A Luminex Approach to Develop an Anti-Tumor-Associated Antigen Autoantibody Panel for the Detection of Prostate Cancer in Racially/Ethnically Diverse Populations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. Luminex Immunoassay
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Statistical Analysis
3. Results
3.1. Higher Frequencies of Anti-TAA Autoantibodies in PCa Patients Compared to Normal Controls
3.2. Performance of Anti-TAA Autoantibodies in Distinguishing PCa from Normal Controls
3.3. ELISA Validation of Anti-HSP60 Autoantibody in a Large Sample
3.4. Developing an Optimal Anti-TAA Autoantibody Panel for PCa Identification
3.5. Changes in Autoantibodies in Patients with PCa before and after Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [PubMed]
- Kohaar, I.; Petrovics, G.; Srivastava, S. A rich array of prostate cancer molecular biomarkers: Opportunities and challenges. Int. J. Mol. Sci. 2019, 20, 1813. [Google Scholar] [PubMed] [Green Version]
- Merriel, S.W.D.; Pocock, L.; Gilbert, E.; Creavin, S.; Walter, F.M.; Spencer, A.; Hamilton, W. Systematic review and meta-analysis of the diagnostic accuracy of prostate-specific antigen (PSA) for the detection of prostate cancer in symptomatic patients. BMC Med. 2022, 20, 54. [Google Scholar]
- Tan, E.M.; Zhang, J. Autoantibodies to tumor-associated antigens: Reporters from the immune system. Immunol. Rev. 2008, 222, 328–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.-Y.; Tan, E.M. Autoantibodies to tumor-associated antigens as diagnostic biomarkers in hepatocellular carcinoma and other solid tumors. Expert Rev. Mol. Diagn. 2010, 10, 321–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monroy-Iglesias, M.J.; Crescioli, S.; Beckmann, K.; Le, N.; Karagiannis, S.N.; Van Hemelrijck, M.; Santaolalla, A. Antibodies as biomarkers for cancer risk: A systematic review. Clin. Exp. Immunol. 2022, 209, 46–63. [Google Scholar]
- Zhang, J.-Y.; Casiano, C.A.; Peng, X.-X.; Koziol, J.A.; Chan, E.K.; Tan, E.M. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epidemiol. Biomark. Prev. 2003, 12, 136–143. [Google Scholar]
