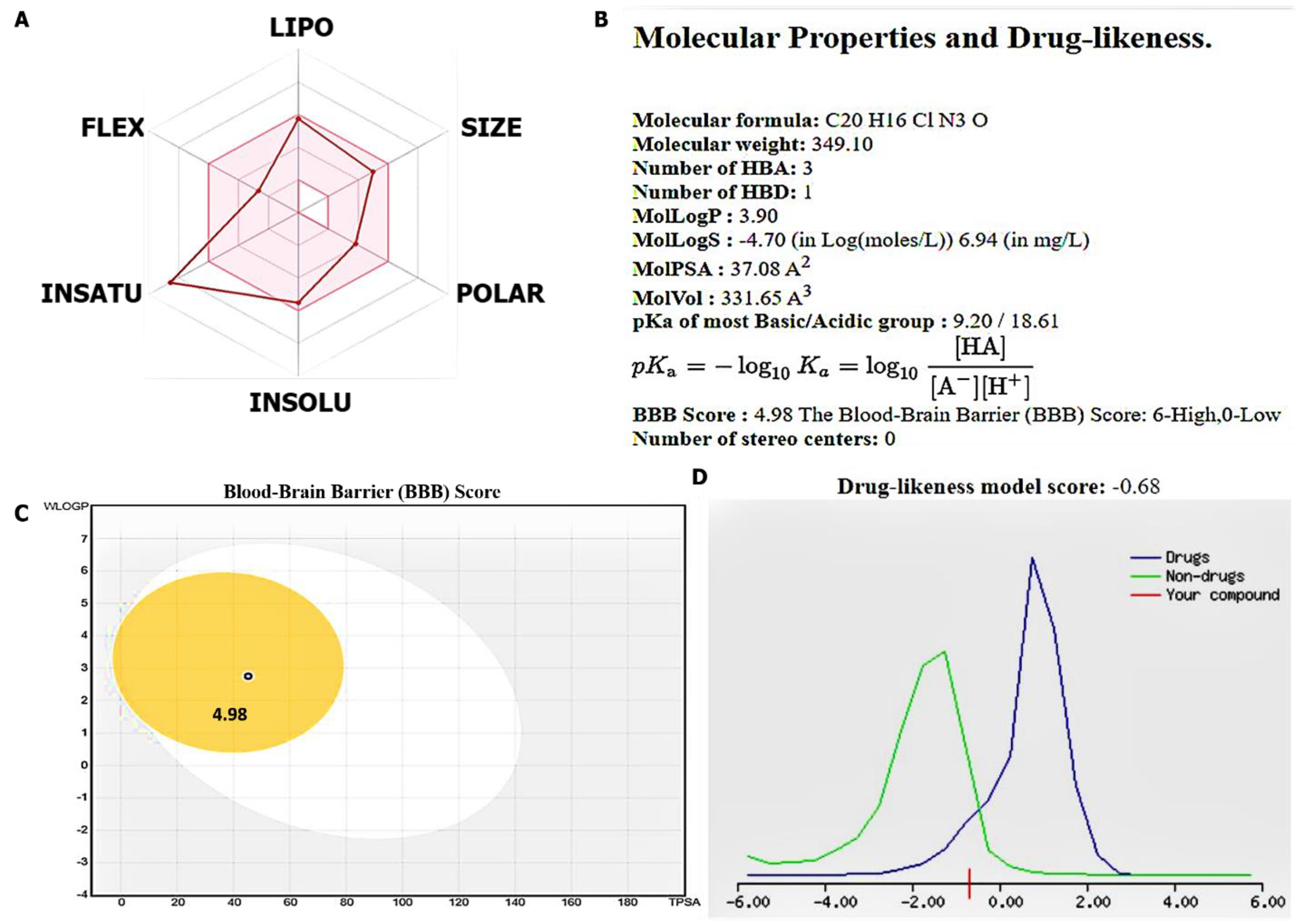
3.2. Evaluation of Drug Likeness, PKs, and Medicinal Chemistry of the SJ10 Compou
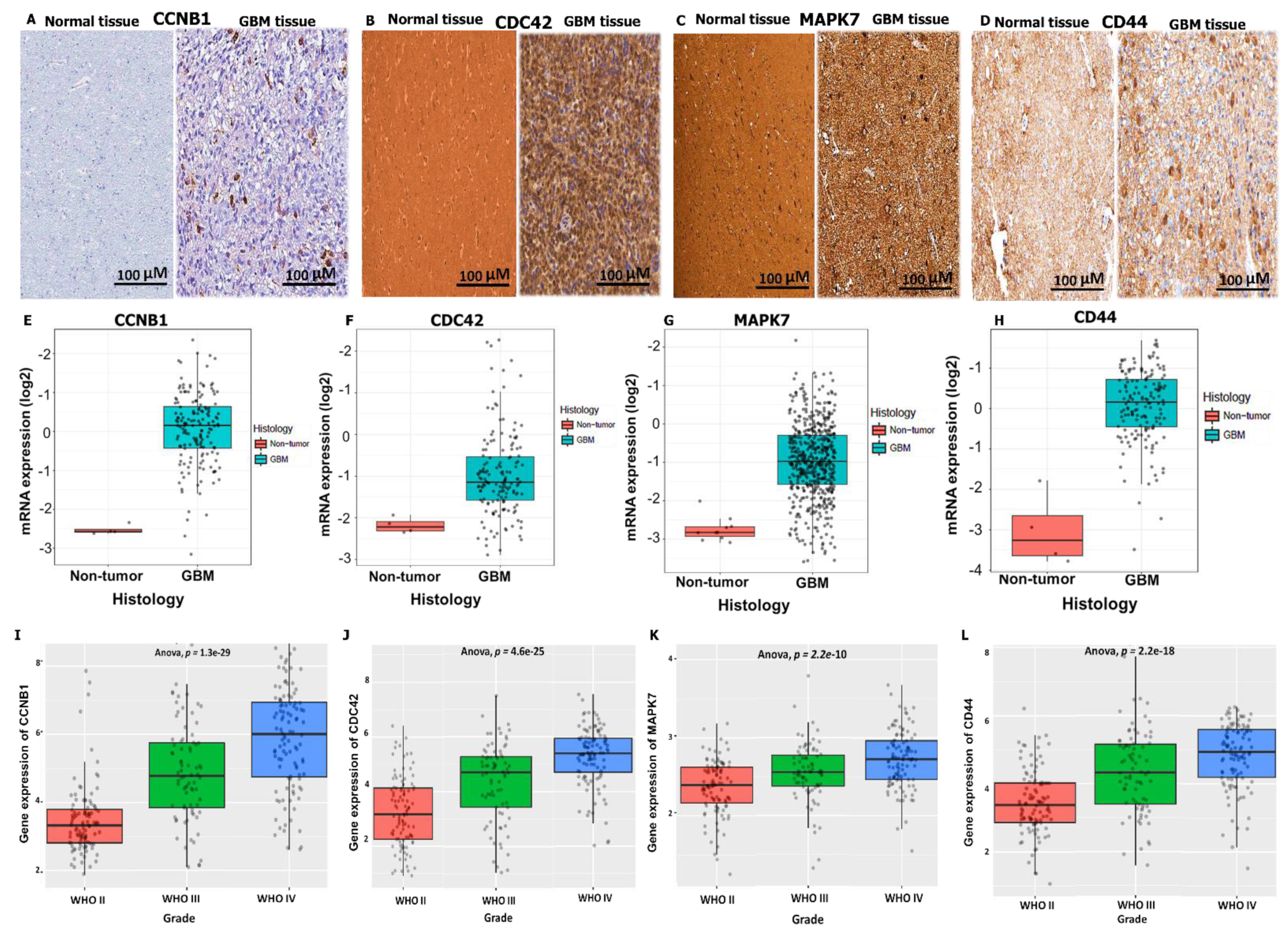
3.3. CCNB1/CDC42/MAPK7/CD44 Oncogenic Signatures Are Overexpressed in GBM
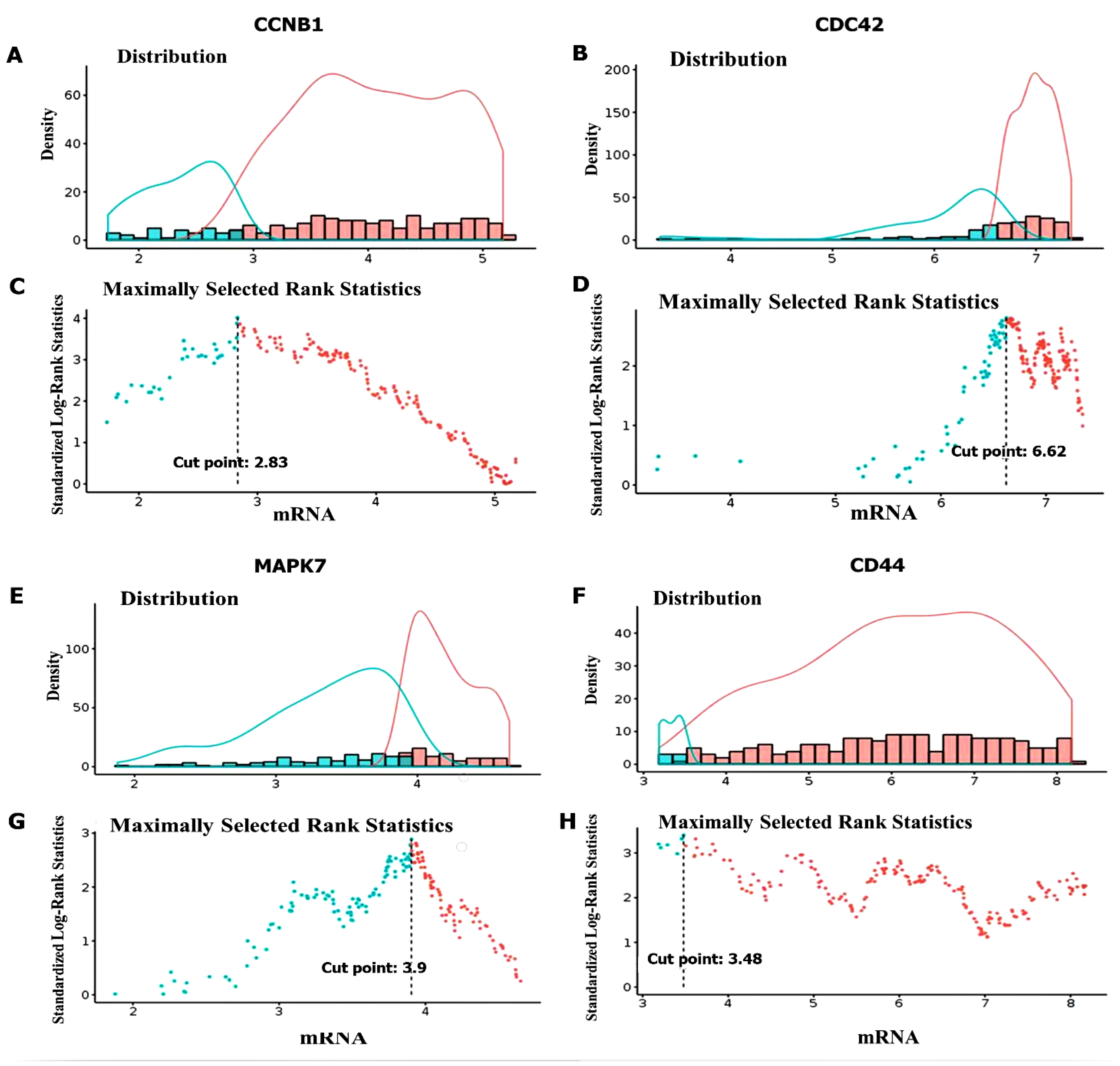
3.4. Validation of CCNB1/CDC42/MAPK7/CD44 Oncogenic Signature Expressions in GBM
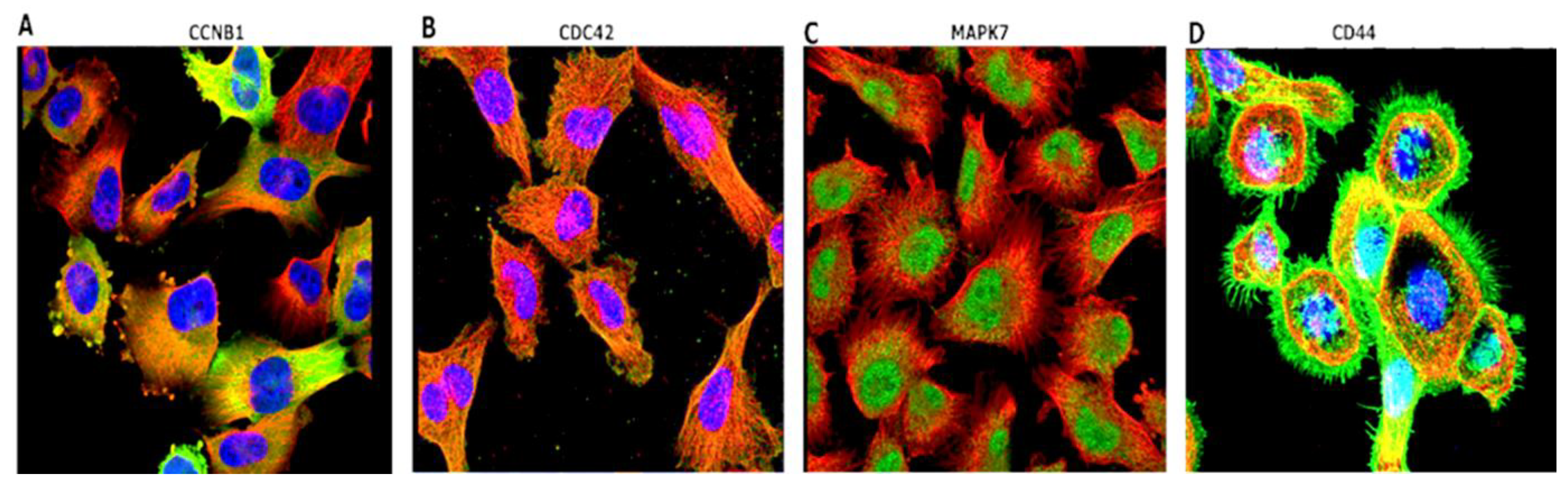
3.5. Immunofluorescent (IF) Staining of the U251-MG GBM Human Cell Line
3.6. PPI Network Construction and Functional Enrichment Analysis
3.7. Predictions of Patient Clinical Outcomes with Radiomics Signature Construction
3.8. High Expressions of CCNB1, CDC42, MAPK7, and CD44 Were Associated with a Poor Prognosis in GBM
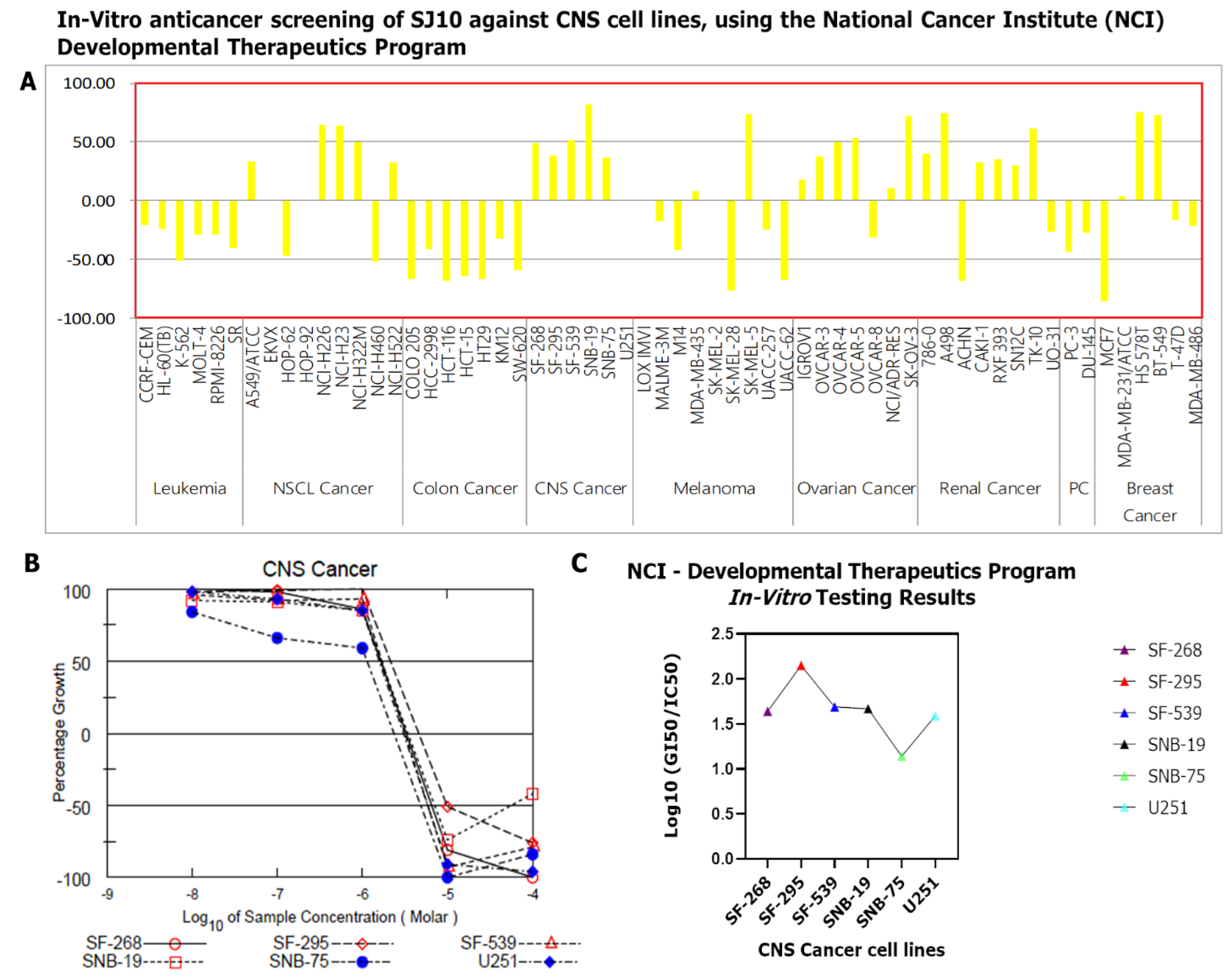
3.9. In Vitro Anticancer Screening of SJ10 against NC1-60 CNS Cell Lines
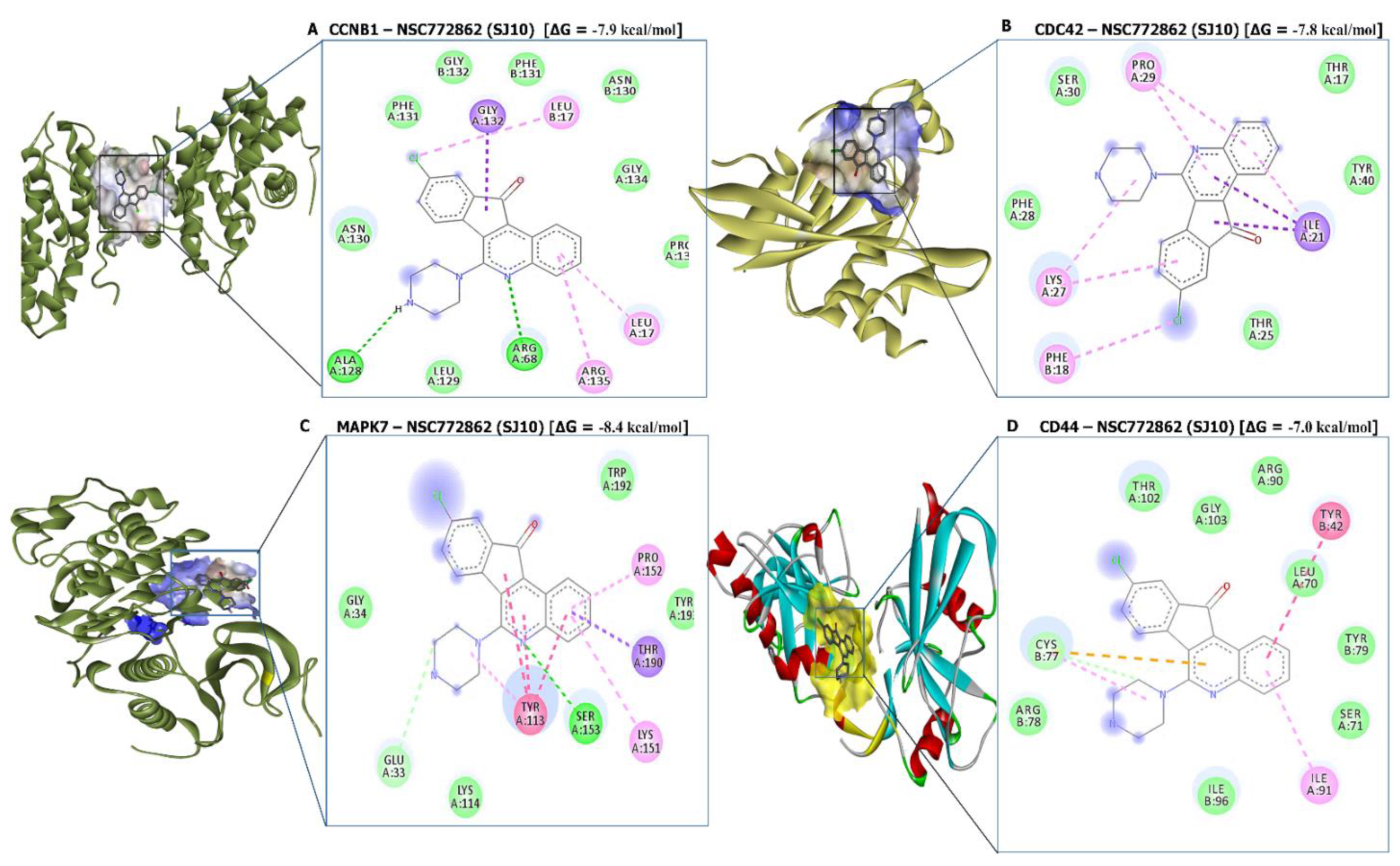
3.10. Molecular Docking Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montemurro, N. Glioblastoma multiforme and genetic mutations: The issue is not over yet. An overview of the current literature. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2020, 81, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef]
- Manikandan, C.; Kaushik, A.; Sen, D. Viral vector: Potential therapeutic for glioblastoma multiforme. Cancer Gene Ther. 2020, 27, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Gilard, V.; Tebani, A.; Dabaj, I.; Laquerrière, A.; Fontanilles, M.; Derrey, S.; Marret, S.; Bekri, S. Diagnosis and management of glioblastoma: A comprehensive perspective. J. Pers. Med. 2021, 11, 258. [Google Scholar] [CrossRef]
- Shabason, J.E.; Sutton, D.; Kenton, O.; Guttmann, D.M.; Lustig, R.A.; Hill-Kayser, C. Patterns of failure for pediatric glioblastoma multiforme following radiation therapy. Pediatr. Blood Cancer 2016, 63, 1465–1467. [Google Scholar] [CrossRef]
- Pearson, J.R.D.; Cuzzubbo, S.; McArthur, S.; Durrant, L.G.; Adhikaree, J.; Tinsley, C.J.; Pockley, A.G.; McArdle, S.E.B. Immune escape in glioblastoma multiforme and the adaptation of immunotherapies for treatment. Front. Immunol. 2020, 11, 582106. [Google Scholar] [CrossRef]
