1. Introduction
Breast cancer is one of the leading causes of cancer morbidity and mortality. A recent review reported that it is the second most diagnosed malignancy, accounting for more than 11.6% of female cancers [
1,
2], and the fifth most common cause of cancer deaths, making up 6.6% of all cancer mortality worldwide. The same review reports that breast cancer induces a substantial public health burden, leading to a loss of 14.8 million Disability Adjusted Life Years (DALYs). Despite progress in early diagnosis of the disease and the variety of therapeutic approaches available, it is still the most frequently reported cause of female cancer deaths.
Nowadays, primary mammary tumors can be treated by surgery, followed by chemotherapy or radiotherapy—an invasive and debilitating approach, contributing to the above-mentioned public health burden. Chemotherapy and radiotherapy can damage healthy tissues, while surgery entails risks of infection and bleeding and is not always curative for metastatic tumors. Innovative, minimally invasive treatment methods, devoid of negative impacts, are desirable to reduce mortality and morbidity in breast cancer patients. One candidate is hyperthermia, which can be generated from sources such as ultrasound, RF waves and laser beams [
3,
4]. Recent advances in nanotechnology have paved the way for magnetic fluid hyperthermia (MFH), combining magnetic nanoparticles (MNPs) with alternating magnetic fields (AMFs). MFH has shown great promise in cancer research, with the potential to overcome many of the limitations presented by other hyperthermia techniques [
5].
The challenges of MFH are the need to deliver a large quantity of MNPs to the tumor tissue, and the risk of overheating healthy peritumoral tissue [
6]. In currently applied preclinical and clinical protocols, nanoparticles are injected directly into the tumor tissue. For tumors in anatomical regions not readily accessible by injection, an invasive procedure entailing surgery and anesthesia may be required. This is not the case for breast tumors: given that image-guided biopsy is routinely performed in breast cancer, the intratumoral injection of MNPs for MFH treatment could be easily carried out as an outpatient procedure, thus avoiding invasive surgery.
For the treatment of tumors located deep in the body, systemic administration of MNPs could be envisaged, although considerably limited by their non-specific uptake to the liver and spleen, typically exceeding specific uptake to the tumor. In this respect, the application of magnetic particle imaging (MPI) to excite MNPs in specific regions of the body has been reported [
4,
5,
7]. The field-free region of MPI instrumentation, which can be localized over the tumor with millimetric precision, allows space-selective irradiation of the tumor region. This makes MFH by MPI apparatus compatible with systemic MNP administration, without risk of damage to the liver and spleen, provided sufficient MNPs are delivered to tumor tissue.
The risk of overheating healthy peritumoral tissue has been previously addressed by us and by other groups [
6,
8] using self-regulating temperature MNPs [
9]. Gerosa et al. report an extensive investigation of a self-regulating temperature nanomaterial, synthesized by MBN Nanomaterialia S.p.A. (Treviso, Italy). Such nanomaterials undergo a stable temperature increase when exposed to an AMF, up to a maximum value (TSR, self-regulating temperature) lower than the Curie temperature (T
C). T
C values can be modulated by adjusting the composition of the nanomaterial. Above the T
C, magnetization is negligible and the heating effect of the AMF ceases. Interestingly, above Tc, a sharp decrease in transverse MRI relaxivity occurs, meaning that the MNP is suitable for use as a temperature-sensitive contrast agent [
6]. In principle, these properties are particularly useful when the nanomaterial is integrated into biological matrices, making it possible to limit the maximum temperature and, at the same time, monitor temperature changes by means of imaging.