- Ma, Y.; Qiu, C.; Wang, B.; Zhang, X.; Wang, X.; Aguilera, R.J.; Zhang, J.-Y. Autoantibody against Tumor-Associated Antigens as Diagnostic Biomarkers in Hispanic Patients with Hepatocellular Carcinoma. Cells 2022, 11, 3227. [Google Scholar] [CrossRef]
- Dai, L.; Ren, P.; Liu, M.; Imai, H.; Tan, E.M.; Zhang, J.-Y. Using immunomic approach to enhance tumor-associated autoantibody detection in diagnosis of hepatocellular carcinoma. Clin. Immunol. 2014, 152, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-Y. Mini-array of multiple tumor-associated antigens to enhance autoantibody detection for immunodiagnosis of hepatocellular carcinoma. Autoimmun. Rev. 2007, 6, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.U.A.; Sun, C.; Ren, P.; Dai, L.; Peng, B.O.; Wang, K.; Qian, W.E.I.; Zhang, J. Mini-array of multiple tumor-associated antigens (TAAs) in the immunodiagnosis of breast cancer. Oncol. Lett. 2013, 5, 663–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, L.; Tsay, J.-C.J.; Li, J.; Yie, T.-A.; Munger, J.S.; Pass, H.; Rom, W.N.; Zhang, Y.; Tan, E.M.; Zhang, J.-Y. Autoantibodies against tumor-associated antigens in the early detection of lung cancer. Lung Cancer 2016, 99, 172–179. [Google Scholar] [PubMed]
- Dai, L.; Li, J.; Ortega, R.; Qian, W.; Casiano, C.A.; Zhang, J.-Y. Preferential autoimmune response in prostate cancer to cyclin B1 in a panel of tumor-associated antigens. J. Immunol. Res. 2014, 2014, 827827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, T.W.; Zhang, G.; Li, J.; Dai, L.; Mirshahidi, S.; Wall, N.R.; Yates, C.; Wilson, C.; Montgomery, S.; Zhang, J.-Y. Immunoseroproteomic profiling in African American men with prostate cancer: Evidence for an autoantibody response to glycolysis and plasminogen-associated proteins. Mol. Cell. Proteom. 2016, 15, 3564–3580. [Google Scholar]
- Soussi, T. p53 Antibodies in the sera of patients with various types of cancer: A review. Cancer Res. 2000, 60, 1777–1788. [Google Scholar] [PubMed]