- Lopes, M.B.S. The 2017 World Health Organization classification of tumors of the pituitary gland: A summary. Acta Neuropathol. 2017, 134, 521–535. [Google Scholar] [CrossRef]
- Petrecca, K.; Guiot, M.C.; Panet-Raymond, V.; Souhami, L. Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with glioblastoma. J. Neurooncol. 2013, 111, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Brandes, A.A.; Tosoni, A.; Franceschi, E.; Sotti, G.; Frezza, G.; Amistà, P.; Morandi, L.; Spagnolli, F.; Ermani, M. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: Correlation with MGMT promoter methylation status. J. Clin. Oncol. 2009, 27, 1275–1279. [Google Scholar] [CrossRef] [Green Version]
- Jastaniyah, N.; Murtha, A.; Pervez, N.; Le, D.; Roa, W.; Patel, S.; Mackenzie, M.; Fulton, D.; Field, C.; Ghosh, S.; et al. Phase I study of hypofractionated intensity modulated radiation therapy with concurrent and adjuvant temozolomide in patients with glioblastoma multiforme. Radiat. Oncol. 2013, 8, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Phillips, T.M.; McBride, W.H.; Pajonk, F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J. Natl. Cancer Inst. 2006, 98, 1777–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Kim, R.K.; Yoon, C.H.; An, S.; Hwang, S.G.; Suh, Y.; Park, M.J.; Chung, H.Y.; Kim, I.G.; Lee, S.J. Importance of PKCδ signaling in fractionated-radiation-induced expansion of glioma-initiating cells and resistance to cancer treatment. J. Cell Sci. 2011, 124, 3084–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Klank, R.L.; Decker Grunke, S.A.; Bangasser, B.L.; Forster, C.L.; Price, M.A.; Odde, T.J.; SantaCruz, K.S.; Rosenfeld, S.S.; Canoll, P.; Turley, E.A.; et al. Biphasic dependence of glioma survival and cell migration on cd44 expression level. Cell Rep. 2017, 18, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Webb, B.; Gerson, S.L. CD133+ cells contribute to radioresistance via altered regulation of DNA repair genes in human lung cancer cells. Radiother. Oncol. 