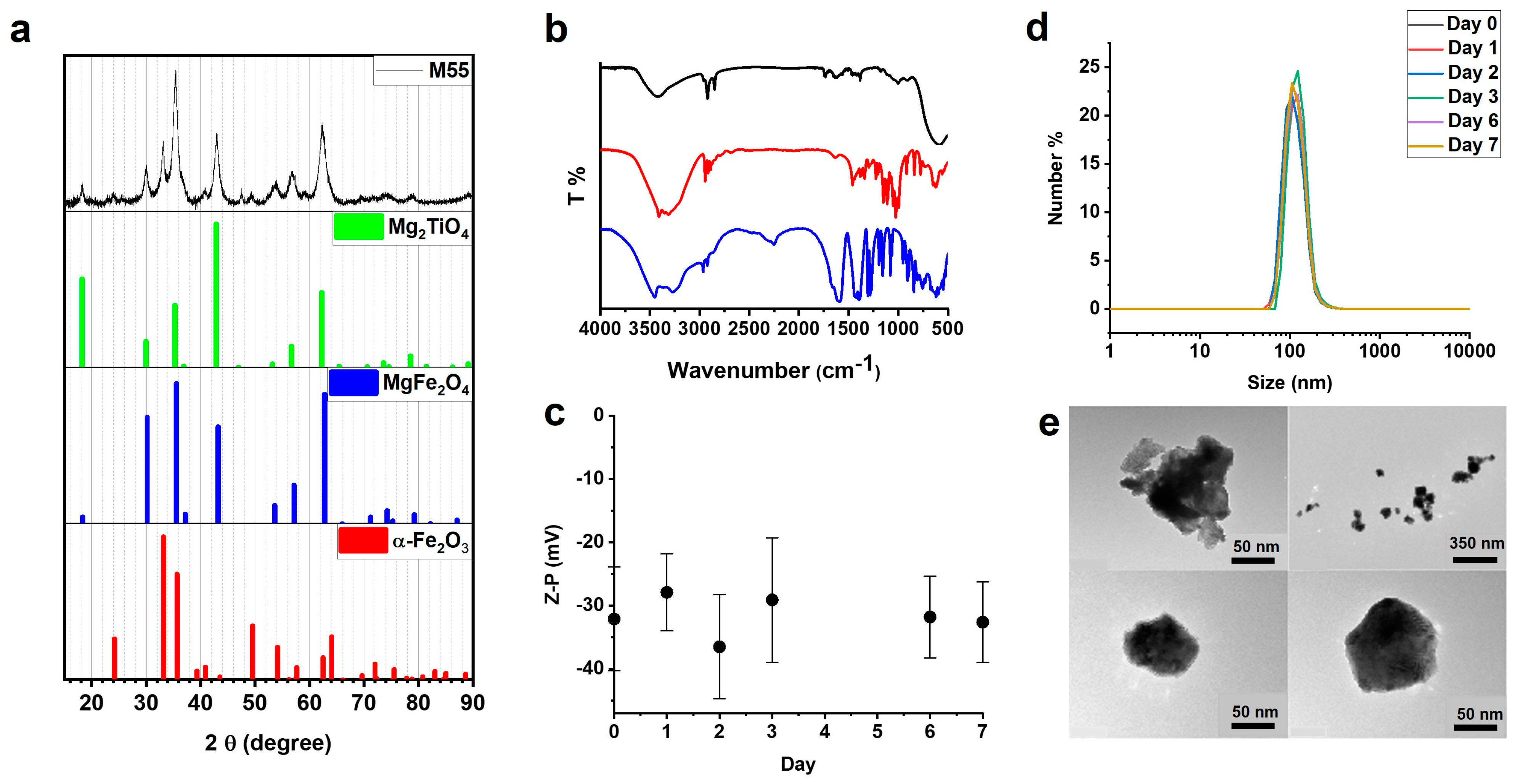
M55, the MNP studied in the present paper, is obtained from previously described M48 material [
6], by implementing an additional purification step in the synthesis process so as to remove potential paramagnetic by-products from the liquid suspension. In investigating this class of materials, we have already considered their properties as theranostic agents for MPI, in view of the foreseen application of MPI in MFH [
4,
7]. The capability of M55 as a bimodal contrast agent was demonstrated in vitro, and in a cellular imaging experiment. In addition, we demonstrated the marked therapeutic efficacy of M55 in an experimental protocol, in which four MFH treatments are applied after a single intratumoral injection of MNPs.
