Current Perspectives on the Surgical Management of Perihilar Cholangiocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Preoperative Evaluation and Optimization
2.1. Workup
2.2. Biliary Drainage
2.3. Determining Resectability
2.4. Future Liver Remnant
3. Surgical Resection
3.1. Minimally Invasive Surgery
3.2. Liver Transplantation
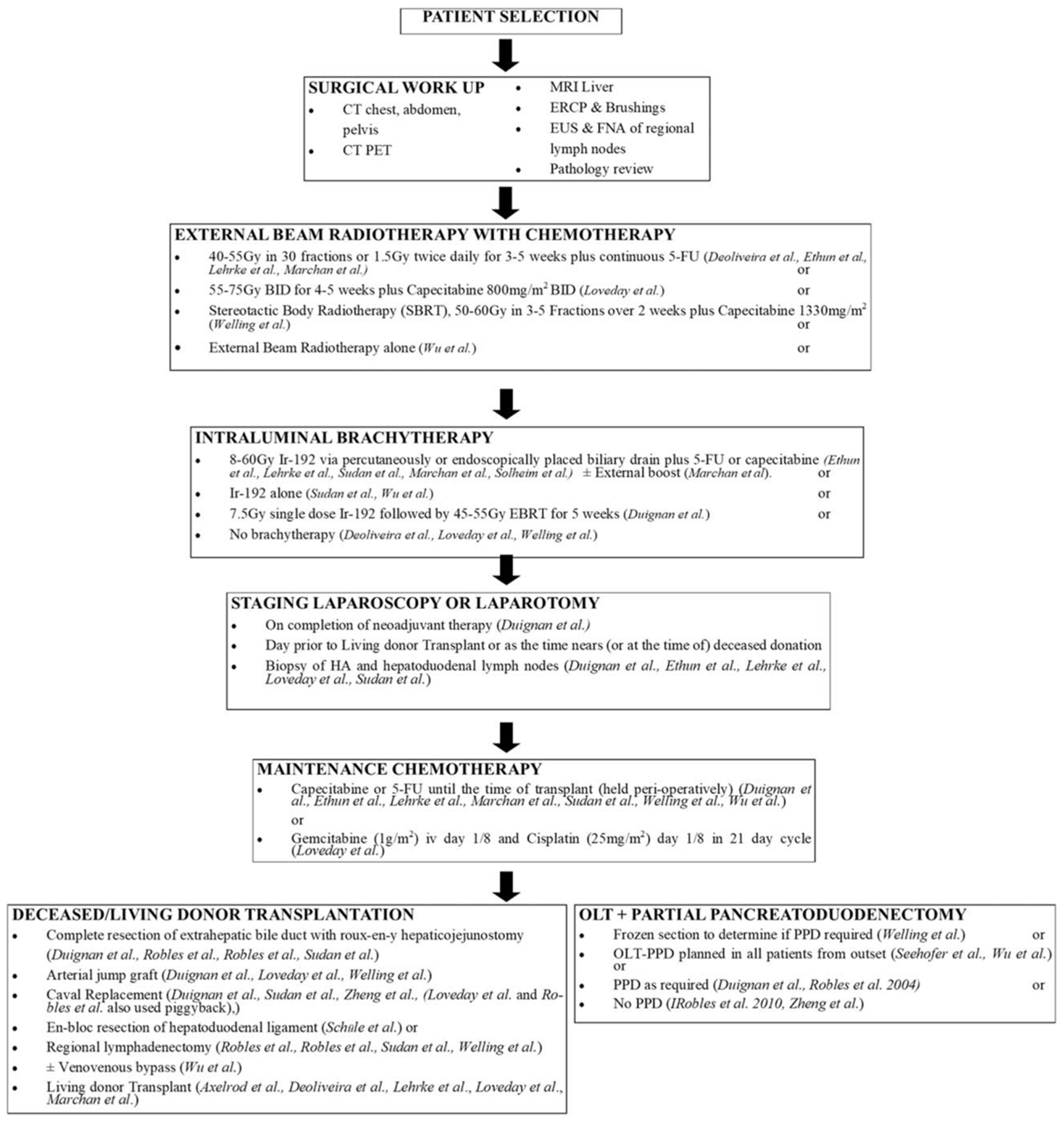
3.3. Neoadjuvant Systemic Therapy and Radiation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, V. Classifications and misclassification in cholangiocarcinoma. Liver Int. 2019, 39, 260–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Komaki, Y.; Komaki, F.; Micic, D.; Zullow, S.; Sakuraba, A. Risk of gastrointestinal cancers in patients with cystic fibrosis: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 758–767. [Google Scholar] [CrossRef]
- Klatskin, G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis: An unusual tumor with distinctive clinical and pathological features. Am. J. Med. 1965, 38, 241–256. [Google Scholar] [CrossRef]
- Khan, A.S.; Dageforde, L.A. Cholangiocarcinoma. Surg. Clin. N. Am. 2019, 99, 315–335. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Rizvi, S.; Gores, G.J. Current diagnostic and management options in perihilar cholangiocarcinoma. Digestion 2014, 89, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Van Keulen, A.M.; Franssen, S.; van der Geest, L.G.; de Boer, M.T.; Coenraad, M.; van Driel, L.M.J.W.; Erdmann, J.I.; Haj Mohammad, N.; Heij, L.; Klümpen, H.J.; et al. Nationwide treatment and outcomes of perihilar cholangiocarcinoma. Liver Int. 2021, 41, 1945–1953. [Google Scholar] [CrossRef]
- Sharma, P.; Yadav, S. Demographics, tumor characteristics, treatment, and survival of patients with Klatskin tumors. Ann. Gastroenterol. 2018, 31, 231–236. [Google Scholar] [CrossRef]
- Gad, M.M.; Saad, A.M.; Faisaluddin, M.; Gaman, M.A.; Ruhban, I.A.; Jazieh, K.A.; Al-Husseini, M.J.; Simons-Linares, C.R.; Sonbol, M.B.; Estfan, B.N. Epidemiology of Cholangiocarcinoma; United States Incidence and Mortality Trends. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 885–893. [Google Scholar] [CrossRef]
- Nagino, M.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Takahashi, Y.; Nimura, Y. Evolution of surgical treatment for perihilar cholangiocarcinoma: A single-center 34-year review of 574 consecutive resections. Ann. Surg. 2013, 258, 129–140. [Google Scholar] [CrossRef]
- Mansour, J.C.; Aloia, T.A.; Crane, C.H.; Heimbach, J.K.; Nagino, M.; Vauthey, J.N. Hilar cholangiocarcinoma: Expert consensus statement. HPB 2015, 17, 691–699. [Google Scholar] [CrossRef] [Green Version]
- Blechacz, B.; Komuta, M.; Roskams, T.; Gores, G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 512–522. [Google Scholar] [CrossRef] [Green Version]
- Loosen, S.H.; Roderburg, C.; Kauertz, K.L.; Koch, A.; Vucur, M.; Schneider, A.T.; Binnebösel, M.; Ulmer, T.F.; Lurje, G.; Schoening, W.; et al. CEA but not CA19-9 is an independent prognostic factor in patients undergoing resection of cholangiocarcinoma. Sci. Rep. 2017, 7, 16975. [Google Scholar] [CrossRef] [Green Version]
- Coelho, R.; Silva, M.; Rodrigues-Pinto, E.; Cardoso, H.; Lopes, S.; Pereira, P.; Vilas-Boas, F.; Santos-Antunes, J.; Costa-Maia, J.; Macedo, G. CA 19-9 as a Marker of Survival and a Predictor of Metastization in Cholangiocarcinoma. GE Port. J. Gastroenterol. 2017, 24, 114–121. [Google Scholar] [CrossRef]