2014, 110, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Lai, G.; Wan, F.; Xiao, Z.; Zeng, L.; Wang, X.; Ye, F.; Lei, T. Knockdown of checkpoint kinase 1 is associated with the increased radiosensitivity of glioblastoma stem-like cells. Tohoku J. Exp. Med. 2012, 226, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, Y.C.; Roberts, T.L.; Day, B.W.; Stringer, B.W.; Kozlov, S.; Fazry, S.; Bruce, Z.C.; Ensbey, K.S.; Walker, D.G.; Boyd, A.W.; et al. Increased sensitivity to ionizing radiation by targeting the homologous recombination pathway in glioma initiating cells. Mol. Oncol. 2014, 8, 1603–1615. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Jiang, J.; Zhang, Y.; Wang, X.; Zhang, Q.; Wang, Y.; Liu, C.; Li, F. CDK1 and CCNB1 as potential diagnostic markers of rhabdomyosarcoma: Validation following bioinformatics analysis. BMC Med. Genom. 2019, 12, 198. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yang, L.; Zhang, X.; Chen, R.; Chen, X.; Tang, W.; Zhang, M. Identification of potential biomarkers in glioblastoma through bioinformatic analysis and evaluating their prognostic value. Biomed. Res. Int. 2019, 2019, 6581576. [Google Scholar] [CrossRef]
- Bo, L.; Wei, B.; Li, C.; Wang, Z.; Gao, Z.; Miao, Z. Identification of potential key genes associated with glioblastoma based on the gene expression profile. Oncol. Lett. 2017, 14, 2045–2052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malumbres, M.; Barbacid, M. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 2005, 30, 630–641. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Q.; Lin, J. Identification of the potential oncogenes in glioblastoma based on bioinformatic analysis and elucidation of the underlying mechanisms. Oncol. Rep. 2018, 40, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Koizumi, H.; Uchikoshi, T. Expression of the G2-M checkpoint regulators cyclin B1 and cdc2 in nonmalignant and malignant human breast lesions: Immunocytochemical and quantitative image analyses. Am. J. Pathol. 1997, 150, 15–23. [Google Scholar]
- Dutta, A.; Chandra, R.; Leiter, L.M.; Lester, S. Cyclins as markers of tumor proliferation: Immunocytochemical studies in breast cancer. Proc. Natl. Acad. Sci. USA 1995, 92, 5386–5390. [Google Scholar] [CrossRef] [Green Version]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.J.; Cobb, M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997, 9, 180–186. [Google Scholar] [CrossRef]