2. Materials and Methods
2.1. Synthesis of the Iron-Based NPs
The NPs were prepared by MBN Nanomaterialia S.p.A. (Treviso, Italy) following a proprietary mechanochemical process [
9]. Briefly, MgO, Fe
2O
3, and TiO
2 powders with a 4:2:1 molar ratio, respectively, were thoroughly mixed, and the reaction was carried out using ball milling equipment [
10] for 6 h. A heat treatment at 1200 °C for 4 h in the air was then carried out. The obtained powders were finely ball-milled for 1 h, then homogeneously dispersed in isopropanol and ultrasonicated to promote de-aggregation. Centrifugation was used to extract nanosized particles (<200 nm). This process further improves the one previously used [
6]: detailed processing parameters are provided courtesy of MBN Nanomaterialia S.p.A.
2.2. Structural Characterization
The phase and crystal characterization were analyzed by X-ray powder diffraction (XRPD) technique. The X-ray diffraction pattern was measured using an X-ray powder diffractometer (Thermo ARLX’TRA, Waltham, MA, USA) in Bragg–Brentano geometry (Cu source, Si (Li)-cooled solid-state detector) with a scan rate of 0.09°/min. The sample was homogenized in an agate mortar adding some drops of ethanol. The sample was then deposited on a low background stage. The ICSD database was used for phase analysis.
2.3. Morphological Analysis and Dimensional Characterization
The morphology of the NPs was analyzed with transmission electron microscopy (TEM, FEI TECNAI G2). The sample was deposited on a copper grid and then desiccated for one day before the measurements.
The hydrodynamic size and zeta potential of the coated nanoparticles were determined with a Malvern Zetasizer Nano instrument. The sample was prepared in an appropriate plastic cuvette, repeating the size and zeta potential measurements for the following 7 days to assess the stability of the coating.
2.4. Coating of the Iron-Based NPs
The NPs were coated with citrate ions, using sodium citrate dihydrate (99%—Alfa Aesar, Haverhill, MA, USA) and glucose (D-(+)-glucose SigmaUltra 99.5%) with a procedure described in [
6]. The citrate- and glucose-coated NPs will be referred to below as G-M55.
2.5. Analysis of the NPs Coating
The NPs coating was analyzed by FT-IR spectroscopy with a JASCO FT/IR-660 plus spectrometer. The sample was prepared by dispersing 3 mg of powders of G-M55 in 300 mg KBr and preparing a pellet of 1 cm diameter.
2.6. Cytotoxicity Assay
MDA-MB-231 cells were cultured in complete culture medium Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% Fetal Bovine Serum (FBS), 1% of a mix of penicillin/streptomycin 1:1 (GIBCO Life Technology, Monza, Italy), and 1% L-glutamine 200 mM (GIBCO Life Technology, Monza, Italy). Then, 3000 cells were seeded in each well of a 96-well plate and incubated for 24 h at 37 °C in humidified air with 5% CO2. The medium was then replaced with fresh medium, containing 10, 50, 100, 150, and 300 µg/mL of G-M55.
Cytotoxicity was assessed through the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay after 2, 24, and 48 h of incubation. Then, 100 µL of MTT solution (5 mg/mL) was added to each well and incubated for an additional 4 h. Then, the MTT solution was removed and 100 µL of DMSO was added to each well to dissolve the formazan crystal. Absorbance was read using an HTX microplate reader (BioTek Instruments, Winooski, VT, USA) at a wavelength of 570 nm. Four measurements of optical density (OD) were recorded for each sample, and cell viability (%) was calculated with the following equation: CV% = (ODsample/ODcontrol) × 100.