- Juntermanns, B.; Radunz, S.; Heuer, M.; Hertel, S.; Reis, H.; Neuhaus, J.P.; Vernadakis, S.; Trarbach, T.; Paul, A.; Kaiser, G.M. Tumor markers as a diagnostic key for hilar cholangiocarcinoma. Eur. J. Med. Res. 2010, 15, 357–361. [Google Scholar] [CrossRef] [Green Version]
- Mann, D.V.; Edwards, R.; Ho, S.; Lau, W.Y.; Glazer, G. Elevated tumour marker CA19-9: Clinical interpretation and influence of obstructive jaundice. Eur. J. Surg. Oncol. 2000, 26, 474–479. [Google Scholar] [CrossRef]
- Rompianesi, G.; Di Martino, M.; Gordon-Weeks, A.; Montalti, R.; Troisi, R. Liquid biopsy in cholangiocarcinoma: Current status and future perspectives. World J. Gastrointest. Oncol. 2021, 13, 332–350. [Google Scholar] [CrossRef]
- Yang, J.D.; Campion, M.B.; Liu, M.C.; Chaiteerakij, R.; Giama, N.H.; Ahmed Mohammed, H.; Zhang, X.; Hu, C.; Campion, V.L.; Jen, J.; et al. Circulating tumor cells are associated with poor overall survival in patients with cholangiocarcinoma. Hepatology 2016, 63, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Eaton, J.; Yang, J.D.; Chandrasekhara, V.; Gores, G.J. Emerging Technologies for the Diagnosis of Perihilar Cholangiocarcinoma. Semin. Liver Dis. 2018, 38, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Sainani, N.I.; Catalano, O.A.; Holalkere, N.S.; Zhu, A.X.; Hahn, P.F.; Sahani, D.V. Cholangiocarcinoma: Current and novel imaging techniques. Radiographics 2008, 28, 1263–1287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Ke, F.; Weng, M.; Wu, X.; Li, M.; Quan, Z.; Liu, Y.; Zhang, Y.; Gong, W. Radiological Imaging for Assessing the Respectability of Hilar Cholangiocarcinoma: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2015, 2015, 497942. [Google Scholar] [CrossRef]
- Vogl, T.J.; Schwarz, W.O.; Heller, M.; Herzog, C.; Zangos, S.; Hintze, R.E.; Neuhaus, P.; Hammerstingl, R.M. Staging of Klatskin tumours (hilar cholangiocarcinomas): Comparison of MR cholangiography, MR imaging, and endoscopic retrograde cholangiography. Eur. Radiol. 2006, 16, 2317–2325. [Google Scholar] [CrossRef]
- Bird, N.; Elmasry, M.; Jones, R.; Elniel, M.; Kelly, M.; Palmer, D.; Fenwick, S.; Poston, G.; Malik, H. Role of staging laparoscopy in the stratification of patients with perihilar cholangiocarcinoma. Br. J. Surg. 2017, 104, 418–425. [Google Scholar] [CrossRef]
- Coelen, R.J.; Ruys, A.T.; Besselink, M.G.; Busch, O.R.; van Gulik, T.M. Diagnostic accuracy of staging laparoscopy for detecting metastasized or locally advanced perihilar cholangiocarcinoma: A systematic review and meta-analysis. Surg. Endosc. 2016, 30, 4163–4173. [Google Scholar] [CrossRef]
- Coelen, R.J.; Ruys, A.T.; Wiggers, J.K.; Nio, C.Y.; Verheij, J.; Gouma, D.J.; Besselink, M.G.; Busch, O.R.; van Gulik, T.M. Development of a Risk Score to Predict Detection of Metastasized or Locally Advanced Perihilar Cholangiocarcinoma at Staging Laparoscopy. Ann. Surg. Oncol. 2016, 23, 904–910. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Kim, M.H.; Lee, T.Y.; Hwang, C.Y.; Kim, J.S.; Yun, S.C.; Lee, S.S.; Seo, D.W.; Lee, S.K. Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: A prospective study compared with conventional imaging. Am. J. Gastroenterol. 2008, 103, 1145–1151. [Google Scholar] [CrossRef]