- Kathrikolly, T.; Nair, S.N.; Mathew, A.; Saxena, P.P.U.; Nair, S. Can serum autoantibodies be a potential early detection biomarker for breast cancer in women? A diagnostic test accuracy review and meta-analysis. Syst. Rev. 2022, 11, 215. [Google Scholar] [PubMed]
- Qiu, C.; Wang, P.; Wang, B.; Shi, J.; Wang, X.; Li, T.; Qin, J.; Dai, L.; Ye, H.; Zhang, J. Establishment and validation of an immunodiagnostic model for prediction of breast cancer. Oncoimmunology 2020, 9, 1682382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jonge, H.; Iamele, L.; Maggi, M.; Pessino, G.; Scotti, C. Anti-cancer auto-antibodies: Roles, applications and open issues. Cancers 2021, 13, 813. [Google Scholar]
- Kobayashi, M.; Katayama, H.; Fahrmann, J.F.; Hanash, S.M. Development of autoantibody signatures for common cancers. Semin. Immunol. 2020, 47, 101388. [Google Scholar]
- Lee, K.E.; Spata, M.; Bayne, L.J.; Buza, E.L.; Durham, A.C.; Allman, D.; Vonderheide, R.H.; Simon, M.C. Hif1a Deletion Reveals Pro-Neoplastic Function of B Cells in Pancreatic NeoplasiaHIF1α and B Cells in Pancreatic Neoplasia. Cancer Discov. 2016, 6, 256–269. [Google Scholar] [CrossRef] [Green Version]
- Nanami, T.; Hoshino, I.; Shiratori, F.; Yajima, S.; Oshima, Y.; Suzuki, T.; Ito, M.; Hiwasa, T.; Kuwajima, A.; Shimada, H. Prevalence of serum galectin-1 autoantibodies in seven types of cancer: A potential biomarker. Mol. Clin. Oncol. 2021, 15, 179. [Google Scholar]
- Clark, A.G.; Weston, M.L.; Foster, M.H. Lack of galectin-1 or galectin-3 alters B cell deletion and anergy in an autoantibody transgene model. Glycobiology 2013, 23, 893–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villar-Vázquez, R.; Padilla, G.; Fernández-Aceñero, M.J.; Suarez, A.; Fuente, E.; Pastor, C.; Calero, M.; Barderas, R.; Casal, J.I. Development of a novel multiplex beads-based assay for autoantibody detection for colorectal cancer diagnosis. Proteomics 2016, 16, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.R.; Kemp, T.J.; Haynesworth, K.; Loftus, S.A.; Pinto, L.A. Development, Validation, and Utilization of a Luminex-Based SARS-CoV-2 Multiplex Serology Assay. Microbiol. Spectr. 