- Cude, K.; Wang, Y.; Choi, H.J.; Hsuan, S.L.; Zhang, H.; Wang, C.Y.; Xia, Z. Regulation of the G2-M cell cycle progression by the ERK5-NFkappaB signaling pathway. J. Cell Biol. 2007, 177, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Carmell, N.; Rominiyi, O.; Myers, K.N.; McGarrity-Cottrell, C.; Vanderlinden, A.; Lad, N.; Perroux-David, E.; El-Khamisy, S.F.; Fernando, M.; Finegan, K.G.; et al. Identification and validation of ERK5 as a DNA damage modulating drug target in glioblastoma. Cancers 2021, 13, 944. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Jin, G.; Cai, F.; Chen, X.; Cao, N.; Zhang, X.; Liu, J.; Chen, F.; Wang, F.; Dong, W.; et al. Extracellular signal-regulated kinase 5 increases radioresistance of lung cancer cells by enhancing the DNA damage response. Exp. Mol. Med. 2019, 51, 1–20. [Google Scholar] [CrossRef]
- Pereira, D.M.; Gomes, S.E.; Borralho, P.M.; Rodrigues, C.M.P. MEK5/ERK5 activation regulates colon cancer stem-like cell properties. Cell Death Discov. 2019, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Simões, A.E.; Rodrigues, C.M.; Borralho, P.M. The MEK5/ERK5 signalling pathway in cancer: A promising novel therapeutic target. Drug Discov Today 2016, 21, 1654–1663. [Google Scholar] [CrossRef]
- Cui, X.; Song, L.; Bai, Y.; Wang, Y.; Wang, B.; Wang, W. Elevated IQGAP1 and CDC42 levels correlate with tumor malignancy of human glioma. Oncol. Rep. 2017, 37, 768–776. [Google Scholar] [CrossRef] [Green Version]
- Okura, H.; Golbourn, B.J.; Shahzad, U.; Agnihotri, S.; Sabha, N.; Krieger, J.R.; Figueiredo, C.A.; Chalil, A.; Landon-Brace, N.; Riemenschneider, A.; et al. A role for activated Cdc42 in glioblastoma multiforme invasion. Oncotarget 2016, 7, 56958–56975. [Google Scholar] [CrossRef] [PubMed]
- Ekins, S.; Mestres, J.; Testa, B. In silico pharmacology for drug discovery: Methods for virtual ligand screening and profiling. Br. J Pharmacol. 2007, 152, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Lawal, B.; Liu, Y.L.; Mokgautsi, N.; Khedkar, H.; Sumitra, M.R.; Wu, A.T.H.; Huang, H.S. Pharmacoinformatics and preclinical studies of NSC765690 and NSC765599, potential STAT3/CDK2/4/6 inhibitors with antitumor activities against NCI60 human tumor cell lines. Biomedicines 2021, 9, 92. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, F.; Zhou, Z.W.; Xia, H.C.; Wang, X.Y.; Yang, Y.X.; He, Z.X.; Sun, T.; Zhou, S.F. Alisertib induces G(2)/M arrest, apoptosis, and autophagy via PI3K/Akt/mTOR- and p38 MAPK-mediated pathways in human glioblastoma cells. Am. J. Transl. Res. 2017, 9, 845–873. [Google Scholar]
- Chen, T.-C.; Yu, D.-S.; Chen, S.-J.; Chen, C.-L.; Lee, C.-C.; Hsieh, Y.-Y.; Chang, L.-C.; Guh, J.-H.; Lin, J.-J.; Huang, H.-S. Design, synthesis and biological evaluation of tetracyclic azafluorenone derivatives with topoisomerase I inhibitory properties as potential anticancer agents. Arab. J. Chem. 2019, 12, 4348–4364. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.S.; Chen, T.C.; Chen, R.H.; Huang, K.F.; Huang, F.C.; Jhan, J.R.; Chen, C.L.; Lee, C.C.; Lo, Y.; Lin, J.J. Synthesis, cytotoxicity and human telomerase inhibition activities of a series of 1,2-heteroannelated anthraquinones and anthra[1,2-d]imidazole-6,11-dione homologues. Bioorg. Med. Chem. 2009, 17, 7418–7428. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Bow, Y.D.; Wang, C.Y.; Chen, Y.C.; Fu, P.R.; Chang, K.F.; Wang, T.W.; Tseng, C.H.; Chen, Y.L.; Chiu, C.C. DFIQ, a novel quinoline derivative, shows anticancer potential by inducing apoptosis and autophagy in NSCLC cell and in vivo zebrafish xenograft models. Cancers 2020, 12, 1348. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets--update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogodin, P.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.V. PASS Targets: Ligand-based multi-target computational system based on a public data and naïve Bayes approach. SAR QSAR Environ. Res. 2015, 26, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef]
- Mokgautsi, N.; Wang, Y.C.; Lawal, B.; Khedkar, H.; Sumitra, M.R.; Wu, A.T.H.; Huang, H.S. Network pharmacological analysis through a bioinformatics approach of novel NSC765600 and NSC765691 compounds as potential inhibitors of CCND1/CDK4/PLK1/CD44 in cancer types. Cancers 2021, 13, 2523. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Jia, D.; Li, S.; Li, D.; Xue, H.; Yang, D.; Liu, Y. Mining TCGA database for genes of prognostic value in glioblastoma microenvironment. Aging 2018, 10, 592–605. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Xia, J.; Benner, M.J.; Hancock, R.E. NetworkAnalyst—Integrative approaches for protein-protein interaction network analysis and visual exploration. Nucleic Acids Res. 2014, 42, W167–W174. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.W.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef] [Green Version]
- Bowman, R.L.; Wang, Q.; Carro, A.; Verhaak, R.G.; Squatrito, M. GlioVis data portal for visualization and analysis of brain tumor expression datasets. Neuro. Oncol. 2017, 19, 139–141. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, T.J.; Ertl, P.; Lewis, R. The graphical representation of ADME-related molecule properties for medicinal chemists. Drug Discov. Today 2011, 16, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Mokgautsi, N.; Wen, Y.T.; Lawal, B.; Khedkar, H.; Sumitra, M.R.; Wu, A.T.H.; Huang, H.S. An integrated bioinformatics study of a novel Niclosamide derivative, NSC765689, a Potential GSK3β/β-Catenin/STAT3/CD44 suppressor with anti-glioblastoma properties. Int J. Mol. Sci. 2021, 22, 2464. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Lawal, B.; Lo, W.C.; Mokgautsi, N.; Sumitra, M.R.; Khedkar, H.; Wu, A.T.; Huang, H.S. A preclinical report of a cobimetinib-inspired novel anticancer small-molecule scaffold of isoflavones, NSC777213, for targeting PI3K/AKT/mTOR/MEK in multiple cancers. Am. J. Cancer Res. 2021, 11, 2590–2617. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg To predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [Green Version]
- Martin, Y.C. A bioavailability score. J. Med. Chem. 2005, 48, 3164–3170. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Lim-Wilby, M. Molecular docking. Methods Mol. Biol 2008, 443, 365–382. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temml, V.; Kaserer, T.; Kutil, Z.; Landa, P.; Vanek, T.; Schuster, D. Pharmacophore modeling for COX-1 and -2 inhibitors with LigandScout in comparison to Discovery Studio. Future Med. Chem. 2014, 6, 1869–1881. [Google Scholar] [CrossRef] [PubMed]