- Corvera, C.U.; Blumgart, L.H.; Akhurst, T.; DeMatteo, R.P.; D’Angelica, M.; Fong, Y.; Jarnagin, W.R. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J. Am. Coll. Surg. 2008, 206, 57–65. [Google Scholar] [CrossRef]
- Ruys, A.T.; Bennink, R.J.; van Westreenen, H.L.; Engelbrecht, M.R.; Busch, O.R.; Gouma, D.J.; van Gulik, T.M. FDG-positron emission tomography/computed tomography and standardized uptake value in the primary diagnosis and staging of hilar cholangiocarcinoma. HPB 2011, 13, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Heimbach, J.K.; Sanchez, W.; Rosen, C.B.; Gores, G.J. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB 2011, 13, 356–360. [Google Scholar] [CrossRef] [Green Version]
- Zaydfudim, V.M.; Wang, A.Y.; de Lange, E.E.; Zhao, Z.; Moskaluk, C.A.; Bauer, T.W.; Adams, R.B. IgG4-Associated Cholangitis Can Mimic Hilar Cholangiocarcinoma. Gut. Liver 2015, 9, 556–560. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.C.; Kim, M.H.; Lee, K.T.; Lee, J.K.; Moon, S.H.; Song, T.J.; Eum, J.; Park, D.H.; Lee, S.S.; Seo, D.W.; et al. Clinical clues to suspicion of IgG4-associated sclerosing cholangitis disguised as primary sclerosing cholangitis or hilar cholangiocarcinoma. J. Gastroenterol. Hepatol. 2010, 25, 1831–1837. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Hepatobiliary Cancers. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (accessed on 20 March 2022).
- She, W.H.; Cheung, T.T.; Ma, K.W.; Tsang, S.H.Y.; Dai, W.C.; Chan, A.C.Y.; Lo, C.M. Defining the optimal bilirubin level before hepatectomy for hilar cholangiocarcinoma. BMC Cancer 2020, 20, 914. [Google Scholar] [CrossRef]
- Olthof, P.B.; Wiggers, J.K.; Groot Koerkamp, B.; Coelen, R.J.; Allen, P.J.; Besselink, M.G.; Busch, O.R.; D’Angelica, M.I.; DeMatteo, R.P.; Kingham, T.P.; et al. Postoperative Liver Failure Risk Score: Identifying Patients with Resectable Perihilar Cholangiocarcinoma Who Can Benefit from Portal Vein Embolization. J. Am. Coll. Surg. 2017, 225, 387–394. [Google Scholar] [CrossRef]
- Farges, O.; Regimbeau, J.M.; Fuks, D.; Le Treut, Y.P.; Cherqui, D.; Bachellier, P.; Mabrut, J.Y.; Adham, M.; Pruvot, F.R.; Gigot, J.F. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br. J. Surg. 2013, 100, 274–283. [Google Scholar] [CrossRef]
- Iacono, C.; Ruzzenente, A.; Campagnaro, T.; Bortolasi, L.; Valdegamberi, A.; Guglielmi, A. Role of preoperative biliary drainage in jaundiced patients who are candidates for pancreatoduodenectomy or hepatic resection: Highlights and drawbacks. Ann. Surg. 2013, 257, 191–204. [Google Scholar] [CrossRef]
- Ferrero, A.; Lo Tesoriere, R.; Viganò, L.; Caggiano, L.; Sgotto, E.; Capussotti, L. Preoperative biliary drainage increases infectious complications after hepatectomy for proximal bile duct tumor obstruction. World J. Surg. 2009, 33, 318–325. [Google Scholar] [CrossRef]
- Kennedy, T.J.; Yopp, A.; Qin, Y.; Zhao, B.; Guo, P.; Liu, F.; Schwartz, L.H.; Allen, P.; D’Angelica, M.; Fong, Y.; et al. Role of preoperative biliary drainage of liver remnant prior to extended liver resection for hilar cholangiocarcinoma. HPB 2009, 11, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Ribero, D.; Zimmitti, G.; Aloia, T.A.; Shindoh, J.; Fabio, F.; Amisano, M.; Passot, G.; Ferrero, A.; Vauthey, J.N. Preoperative Cholangitis and Future Liver Remnant Volume Determine the Risk of Liver Failure in Patients Undergoing Resection for Hilar Cholangiocarcinoma. J. Am. Coll. Surg. 2016, 223, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krähenbühl, L.; Schäfer, M.; Krähenbühl, S. Reversibility of hepatic mitochondrial damage in rats with long-term cholestasis. J. Hepatol. 1998, 28, 1000–1007. [Google Scholar] [CrossRef]