2023, 11, e03898-22. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Ma, Y.; Wang, B.; Zhang, X.; Wang, X.; Zhang, J.-Y. Autoantibodies to PAX5, PTCH1, and GNA11 as Serological Biomarkers in the Detection of Hepatocellular Carcinoma in Hispanic Americans. Int. J. Mol. Sci. 2023, 24, 3721. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, I.K.; Parsy-Kowalska, C.B.; Chapman, C.J. Autoantibodies: Opportunities for Early Cancer Detection. Trends Cancer 2017, 3, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Zaenker, P.; Ziman, M.R. Serologic autoantibodies as diagnostic cancer biomarkers—A review. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2161–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanami, T.; Hoshino, I.; Shiratori, F.; Yajima, S.; Oshima, Y.; Suzuki, T.; Ito, M.; Hiwasa, T.; Kuwajima, A.; Shimada, H. Presence of serum RalA and serum p53 autoantibodies in 1833 patients with various types of cancers. Int. J. Clin. Oncol. 2022, 27, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, N.; Roviello, G.; D’angelo, A.; Roudi, R.; Neeli, P.K.; Generali, D. p53 antibodies as a diagnostic marker for cancer: A meta-analysis. Molecules 2021, 26, 6215. [Google Scholar] [CrossRef]
- Sun, B.; Li, G.; Yu, Q.; Liu, D.; Tang, X. HSP60 in cancer: A promising biomarker for diagnosis and a potentially useful target for treatment. J. Drug Target. 2022, 30, 31–45. [Google Scholar] [CrossRef]
- Castilla, C.; Congregado, B.; Conde, J.M.; Medina, R.; Torrubia, F.J.; Japón, M.A.; Sáez, C. Immunohistochemical expression of Hsp60 correlates with tumor progression and hormone resistance in prostate cancer. Urology 2010, 76, 1017.e1–1017.e6. [Google Scholar] [CrossRef]
- Lianos, G.D.; Alexiou, G.A.; Mangano, A.; Mangano, A.; Rausei, S.; Boni, L.; Dionigi, G.; Roukos, D.H. The role of heat shock proteins in cancer. Cancer Lett. 2015, 360, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Looi, K.S.; Nakayasu, E.S.; Diaz, R.A.; Tan, E.M.; Almeida, I.C.; Zhang, J.Y. Using proteomic