- Rapp, C.; Warta, R.; Stamova, S.; Nowrouzi, A.; Geisenberger, C.; Gal, Z.; Roesch, S.; Dettling, S.; Juenger, S.; Bucur, M.; et al. Identification of T cell target antigens in glioblastoma stem-like cells using an integrated proteomics-based approach in patient specimens. Acta Neuropathol. 2017, 134, 297–316. [Google Scholar] [CrossRef] [PubMed]



| SwissTarget Prediction | PASS Prediction Results | |||
|---|---|---|---|---|
| Target Gene | Target Class | PA | PI | Activities |
| AKT1 | Kinase | 0.703 | 0.054 | MAP kinase kinase 4 inhibitor |
| MAPK8 | Kinase | 0.652 | 0.014 | Histidine kinase inhibitor |
| TTK | Kinase | 0.598 | 0.088 | Cyclic AMP phosphodiesterase inhibitor |
| PIM2 | Kinase | 0.513 | 0.024 | MAP3K5 inhibitor |
| CDK9 | Kinase | 0.487 | 0.037 | Antineoplastic (glioblastoma multiforme) |
| SLC6A3 | ECT | 0.529 | 0.091 | Protein kinase inhibitor |
| EGFR | Kinase | 0.422 | 0.004 | Focal adhesion kinase inhibitor |
| CDK2 | Kinase | 0.445 | 0.040 | Cyclin B1 inhibitor |
| MAPK7 | Kinase | 0.435 | 0.037 | Apoptosis agonist |
| MAPK9 | Kinase | 0.415 | 0.021 | Protein kinase B gamma inhibitor |
| CCNA2 CDK2 | Kinase | 0.541 | 0.152 | MAP kinase kinase 7 inhibitor |
| CCND1 CDK4 | Kinase | 0.395 | 0.020 | Transcription factor STAT3 inhibitor |
| CDK1 CCNB1 | Other cytosolic protein | 0.407 | 0.049 | T cell inhibitor |
| CDK2 CCNA1 CCNA2 | Other cytosolic protein | 0.407 | 0.055 | Wee-1 tyrosine kinase inhibitor |
| MAPK1 | Kinase | 0.353 | 0.042 | CDC42 inhibitor |
| MAPK3 | Kinase | 0.406 | 0.102 | Check point kinase 2 inhibitor |
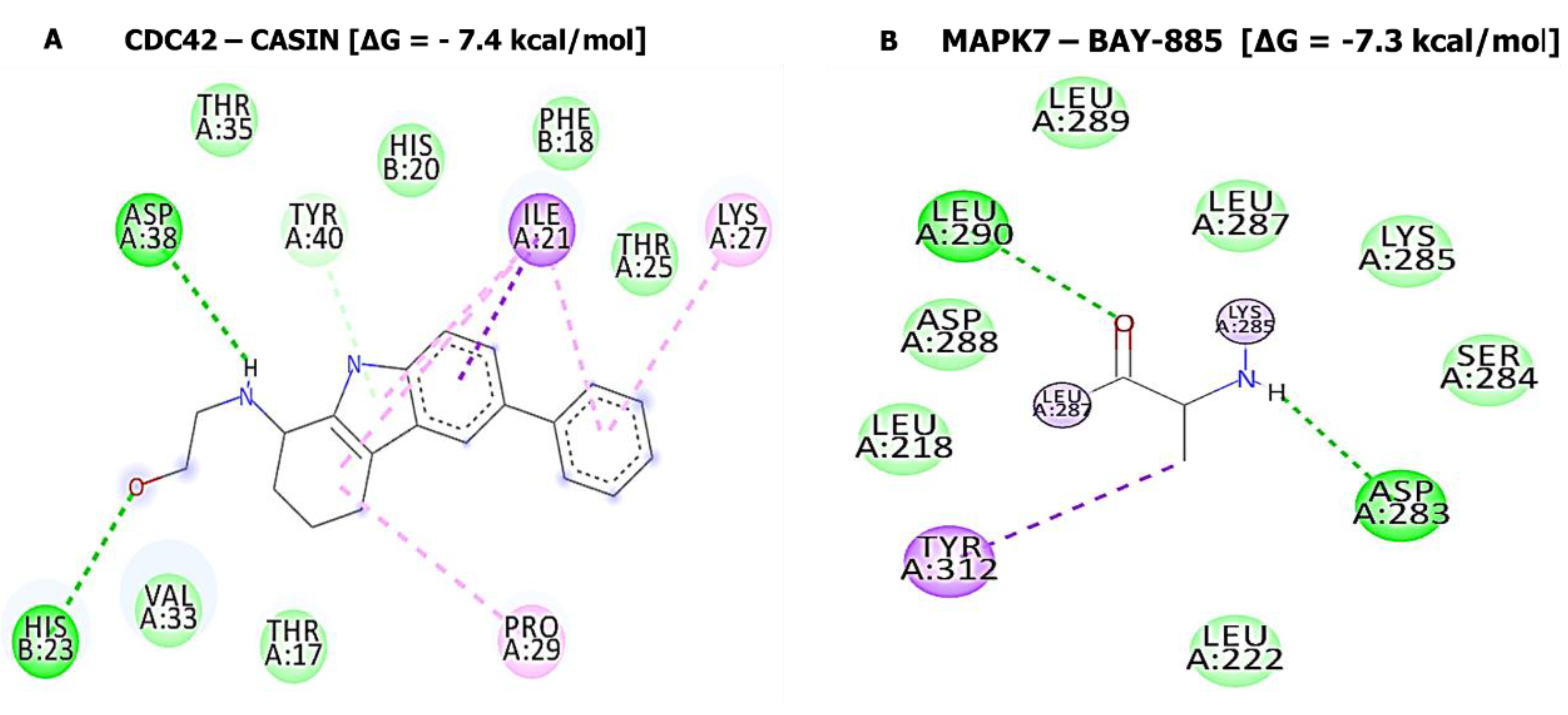
| SJ10-CCNB1 Complex (=−7.9 kcal/mol) | SJ10-CDC42 Complex (=−7.6 kcal/mol) | ||
| Type of interactions and number of bonds | distance of interacting Amino acids | Type of interactions and number of bonds | distance of interacting Amino acids |
| Conventional Hydrogen bond (2) | ALA128 (2.07 Å) and ARG68 (2.73Å) | Van der Waals forces | THR25, PHE28, SER30, THR17, and TYR40 |
| Van der Waals forces | ASN130, LEU129, PHE131, GLY132, PHE131, ASN130, GLY134, and PRO136 | Pi-Sigma | ILE21 |
| Pi-Sigma | GLY132 | Pi-alkyl | PHE18, LYS27, PRO29 |
| Pi-Alkyl | LEU17, LEU17, and ARG135 | ||