- Yim, J.; Hyun, D.; Cho, S.K.; Park, K.B.; Park, H.S.; Shin, S.W.; Choi, D.W.; Kim, S.; Baek, S.Y.; Lee, S.Y. Effect of Hyperbilirubinemia on Hepatic Hypertrophy after Portal Vein Embolization and Liver Failure after Hepatectomy in Primary Biliary Malignancy. J. Vasc. Interv. Radiol. 2019, 30, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, M.; Kato, A.; Shimizu, H.; Ohtsuka, M.; Yoshitomi, H.; Furukawa, K.; Miyazaki, M. Preoperative Portal Vein Embolization before Major Hepatectomy in Patients with Excess Bilirubin does not Affect Hypertrophy of Remnant Liver and Postoperative Outcomes. Hepatogastroenterology 2014, 61, 908–915. [Google Scholar]
- Teng, F.; Tang, Y.Y.; Dai, J.L.; Li, Y.; Chen, Z.Y. The effect and safety of preoperative biliary drainage in patients with hilar cholangiocarcinoma: An updated meta-analysis. World J. Surg. Oncol. 2020, 18, 174. [Google Scholar] [CrossRef]
- Mehrabi, A.; Khajeh, E.; Ghamarnejad, O.; Nikdad, M.; Chang, D.H.; Büchler, M.W.; Hoffmann, K. Meta-analysis of the efficacy of preoperative biliary drainage in patients undergoing liver resection for perihilar cholangiocarcinoma. Eur. J. Radiol. 2020, 125, 108897. [Google Scholar] [CrossRef] [Green Version]
- Coelen, R.J.S.; Roos, E.; Wiggers, J.K.; Besselink, M.G.; Buis, C.I.; Busch, O.R.C.; Dejong, C.H.C.; van Delden, O.M.; van Eijck, C.H.J.; Fockens, P.; et al. Endoscopic versus percutaneous biliary drainage in patients with resectable perihilar cholangiocarcinoma: A multicentre, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2018, 3, 681–690. [Google Scholar] [CrossRef]
- Odisio, B.C.; Krampitz, G.W.; Murthy, R.; Mahvash, A.; Javle, M.; Vauthey, J.N. Preoperative drainage for perihilar cholangiocarcinoma. Lancet Gastroenterol. Hepatol. 2019, 4, 10–11. [Google Scholar] [CrossRef]
- Al Mahjoub, A.; Menahem, B.; Fohlen, A.; Dupont, B.; Alves, A.; Launoy, G.; Lubrano, J. Preoperative Biliary Drainage in Patients with Resectable Perihilar Cholangiocarcinoma: Is Percutaneous Transhepatic Biliary Drainage Safer and More Effective than Endoscopic Biliary Drainage? A Meta-Analysis. J. Vasc. Interv. Radiol. 2017, 28, 576–582. [Google Scholar] [CrossRef]
- Wang, L.; Lin, N.; Xin, F.; Ke, Q.; Zeng, Y.; Liu, J. A systematic review of the comparison of the incidence of seeding metastasis between endoscopic biliary drainage and percutaneous transhepatic biliary drainage for resectable malignant biliary obstruction. World J. Surg. Oncol. 2019, 17, 116. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, H.W.; Lee, J.C.; Paik, K.H.; Seong, N.J.; Yoon, C.J.; Hwang, J.H.; Kim, J. Percutaneous transhepatic versus EUS-guided gallbladder drainage for malignant cystic duct obstruction. Gastrointest. Endosc. 2017, 85, 357–364. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Hepatobiliary Cancers v2.2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (accessed on 12 June 2021).