approach to identify tumor-associated antigens as markers in hepatocellular carcinoma. J. Proteome Res. 2008, 7, 4004–4012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodzek, P.; Partyka, R.; Damasiewicz-Bodzek, A. Antibodies against Hsp60 and Hsp65 in the sera of women with ovarian cancer. J. Ovarian Res. 2014, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Wu, Y.; Mou, Z.; Li, W.; Zou, L.; Fu, T.; Zhang, A.; Xiang, D.; Xiao, H.; Wang, X. Proteomics-based identification of HSP60 as a tumor-associated antigen in colorectal cancer. Proteom. Clin. Appl. 2007, 1, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wadhwa, P.D.; Culhane, J.F.; Nelson, E.L. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: Determinations of a panel of nine cytokines in clinical sample culture supernatants. J. Reprod. Immunol. 2005, 66, 175–191. [Google Scholar]
- Tait, B.D.; Hudson, F.; Cantwell, L.; Brewin, G.; Holdsworth, R.; Bennett, G.; Jose, M. Luminex technology for HLA antibody detection in organ transplantation. Nephrology 2009, 14, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.; Oaxaca, D.; Di Desidero, T.; Parra, K.; Rodriguez, G.; Manciu, M.; Allegrini, G.; Falcone, A.; Bocci, G.; Kirken, R.A. Pharmacodynamic biomarkers in metronomic chemotherapy: Multiplex cytokine measurements in gastrointestinal cancer patients. Clin. Exp. Med. 2021, 21, 149–159. [Google Scholar] [PubMed]
- Qiu, C.; Wang, B.; Wang, P.; Wang, X.; Ma, Y.; Dai, L.; Shi, J.; Wang, K.; Sun, G.; Ye, H. Identification of novel autoantibody signatures and evaluation of a panel of autoantibodies in breast cancer. Cancer Sci. 2021, 112, 3388–3400. [Google Scholar] [CrossRef]
- Murray, A.; Chapman, C.J.; Healey, G.; Peek, L.J.; Parsons, G.; Baldwin, D.; Barnes, A.; Sewell, H.F.; Fritsche, H.A.; Robertson, J.F.R. Technical validation of an autoantibody test for lung cancer. Ann. Oncol. 2010, 21, 1687–1693. [Google Scholar] [CrossRef]