2.7. Magnetic Characterization
The efficiency of G-M55 as a multimodal contrast agent was assessed in MPI by using a RELAX module (Magnetic Insight Inc., Alameda, CA, USA), and in MRI by using a 7T Bruker Biospec system. In MPI, two components of the measurement were assessed: the signal efficiency was measured through the amount of MPI signal per mass of iron (high signal efficiency results in improved SNR and lower detection limits) and full width at half-maximum (FWHM), which is related to the imaging resolution (lower FWHM results in less signal spread and improved imaging resolution). The resolution, defined as the distance for 2 objects separated at 50%, was estimated according to the following relationship: Resolution (mm) = FWHM (mT)/Gradient (T/m). A sample containing G-M55 with a concentration of 5.5 μg of Fe in 10.0 μL volume was vortexed for 5 min and sonicated in a room temperature water bath sonicator for 5 min before testing. Relaxometry measurements were performed, using the RELAX module on naked and coated nanoparticles at the same concentration, and compared to VivoTrax (Ferucarbotran, Magnetic Insight Inc., Alameda, CA, USA). The 1D signal acquisition mode was used for relaxometry measurements. A 2D projection image was used to test the nanoparticles with the following imaging parameters: standard mode, coronal acquisition, 2-channel, FOV = 6 × 6 cm2.
In order to measure transverse relaxivity (spin-spin relaxation process) in MRI, the transverse relaxation time
T2 of water containing different amounts of G-M55 was measured by acquiring a MSME (multi-slice multi-echo) pulse sequence, with the following parameters: TR = 2000 ms, TE from 6.5 to 170 ms, FOV = 55 × 55 mm, matrix size = 128 × 128, slice thickness = 1 mm, number of echoes = 25. The signals coming from each phantom at different TEs were averaged to obtain the relaxation curve decay, which was fitted with a single exponential decay function to obtain the
T2 value. Transverse relaxivity
r2 was then obtained by measuring the modified relaxation time of water through the presence of G-M55 at different NP concentrations, and extrapolated via linear fit using the following equation:
where
T2,0 is the relaxation time of pure water,
c the concentration of G-M55 (expressed as mM of Fe), and
T2 the modified relaxation time of water.
2.8. Multimodal Imaging of Cells Labeled with NPs
To test the efficiency of G-M55 as an MPI contrast agent in a biologically significant context, it was used in a cellular imaging experiment. MDA-MB-231 cells were seeded and cultured in DMEM with 10% FBS, 1% penicillin/streptomycin 1:1 mix, and 1% L-glutamine 200 mM, in a T-75 flask (5 × 106 cells) and accommodated for 24 h. MDA-MB-231 cells were incubated with 0.1 mgFe/mL (corresponding to 0.256 mg/mL of MNP) of G-M55 for 24 h, to observe iron uptake. Cells were then washed 3 times with 5.0 mL of phosphate buffered saline solution (PBS) and detached with 1.5 mL of 0.25% trypsin, harvested, and centrifuged (1200 rpm for 3 min). After collecting and throwing out the supernatant, the pellet was resuspended in 1.0 mL of PBS. The sample was divided into three portions of 4 × 106 cells in 0.1 mL PBS each, for MPI. Serial dilutions were performed, to obtain samples containing decreasing amounts of cells (from 4 × 106 to 30 × 103 cells).
Images of the pellets were acquired with a Momentum MPI scanner (Magnetic Insight Inc., Alameda, CA, USA), using a 2D projection mode scan with the default mode setting (5.7 T/m gradient). The imaging parameters adopted were: field of view (FOV) = 4 × 12 cm2; single average; acquisition time = 10.0 s for each projection; reconstruction time = 4.0 min.
For quantitative MPI analysis, the cell pellet was outlined in a single slice with a freehand region of interest (ROI). The MPI signal was calculated using the sum function of the VivoQuant software; this function calculates the sum of pixel signal intensity within a given ROI, normalized to the number of pixels. A calibration curve of the tracer was set up by 2D scanning of NPs at different concentrations, expressed as iron content. A duplicate of each dilution was scanned.
After computing the calibration curves, the MPI signal collected from the cell pellets was converted into iron content, using the following equation based on the calibration curves and the doses employed:
Cell pellets were also prepared in the bottom of Eppendorf tubes, for MRI. A gradient echo acquisition sequence was used, with the following parameters: TR = 460 ms, TE = 5 ms, flip angle = 30°, FOV = 43 × 43 mm2, matrix size = 384 × 384, NEX = 6.