- Boulay, B.R.; Birg, A. Malignant biliary obstruction: From palliation to treatment. World J. Gastrointest. Oncol. 2016, 8, 498–508. [Google Scholar] [CrossRef]
- Ding, G.; Yang, Y.; Cao, L.; Chen, W.; Wu, Z.; Jiang, G. A modified Jarnagin-Blumgart classification better predicts survival for resectable hilar cholangiocarcinoma. World J. Surg. Oncol. 2015, 13, 99. [Google Scholar] [CrossRef] [Green Version]
- Jarnagin, W.R.; Fong, Y.; DeMatteo, R.P.; Gonen, M.; Burke, E.C.; Bodniewicz BS, J.; Youssef BA, M.; Klimstra, D.; Blumgart, L.H. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann. Surg. 2001, 234, 507–517, discussion 517–509. [Google Scholar] [CrossRef]
- Matsuo, K.; Rocha, F.G.; Ito, K.; D’Angelica, M.I.; Allen, P.J.; Fong, Y.; Dematteo, R.P.; Gonen, M.; Endo, I.; Jarnagin, W.R. The Blumgart preoperative staging system for hilar cholangiocarcinoma: Analysis of resectability and outcomes in 380 patients. J. Am. Coll. Surg. 2012, 215, 343–355. [Google Scholar] [CrossRef]
- Chaiteerakij, R.; Harmsen, W.S.; Marrero, C.R.; Aboelsoud, M.M.; Ndzengue, A.; Kaiya, J.; Therneau, T.M.; Sanchez, W.; Gores, G.J.; Roberts, L.R. A new clinically based staging system for perihilar cholangiocarcinoma. Am. J. Gastroenterol. 2014, 109, 1881–1890. [Google Scholar] [CrossRef] [Green Version]
- Kuriyama, N.; Usui, M.; Gyoten, K.; Hayasaki, A.; Fujii, T.; Iizawa, Y.; Kato, H.; Murata, Y.; Tanemura, A.; Kishiwada, M.; et al. Neoadjuvant chemotherapy followed by curative-intent surgery for perihilar cholangiocarcinoma based on its anatomical resectability classification and lymph node status. BMC Cancer 2020, 20, 405. [Google Scholar] [CrossRef]
- Deoliveira, M.L.; Schulick, R.D.; Nimura, Y.; Rosen, C.; Gores, G.; Neuhaus, P.; Clavien, P.A. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology 2011, 53, 1363–1371. [Google Scholar] [CrossRef]
- Boudjema, K.; Sulpice, L.; Garnier, S.; Bretagne, J.-F.; Gandon, Y.; Rohou, T. A Simple System to Predict Perihilar Cholangiocarcinoma Resectability. J. Gastrointest. Surg. 2013, 17, 1247–1256. [Google Scholar] [CrossRef]
- Sollini, M.; Antunovic, L.; Chiti, A.; Kirienko, M. Towards clinical application of image mining: A systematic review on artificial intelligence and radiomics. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2656–2672. [Google Scholar] [CrossRef] [Green Version]
- Ji, G.W.; Zhu, F.P.; Zhang, Y.D.; Liu, X.S.; Wu, F.Y.; Wang, K.; Xia, Y.X.; Jiang, W.J.; Li, X.C.; Wang, X.H. A radiomics approach to predict lymph node metastasis and clinical outcome of intrahepatic cholangiocarcinoma. Eur. Radiol. 2019, 29, 3725–3735. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Shu, J. Cholangiocarcinoma Evaluation via Imaging and Artificial Intelligence. Oncology 2021, 99, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Lillemoe, H.A.; Vauthey, J.N. Dealing with an insufficient future liver remnant: Portal vein embolization and two-stage hepatectomy. J. Surg. Oncol. 2019, 119, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Ha, T.Y.; Song, G.W.; Jung, D.H.; Ahn, C.S.; Moon, D.B.; Kim, K.H.; Lee, Y.J.; Lee, S.G. Quantified Risk Assessment for Major Hepatectomy via the Indocyanine Green Clearance Rate and Liver Volumetry Combined with Standard Liver Volume. J. Gastrointest. Surg. 2015, 19, 1305–1314. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nishio, H.; Ebata, T.; Igami, T.; Sugawara, G.; Nagino, M. Value of indocyanine green clearance of the future liver remnant in predicting outcome after resection for biliary cancer. Br. J. Surg. 2010, 97, 1260–1268. [Google Scholar] [CrossRef]
- Maruyama, M.; Yoshizako, T.; Araki, H.; Yoshida, R.; Ando, S.; Nakamura, M.; Kitagaki, H. Future Liver Remnant Indocyanine Green Plasma Clearance Rate as a Predictor of Post-hepatectomy Liver Failure After Portal Vein Embolization. Cardiovasc. Interv. Radiol. 2018, 41, 1877–1884. [Google Scholar] [CrossRef]
- Rous, P.; Larimore, L.D. Relation of the portal blood to liver maintenance: A demonstration of liver atrophy conditional on compensation. J. Exp. Med. 1920, 31, 609–632. [Google Scholar] [CrossRef] [Green Version]
- Honjo, I.; Suzuki, T.; Ozawa, K.; Takasan, H.; Kitamura, O. Ligation of a branch of the portal vein for carcinoma of the liver. Am. J. Surg. 1975, 130, 296–302. [Google Scholar] [CrossRef]