- Dai, L.; Li, J.; Xing, M.; Sanchez, T.W.; Casiano, C.A.; Zhang, J.Y. Using serological proteome analysis to identify serum anti-nucleophosmin 1 autoantibody as a potential biomarker in European-American and African-American patients with prostate cancer. Prostate 2016, 76, 1375–1386. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.-Q.; Guo, S.-J.; Guo, W.; Jiang, H.-W.; Li, H.-C.; Tao, S.-C. Longitudinal serum autoantibody repertoire profiling identifies surgery-associated biomarkers in lung adenocarcinoma. EBioMedicine 2020, 53, 102674. [Google Scholar] [CrossRef]
- Häfner, N.; Nicolaus, K.; Weiss, S.; Frey, M.; Diebolder, H.; Rengsberger, M.; Dürst, M.; Runnebaum, I.B. p53-autoantibody may be more sensitive than CA-125 in monitoring microscopic and macroscopic residual disease after primary therapy for epithelial ovarian cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 1207–1210. [Google Scholar] [CrossRef]
- Shaarawy, M.; Sheiba, M. Diagnostic and prognostic significance of circulating tumor suppressor gene p53 autoantibodies in patients with gestational trophoblastic tumors. Acta Oncol. 2004, 43, 43–48. [Google Scholar] [CrossRef] [Green Version]
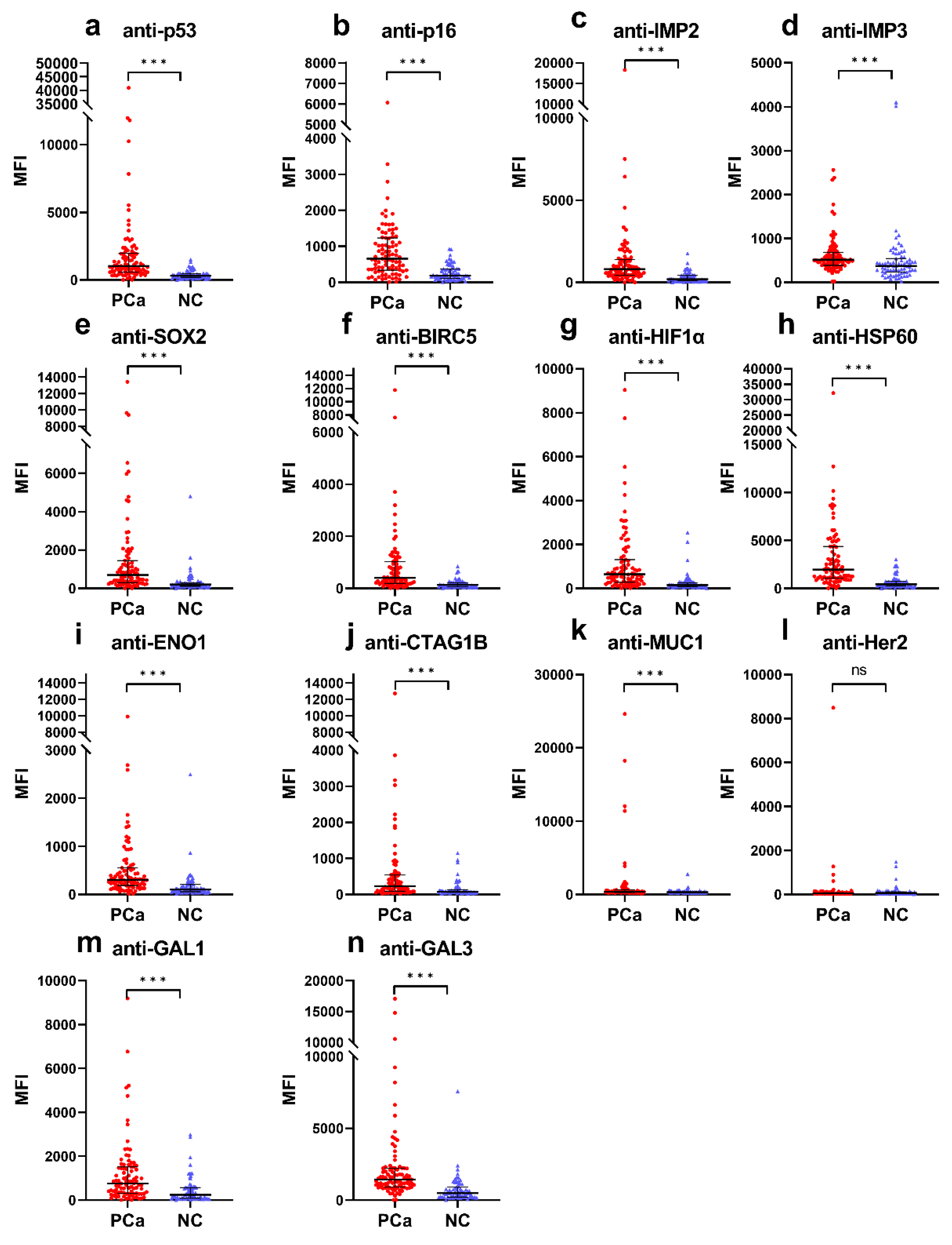
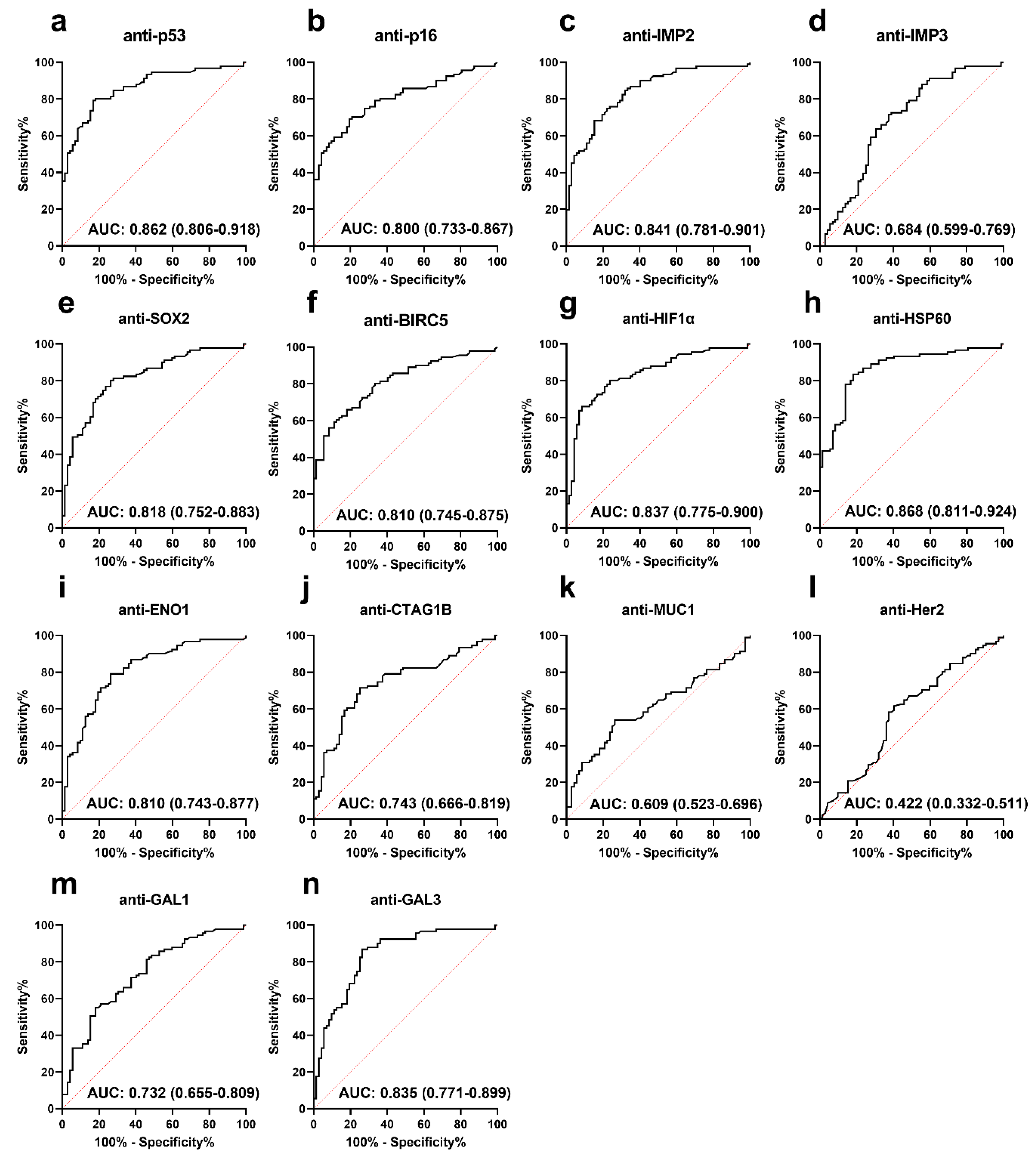
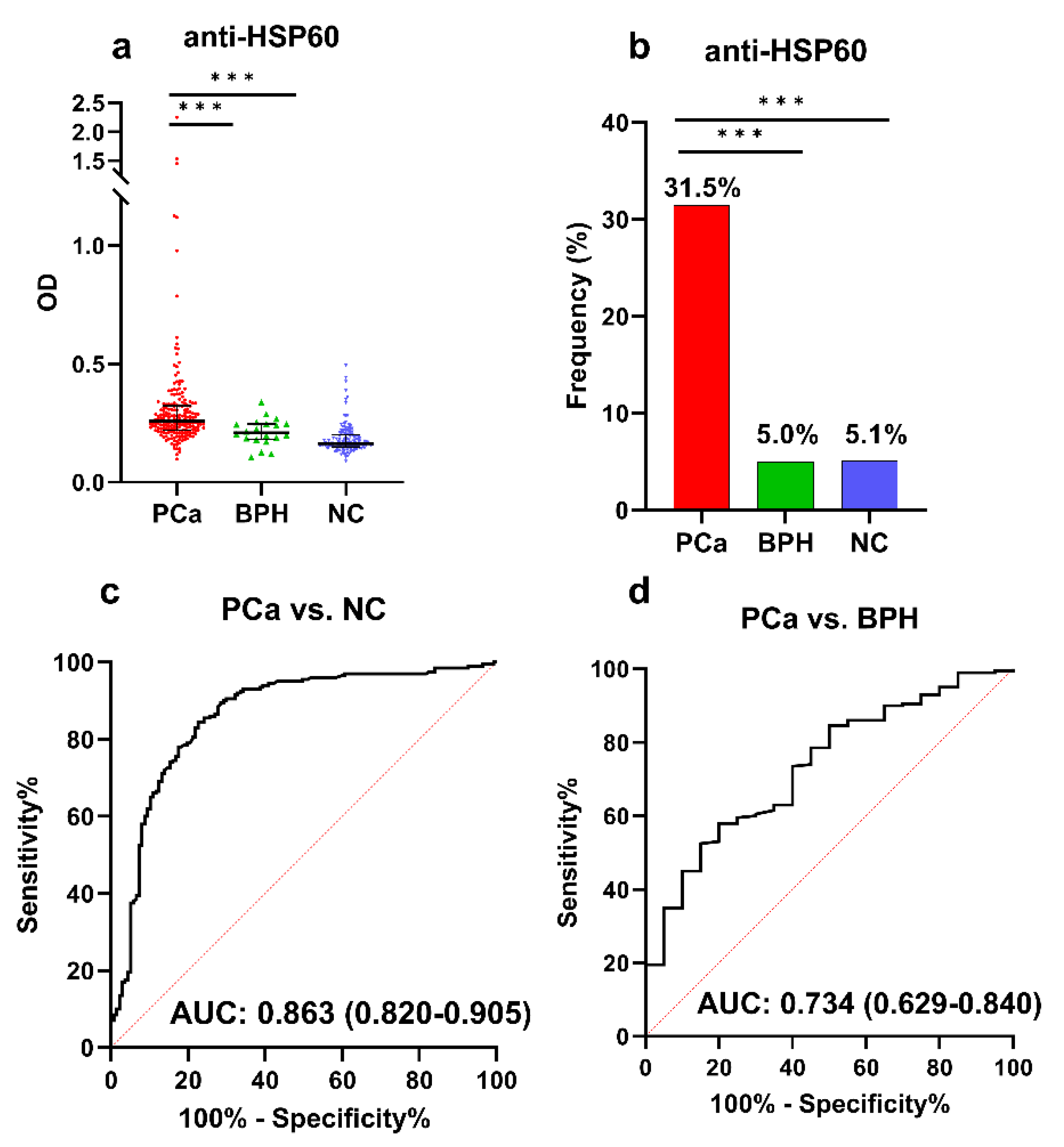

| Autoantibody | Cut-Off (MFI) | Frequency (%) | p | Se (%) | Sp (%) | YI | PPV (%) | NPV (%) | Accuracy (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| PCa (n = 91) | NC (n = 72) | |||||||||
| anti-p53 | 994.3 | 47 (51.6) | 3 (4.2) | <0.001 | 51.6 | 95.8 | 0.5 | 94.0 | 61.1 | 71.2 |
| anti-p16 | 657.5 | 46 (50.5) | 3 (4.2) | <0.001 | 50.5 | 95.8 | 0.5 | 93.9 | 60.5 | 70.6 |
| anti-IMP2 | 834.5 | 44 (48.4) | 3 (4.2) | <0.001 | 48.4 | 95.8 | 0.4 | 93.6 | 59.5 | 69.3 |
| anti-IMP3 | 1086.5 | 8 (8.8) | 3 (4.2) | 0.198 | 8.8 | 95.8 | 0.0 | 72.7 | 45.4 | 47.2 |
| anti-SOX2 | 926.8 | 35 (38.5) | 3 (4.2) | <0.001 | 38.5 | 95.8 | 0.3 | 92.1 | 55.2 | 63.8 |
| anti-BIRC5 | 624.0 | 35 (38.5) | 3 (4.2) | <0.001 | 38.5 | 95.8 | 0.3 | 92.1 | 55.2 | 63.8 |
| anti-HIF1α | 660.3 | 44 (48.4) | 3 (4.2) | <0.001 | 48.4 | 95.8 | 0.4 | 93.6 | 59.5 | 69.3 |