2.9. Magnetic Fluid Hyperthermia and Magnetic Particle Imaging HYPER Module
Magnetic nanoparticles, in the presence of an external AMF, convert magnetic energy into heat. This ability is quantified from the value of the specific absorption rate (
SAR). The
SAR value of nanoparticles in solution is calculated by using the following equation:
where
C is the specific heat capacity of the solvent (4.186 J/g°C), ∆
T is temperature variation (°C), Δ
t is the time interval (s), and mFe is the iron mass fraction in the compound. Temperature variation was calculated over the first 60 s of heating.
SAR was measured using a Nanotherics MagneTherm system (Warrington, UK), with samples diluted in water. The heat dissipation value depends on the frequency and amplitude of the AMF. The AMF apparatus yields a maximum magnetic field intensity of 23.0 kA/m (approximately 29.0 mT). A multichannel thermometer equipped with optical fiber probes (FOTEMP4, Optocon AG, Dresden, Germany) was used to assess temperature variation within the sample, every 10.0 s. The multichannel thermometer was placed inside the sample throughout the entire acquisition time. MNP concentration was 6 mg/mL. The specific time window was chosen considering previous results obtained with G-M48 [
6], as well as translatability to clinical practice. The characteristic parameters of AMF were chosen in order to satisfy the Hergt criterion for clinical translatability [
11]: in particular, magnetic field intensity was set at H = 8.75 kA/m, with a frequency of 473.1 kHz. The samples were kept in a homemade closed box with a thermostatic air stream at 37 °C.
SAR was also measured with the HYPER module of the Magnetic Particle Imager Momentum scanner (Magnetic Insight Inc., Alameda, CA, USA). The change in temperature over time was recorded for both coated and naked nanoparticles, for 300 s (the necessary time window to evaluate SAR), starting at thermostated room temperature with a magnetic field excitation of 12.75 kA/m at a 1600 kA/m2 gradient. The samples used had a volume of 100 µL, with the same Fe concentration as for the measurement with the Nanotherics system.
MFH was also performed in cell culture as previously described [
6]. Briefly, cells were incubated with 150 µg/mL of G-M55, and after 24 h of incubation, cells were placed in a homemade closed box with a thermostatic air stream for 15 min and then an external AMF (MFH treatment) was applied to the cells for an additional 20 min. The efficiency of the MFH treatment was assessed through the MTT assay as described in
Section 2.6. Cells subjected to MFH and cells incubated with 150 µg/mL of G-M55, but not exposed to MFH, were used as controls. Twenty-four hours later than the MFH treatment, the cytotoxicity assays were performed. The viability of MDA-MB-231 cells was evaluated after one and two hyperthermia treatments (24 h apart) and normalized to that of the respective controls.
2.10. In Vivo Studies
The biodistribution of G-M55 was investigated in four normal nude homozygote female mice, 6–8 weeks old (supplied by Envigo, Bresso, Italy). MRI was performed with a Bruker Biospin 7T scanner (Bruker Biospin, Ettlingen, Germany). Animals were placed in a heated animal bed and inserted in a 7.2 cm internal diameter bird-cage coil under anesthesia (1.5% isofluorane in a mixture of O2 and air). G-M55 was injected at a dosage of 4.0 mg Fe/kg (corresponding to 10.3 mg MNPs/kg dissolved in a volume of 150 μL) through the tail vein. Images were acquired before, and 10 min, 30 min, 60 min, 24 h, and 72 h post-injection. The sequences adopted were the following: T2 RARE sequence with TR = 1200 ms, TEeff = 36 ms, FOV = 35 × 35 mm2, MTX = 128 × 128, slice thickness = 1.0 mm.