- Abulkhir, A.; Limongelli, P.; Healey, A.J.; Damrah, O.; Tait, P.; Jackson, J.; Habib, N.; Jiao, L.R. Preoperative portal vein embolization for major liver resection: A meta-analysis. Ann. Surg. 2008, 247, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Masthoff, M.; Katou, S.; Köhler, M.; Schindler, P.; Heindel, W.; Wilms, C.; Schmidt, H.H.; Pascher, A.; Struecker, B.; Wildgruber, M.; et al. Portal and hepatic vein embolization prior to major hepatectomy. Z. Gastroenterol. 2021, 59, 35–42. [Google Scholar] [CrossRef]
- Deshayes, E.; Piron, L.; Bouvier, A.; Lapuyade, B.; Lermite, E.; Vervueren, L.; Laurent, C.; Pinaquy, J.B.; Chevallier, P.; Dohan, A.; et al. Study protocol of the HYPER-LIV01 trial: A multicenter phase II, prospective and randomized study comparing simultaneous portal and hepatic vein embolization to portal vein embolization for hypertrophy of the future liver remnant before major hepatectomy for colo-rectal liver metastases. BMC Cancer 2020, 20, 574. [Google Scholar] [CrossRef]
- Schnitzbauer, A.A.; Lang, S.A.; Goessmann, H.; Nadalin, S.; Baumgart, J.; Farkas, S.A.; Fichtner-Feigl, S.; Lorf, T.; Goralcyk, A.; Hörbelt, R.; et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 2012, 255, 405–414. [Google Scholar] [CrossRef]
- Popescu, G.A.; Alexandrescu, S.T.; Grigorie, R.T.; Stoica, L.; Apavaloaie, C.A.; Hrehoreţ, D. Good to know: The alpps procedure-embracing a new technique. Chirurgia 2017, 112, 332–341. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Gu, S.; Tang, K. A systematic review and meta-analysis of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus traditional staged hepatectomy. Medicine 2019, 98, e15229. [Google Scholar] [CrossRef]
- Olthof, P.B.; Coelen, R.J.S.; Wiggers, J.K.; Groot Koerkamp, B.; Malago, M.; Hernandez-Alejandro, R.; Topp, S.A.; Vivarelli, M.; Aldrighetti, L.A.; Robles Campos, R.; et al. High mortality after ALPPS for perihilar cholangiocarcinoma: Case-control analysis including the first series from the international ALPPS registry. HPB 2017, 19, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, F.A.; Ardiles, V.; de Santibañes, M.; Pekolj, J.; de Santibañes, E. Associating liver partition and portal vein ligation for staged hepatectomy offers high oncological feasibility with adequate patient safety: A prospective study at a single center. Ann. Surg. 2015, 261, 723–732. [Google Scholar] [CrossRef]
- Wu, X.; Rao, J.; Zhou, X.; Deng, R.; Ma, Y. Partial ALPPS versus complete ALPPS for staged hepatectomy. BMC Gastroenterol. 2019, 19, 170. [Google Scholar] [CrossRef] [Green Version]
- Truant, S.; El Amrani, M.; Baillet, C.; Ploquin, A.; Lecolle, K.; Ernst, O.; Hebbar, M.; Huglo, D.; Pruvot, F.R. Laparoscopic Partial ALPPS: Much Better Than ALPPS! Ann. Hepatol. 2019, 18, 269–273. [Google Scholar] [CrossRef]
- Eshmuminov, D.; Raptis, D.A.; Linecker, M.; Wirsching, A.; Lesurtel, M.; Clavien, P.A. Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy. Br. J. Surg. 2016, 103, 1768–1782. [Google Scholar] [CrossRef]
- Hemming, A.W.; Reed, A.I.; Fujita, S.; Foley, D.P.; Howard, R.J. Surgical management of hilar cholangiocarcinoma. Ann. Surg. 2005, 241, 693–699, discussion 699–702. [Google Scholar] [CrossRef]
- Hong, J.C.; Jones, C.M.; Duffy, J.P.; Petrowsky, H.; Farmer, D.G.; French, S.; Finn, R.; Durazo, F.A.; Saab, S.; Tong, M.J.; et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: A 24-year experience in a single center. Arch. Surg. 2011, 146, 683–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuzzo, G.; Giuliante, F.; Ardito, F.; Giovannini, I.; Aldrighetti, L.; Belli, G.; Bresadola, F.; Calise, F.; Dalla Valle, R.; D’Amico, D.F.; et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: Results of an Italian multicenter analysis of 440 patients. Arch. Surg. 2012, 147, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Ikai, I.; Fujii, H.; Hatano, E.; Shimahara, Y. Surgical resection of hilar cholangiocarcinoma: Analysis of survival and postoperative complications. World J. Surg. 2007, 31, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, W.W.; Chen, J.H.; Cui, M.H.; Liu, J.L. The value of caudate lobectomy in hilar cholangiocarcinoma treatment: A meta-analysis. Medicine 2021, 100, e24727. [Google Scholar] [CrossRef]