| anti-HSP60 | 2317.8 | 38 (41.8) | 3 (4.2) | <0.001 | 41.8 | 95.8 | 0.4 | 92.7 | 56.6 | 65.6 |
| anti-ENO1 | 403.8 | 32 (35.2) | 3 (4.2) | <0.001 | 35.2 | 95.8 | 0.3 | 91.4 | 53.9 | 62.0 |
| anti-CTAG1B | 570.5 | 21 (27.1) | 3 (4.2) | <0.001 | 23.1 | 95.8 | 0.2 | 87.5 | 49.6 | 55.2 |
| anti-MUC1 | 841.5 | 18 (19.8) | 3 (4.2) | 0.002 | 19.8 | 95.8 | 0.2 | 85.7 | 48.6 | 53.4 |
| anti-Her2 | 489.8 | 4 (4.4) | 3 (4.2) | 0.628 | 4.4 | 95.8 | 0.0 | 57.1 | 44.2 | 44.8 |
| anti-GAL1 | 1617.0 | 19 (20.9) | 3 (4.2) | 0.001 | 20.9 | 95.8 | 0.2 | 86.4 | 48.9 | 54.0 |
| anti-GAL3 | 1882.5 | 30 (33.0) | 3 (4.2) | <0.001 | 33.0 | 95.8 | 0.3 | 90.9 | 53.1 | 60.7 |
| Subjects | Frequency (%) | p * | Se (%) | Sp (%) | YI | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| All Pca (n = 91) | 65 (71.4) | <0.001 | 71.4 | 95.8 | 0.7 | 95.6 | 72.6 | 82.2 |
| AA (n = 20) | 8 (40.0) | <0.001 | 40.0 | 95.8 | 0.4 | 72.7 | 85.2 | 83.7 |
| CC (n = 51) | 37 (72.5) | <0.001 | 72.5 | 95.8 | 0.7 | 92.5 | 83.1 | 86.2 |
| HA (n = 20) | 19 (95.0) | <0.001 | 95.0 | 95.8 | 0.9 | 86.4 | 98.6 | 95.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, C.; Wang, X.; Batson, S.A.; Wang, B.; Casiano, C.A.; Francia, G.; Zhang, J.-Y. A Luminex Approach to Develop an Anti-Tumor-Associated Antigen Autoantibody Panel for the Detection of Prostate Cancer in Racially/Ethnically Diverse Populations. Cancers 2023, 15, 4064. https://doi.org/10.3390/cancers15164064
Qiu C, Wang X, Batson SA, Wang B, Casiano CA, Francia G, Zhang J-Y. A Luminex Approach to Develop an Anti-Tumor-Associated Antigen Autoantibody Panel for the Detection of Prostate Cancer in Racially/Ethnically Diverse Populations. Cancers. 2023; 15(16):4064. https://doi.org/10.3390/cancers15164064
Chicago/Turabian StyleQiu, Cuipeng, Xiao Wang, Serina A. Batson, Bofei Wang, Carlos A. Casiano, Giulio Francia, and Jian-Ying Zhang. 2023. "A Luminex Approach to Develop an Anti-Tumor-Associated Antigen Autoantibody Panel for the Detection of Prostate Cancer in Racially/Ethnically Diverse Populations" Cancers 15, no. 16: 4064. https://doi.org/10.3390/cancers15164064