| SJ10-MAPK7 Complex (=−8.4 kcal/mol) | SJ10-CD44 Complex (=−7.0 kcal/mol) | ||
| Type of interactions and number of bonds | distance of interacting Amino acids | Type of interactions and number of bonds | distance of interacting Amino acids |
| Conventional Hydrogen bond (1) | SER153 (2.23 Å) | Van der Waals forces | THR102, GLY103, ARG90, LEU70, TYR79, SER71, ILE96, and ARG78 |
| Van der Waals forces | THR102, GLY103, ARG90, LEU70, TYR79, SER71, ILE96, and ARG78 TRP192, THR193 | Carbon hydrogen bond | CYS77 |
| Carbon hydrogen bond | CYS77 | pi-pi T-shaped | TYR42 |
| Pi-sigma | THR190 | Pi-Alkyl | ILE91 |
| Pi-Alkyl | ILE91 | ||
| Pi-Pi stacked | TYR113 | ||
| Pi-alkyl | PRO152 and LYS151 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokgautsi, N.; Kuo, Y.-C.; Tang, S.-L.; Liu, F.-C.; Chen, S.-J.; Wu, A.T.H.; Huang, H.-S. Anticancer Activities of 9-chloro-6-(piperazin-1-yl)-11H-indeno[1,2-c] quinolin-11-one (SJ10) in Glioblastoma Multiforme (GBM) Chemoradioresistant Cell Cycle-Related Oncogenic Signatures. Cancers 2022, 14, 262. https://doi.org/10.3390/cancers14010262
Mokgautsi N, Kuo Y-C, Tang S-L, Liu F-C, Chen S-J, Wu ATH, Huang H-S. Anticancer Activities of 9-chloro-6-(piperazin-1-yl)-11H-indeno[1,2-c] quinolin-11-one (SJ10) in Glioblastoma Multiforme (GBM) Chemoradioresistant Cell Cycle-Related Oncogenic Signatures. Cancers. 2022; 14(1):262. https://doi.org/10.3390/cancers14010262
Chicago/Turabian StyleMokgautsi, Ntlotlang, Yu-Cheng Kuo, Sung-Ling Tang, Feng-Cheng Liu, Shiang-Jiun Chen, Alexander T. H. Wu, and Hsu-Shan Huang. 2022. "Anticancer Activities of 9-chloro-6-(piperazin-1-yl)-11H-indeno[1,2-c] quinolin-11-one (SJ10) in Glioblastoma Multiforme (GBM) Chemoradioresistant Cell Cycle-Related Oncogenic Signatures" Cancers 14, no. 1: 262. https://doi.org/10.3390/cancers14010262
APA StyleMokgautsi, N., Kuo, Y. -C., Tang, S. -L., Liu, F. -C., Chen, S. -J., Wu, A. T. H., & Huang, H. -S. (2022). Anticancer Activities of 9-chloro-6-(piperazin-1-yl)-11H-indeno[1,2-c] quinolin-11-one (SJ10) in Glioblastoma Multiforme (GBM) Chemoradioresistant Cell Cycle-Related Oncogenic Signatures. Cancers, 14(1), 262. https://doi.org/10.3390/cancers14010262