- Hasegawa, K.; Kokudo, N.; Imamura, H.; Sano, K.; Aoki, T.; Miki, K.; Hashimoto, T.; Sugawara, Y.; Makuuchi, M. Bilioenteric reconstruction for small bile ducts without mucosa-to-mucosa alignment: Long-term results. Arch. Surg. 2004, 139, 1050–1054. [Google Scholar] [CrossRef] [Green Version]
- Govil, S.; Reddy, M.S.; Rela, M. Surgical resection techniques for locally advanced hilar cholangiocarcinoma. Langenbeck’s Arch. Surg. 2014, 399, 707–716. [Google Scholar] [CrossRef]
- Serrablo, A.; Serrablo, L.; Alikhanov, R.; Tejedor, L. Vascular Resection in Perihilar Cholangiocarcinoma. Cancers 2021, 13, 5278. [Google Scholar] [CrossRef]
- Abbas, S.; Sandroussi, C. Systematic review and meta-analysis of the role of vascular resection in the treatment of hilar cholangiocarcinoma. HPB 2013, 15, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, T.; Ebata, T.; Yokoyama, Y.; Igami, T.; Yamaguchi, J.; Onoe, S.; Watanabe, N.; Kamei, Y.; Nagino, M. Combined Vascular Resection for Locally Advanced Perihilar Cholangiocarcinoma. Ann. Surg. 2022, 275, 382–390. [Google Scholar] [CrossRef]
- Sugiura, T.; Uesaka, K.; Okamura, Y.; Ito, T.; Yamamoto, Y.; Ashida, R.; Ohgi, K.; Otsuka, S.; Nakagawa, M.; Aramaki, T.; et al. Major hepatectomy with combined vascular resection for perihilar cholangiocarcinoma. BJS Open 2021, 5, zrab064. [Google Scholar] [CrossRef]
- Zhang, X.F.; Squires, M.H.; Bagante, F.; Ethun, C.G.; Salem, A.; Weber, S.M.; Tran, T.; Poultsides, G.; Son, A.Y.; Hatzaras, I.; et al. The Impact of Intraoperative Re-Resection of a Positive Bile Duct Margin on Clinical Outcomes for Hilar Cholangiocarcinoma. Ann. Surg. Oncol. 2018, 25, 1140–1149. [Google Scholar] [CrossRef]
- Lim, J.H.; Choi, G.H.; Choi, S.H.; Kim, K.S.; Choi, J.S.; Lee, W.J. Liver resection for Bismuth type I and Type II hilar cholangiocarcinoma. World J. Surg. 2013, 37, 829–837. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Wu, L.; Li, B. A systematic review of safety and efficacy of hepatopancreatoduodenectomy for biliary and gallbladder cancers. HPB 2016, 18, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Franken, L.C.; van der Poel, M.J.; Latenstein, A.E.J.; Zwart, M.J.; Roos, E.; Busch, O.R.; Besselink, M.G.; van Gulik, T.M. Minimally invasive surgery for perihilar cholangiocarcinoma: A systematic review. J. Robot. Surg. 2019, 13, 717–727. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Qiu, J.G.; Deng, X.; Liu, S.S.; Cheng, L.; Liu, J.R.; Du, C.Y. Minimally invasive versus open radical resection surgery for hilar cholangiocarcinoma: Comparable outcomes associated with advantages of minimal invasiveness. PLoS ONE 2021, 16, e0248534. [Google Scholar] [CrossRef]
- Rahnemai-Azar, A.A.; Abbasi, A.; Tsilimigras, D.I.; Weber, S.M.; Pawlik, T.M. Current Advances in Minimally Invasive Surgical Management of Perihilar Cholangiocarcinoma. J. Gastrointest. Surg. 2020, 24, 2143–2149. [Google Scholar] [CrossRef]
- Cipriani, F.; Ratti, F.; Fiorentini, G.; Reineke, R.; Aldrighetti, L. Systematic review of perioperative and oncologic outcomes of minimally-invasive surgery for hilar cholangiocarcinoma. Updates Surg. 2021, 73, 359–377. [Google Scholar] [CrossRef]
- Meyer, C.G.; Penn, I.; James, L. Liver transplantation for cholangiocarcinoma: Results in 207 patients. Transplantation 2000, 69, 1633–1637. [Google Scholar] [CrossRef]
- Iwatsuki, S.; Todo, S.; Marsh, J.W.; Madariaga, J.R.; Lee, R.G.; Dvorchik, I.; Fung, J.J.; Starzl, T.E. Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J. Am. Coll. Surg. 1998, 187, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Heimbach, J.K.; Gores, G.J.; Haddock, M.G.; Alberts, S.R.; Nyberg, S.L.; Ishitani, M.B.; Rosen, C.B. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 201–207. [Google Scholar] [CrossRef]