The efficacy of G-M55 as a MFH agent was investigated in a group of 18 Balb/c nu/nu female mice (Harlan Laboratories, Udine, Italy). Mice were maintained under standard environmental conditions (temperature, humidity, and 12 h/12 h light/dark cycle, with water and food ad libitum) and veterinary control, in the University of Verona animal facility. Two million cells were injected subcutaneously into the right flank of each mouse. The size of the tumor was measured every 3 days by MRI, starting from 15 days post-inoculation. After 21 days (when tumor volume reached about 200 mm3), the mice were divided into three different intratumoral treatment groups. The first group (CTRL) received saline, the second (G-M55) received G-M55 (1.2 mg Fe/mL, total volume 100 μL), the third group (G-M55 + MFH) received G-M55 (1.2 mg Fe/mL) and MFH treatments. Animals belonging to the third group were exposed to four MFH cycles: immediately, and after 24, 48, and 96 h. Each MFH treatment comprised the application of an AMF for 1200 s, at an intensity of 8.75 kA/m and a frequency of 473.1 kHz.
Tumor size in all mice was measured by MRI before receiving G-M55 (day 0), and 4-, 6-, and 9-days post-injection. Transversal T2 weighted images were acquired using a RARE 3D sequence with TR = 1200 ms, TEeff = 47.5 ms, NEX = 1, field of view = 25 × 25 × 25 cm3, MTX = 256 × 128 × 32, slice thickness = 0.8 mm, FA = 90°, RARE factor = 16. The tumor volume was obtained by manually drawing ROIs slice by slice using the Paravision software. The percentage increase in tumor volume was calculated at each time point as 100 × (Vt − V0)/V0, where Vt is the tumor volume at time t and V0 is the initial volume.
Individual animal body weight and clinical signs (spontaneous movement, head posture, and social behavior) were monitored. The experimental plan received authorization from the Italian Ministry of Health (protocol number 56DC9.38) and was approved by the local ethical committee. Animal experiments were conducted in full compliance with Italian law (D.L. 4 March 2014 no. 26) and European Union regulations (2010/63/EU).
2.11. Histology
Once the in vivo experiments were completed, mice were sacrificed, and relevant tissue (tumor, liver, kidneys, spleen, lungs, stomach, intestine, and heart) was removed for histology. Excised samples were washed with PBS 0.1 M and fixed in Carnoy solution for 2.5 h. Tissues were embedded in paraffin, cut into 8 µm sections with a microtome, and dried at 37 °C for 24 h. Prussian blue staining was performed to evaluate the presence of iron in the tissues: sections were incubated with PB solution (5% hydrochloric acid and 5% potassium ferrocyanide) for 20 min and counterstained with nuclear fast red (Bioptica, Milan, Italy) for 2 min. To evaluate tissue damage and morphology, sections were stained with hematoxylin and eosin. Sections were examined under a light microscope (Olympus BXS1, Hamburg, Germany), equipped with a charge-coupled device camera.
2.12. Immunofluorescence
Immunofluorescence was used to observe the overexpression of HSP70 within the cell culture (MDA-MB-231). After two and four MFH treatments, cells were fixed with 4% PFA solution at room temperature, and then with 70% ethanol at −20 °C overnight. After fixation, cells were permeabilized with 0.1% Triton X-100 in PBS for 10 min at room temperature. Subsequently, cells were incubated in blocking solution for 30 min and immunostained with primary Rabbit Anti HSP70 (Abcam 1:50, Cambridge, UK) antibody, overnight at 4 °C. Secondary rabbit antibody (Alexa Fluor® 488) ab150077 was used at 1:100 dilution. Cells were then washed with PBS, counterstained with Trypan Blue (GIBCO 1:10) for 15 s, washed with PBS, stained for 5 min with 1 μg/mL of Hoechst 33342 (Sigma, Burlington, MA, USA) in PBS, and mounted in PBS:glycerol (1:1) for inspection with a LEICA TCS-SP5 inverted confocal microscope. A diode laser was used for Hoechst 33342, with = 405 nm; a visible laser for HSP70, with = 496 nm; and a He/Ne laser for Trypan Blue, with = 543 nm.
2.13. Statistical Analysis
Statistical analysis was conducted with MatLab (Mathworks, Natick, MA, USA), then confirmed and plotted using Prism software (9, GraphPad Inc., La Jolla, CA, USA). All data reported in the paper are expressed as mean ± standard error of the mean (mean ± SEM). A two-way ANOVA test was applied to evaluate statistically significant differences with multiple comparisons, using the Tukey-Kramer test. The statistical significance level was placed at p < 0.05.