- De Vreede, I.; Steers, J.L.; Burch, P.A.; Rosen, C.B.; Gunderson, L.L.; Haddock, M.G.; Burgart, L.; Gores, G.J. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transplant. 2000, 6, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Sudan, D.; DeRoover, A.; Chinnakotla, S.; Fox, I.; Shaw, B.; McCashland, T.; Sorrell, M.; Tempero, M.; Langnas, A. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am. J. Transplant. 2002, 2, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Darwish Murad, S.; Kim, W.R.; Harnois, D.M.; Douglas, D.D.; Burton, J.; Kulik, L.M.; Botha, J.F.; Mezrich, J.D.; Chapman, W.C.; Schwartz, J.J.; et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012, 143, 88–98.e3, quiz e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cambridge, W.A.; Fairfield, C.; Powell, J.J.; Harrison, E.M.; Søreide, K.; Wigmore, S.J.; Guest, R.V. Meta-analysis and Meta-regression of Survival After Liver Transplantation for Unresectable Perihilar Cholangiocarcinoma. Ann. Surg. 2021, 273, 240–250. [Google Scholar] [CrossRef]
- Ethun, C.G.; Lopez-Aguiar, A.G.; Anderson, D.J.; Adams, A.B.; Fields, R.C.; Doyle, M.B.; Chapman, W.C.; Krasnick, B.A.; Weber, S.M.; Mezrich, J.D.; et al. Transplantation Versus Resection for Hilar Cholangiocarcinoma: An Argument for Shifting Treatment Paradigms for Resectable Disease. Ann. Surg. 2018, 267, 797–805. [Google Scholar] [CrossRef]
- Allen, M.J.; Knox, J.J. A review of current adjuvant and neoadjuvant systemic treatments for cholangiocarcinoma and gallbladder carcinoma. Hepatoma Res. 2021, 7, 73. [Google Scholar] [CrossRef]
- Yadav, S.; Xie, H.; Bin-Riaz, I.; Sharma, P.; Durani, U.; Goyal, G.; Borah, B.; Borad, M.J.; Smoot, R.L.; Roberts, L.R.; et al. Neoadjuvant vs. adjuvant chemotherapy for cholangiocarcinoma: A propensity score matched analysis. Eur. J. Surg. Oncol. 2019, 45, 1432–1438. [Google Scholar] [CrossRef]
- Baltatzis, M.; Jegatheeswaran, S.; Siriwardena, A.K. Neoadjuvant chemoradiotherapy before resection of perihilar cholangiocarcinoma: A systematic review. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 103–108. [Google Scholar] [CrossRef]
- Boscoe, A.N.; Rolland, C.; Kelley, R.K. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: A systematic literature review. J. Gastrointest. Oncol. 2019, 10, 751–765. [Google Scholar] [CrossRef]
- Piha-Paul, S.A.; Oh, D.Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Brandi, G. PD-L1, TMB, MSI, and Other Predictors of Response to Immune Checkpoint Inhibitors in Biliary Tract Cancer. Cancers 2021, 13, 558. [Google Scholar] [CrossRef]


| Trial ID/Name | Location | Trial Type | Tumor Site | Resectability Status | No. of Patients | Intervention | Primary Outcomes | Status |
|---|---|---|---|---|---|---|---|---|
| NCT02232932 | France | Phase III | pCCA | Resectable | 60 | Capecitabine-radiotherapy-liver transplant v resection | OS | Active, not recruiting |
| NCT03673072 (GAIN) | Germany | Phase III | GBC, CCA | Incidental diagnosis post cholecystectomy or advanced CCA | 300 | Cisplatin + gemcitabine (×3 cycles) v nil → surgery → +/- adjuvant cisplatin + gemcitabine (×3 cycles) | OS | Recruiting |
| NCT03603834 | Thailand | Phase II | CCA | Borderline resectable | 25 | mFOLFOXIRI | ORR | Recruiting |
| NCT04308174 (DEBATE) | Korea | Phase II | GBC, CCA | Resectable | 45 | Durvalumab + cisplatin + gemcitabine v cisplatin + gemcitabine | R0 rate | Recruiting |
| NCT04727541 | Germany | Phase II | GBC, CCA | Resectable | 24 | Bintrafusp-alfa ×2 doses | Major pathologic response | Recruiting |
| NCT04480190 | USA | Phase I | GBC, CCA | Resectable | 12 | Gemcitabine + cisplatin + 5-FU/RT | Completion of therapy | Recruiting |
| NCT04378023 | Spain | Phase IV | pCCA | Unresectable | 34 | EBRT + capecitabine → cisplatin + gemcitabine until transplant | OS at 1, 3, and 5 years | Recruiting |
| NCT04824742 | China | Phase II | CCA | Resectable | 50 | PDT | R0, local recurrence, OS 5-year | Not yet recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hewitt, D.B.; Brown, Z.J.; Pawlik, T.M. Current Perspectives on the Surgical Management of Perihilar Cholangiocarcinoma. Cancers 2022, 14, 2208. https://doi.org/10.3390/cancers14092208
Hewitt DB, Brown ZJ, Pawlik TM. Current Perspectives on the Surgical Management of Perihilar Cholangiocarcinoma. Cancers. 2022; 14(9):2208. https://doi.org/10.3390/cancers14092208
Chicago/Turabian StyleHewitt, D. Brock, Zachary J. Brown, and Timothy M. Pawlik. 2022. "Current Perspectives on the Surgical Management of Perihilar Cholangiocarcinoma" Cancers 14, no. 9: 2208. https://doi.org/10.3390/cancers14092208