4. Discussion
Thermal therapy, i.e., a local increase of tumor tissue temperature, is widely applied in cancer treatment. Both hyperthermia, i.e., heating to 39–45 °C to induce sensitization to radiotherapy and chemotherapy, and thermal ablation, where temperatures beyond 50 °C destroy tumor cells directly, are frequently applied in clinical practice [
19]. Among the different approaches that can be used to induce local temperature increase [
3] in tumor tissue, MFH is regarded as a minimally invasive method without systemic toxicity. MFH is based on a limited temperature increase (42–46 °C) of tumor tissue that can lead to apoptosis, avoiding extensive necrosis. It is well known [
20], that cancer cells are more sensitive to temperature increase than non-tumoral cells, and that temperatures around 46 °C can usually induce tumor cell apoptosis.
The use of self-regulating temperature materials, which heat only to the T
C, i.e., the temperature at which a magnetic phase transition from ferromagnetic to paramagnetic occurs, is seen as an undoubted advantage of this technique: it affords an intrinsic safety mechanism, guarding against the risk of damage to surrounding tissues [
6,
8]. The innovative nanomaterial—developed and patented by MBN, and functionalized by the University of Verona—has a T
C that can be modulated by synthesis, providing a temperature-limiting effect during AMF heating. M55, the magnetic core of the NP investigated in the present study, has a T
C value of about 95 °C (see Gerosa et al. [
6]). Since MFH ideally produces a limited increase in tissue temperature (42–46 °C), this T
C value may seem too high. However, recent experimental results have demonstrated that the very marked temperature increase, up to Δ
t = 45 °C within a distance of 0.5 nm from the MNPs’ surface, falls off exponentially as this distance increases [
21]. Therefore, a T
C value of about 95 °C could ensure that NPs reach a sufficiently high temperature to be effective MFH agents while remaining intrinsically safe for healthy tissues. Indeed, no damage to tumor-neighboring organs was observed in the present study.
We have demonstrated that G-M55 has diagnostic capabilities in bimodal imaging. G-M55 displays r2 relaxivity comparable to commercial MRI contrast agents such as Endorem®. As far as MPI is concerned, the signal efficiency of G-M55 is about 10% of that of a commercial MPI agent (such as VivoTrax), with comparable spatial resolution. G-M55 thus has good properties as a bimodalcontrast agent. In addition, in a biologically significant context, namely in a cellular imaging experiment, G-M55 allowed the labeling and detection of cells in vitro. Cells labeled by using G-M55 were detectable by both MPI and MRI. MPI allowed the detection of a lower number of cells than MRI, this result being consistent with the greater sensitivity of the former. Although preliminary, this experiment indicates that G-M55 has potential for application in the field of cellular imaging.
Another interesting characteristic of G-M55 is that it can be detected by both MRI and MPI. Moreover, with a view to future clinical applications, the finding that SAR values with standard MFH and MPI measurements are similar is of particular interest for the prospective use of G-M55 as a magnetic fluid hyperthermia agent, in both classical and advanced applications [
5]. In addition, the experimental conditions adopted are transferable to clinical protocols.
The timing of the in vivo experiment was decided with potential clinical application in mind—i.e., a single injection of G-M55, followed by multiple applications of AMF in rapid succession. This protocol was very effective in reducing tumor growth rate. Moreover, MRI showed the substantial presence of MNPs within the tumor up to 9 days after injection, enabling delayed AMF without further injections of MNPs.
In this paper, we have examined the application of a synthetic MNP, namely G-M55, in MFH in preclinical studies with reference to both diagnostic and therapeutic capabilities. G-M55 revealed good performances as a bimodal diagnostic contrast agent and as a therapeutic agent in MFH, with either standard or MPI-based systems. Our results demonstrate the efficacy of MFH in the treatment of breast cancer [
8,
22,
23,
24].