Phase II Trial Evaluating Olaparib Maintenance in Patients with Metastatic Castration-Resistant Prostate Cancer Responsive or Stabilized on Docetaxel Treatment: SOGUG-IMANOL Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Intervention
2.4. Study Assessments
2.5. Endpoints
2.6. Statistical Analysis
3. Results
3.1. Patient Disposition and Characteristics and Treatment with Olaparib
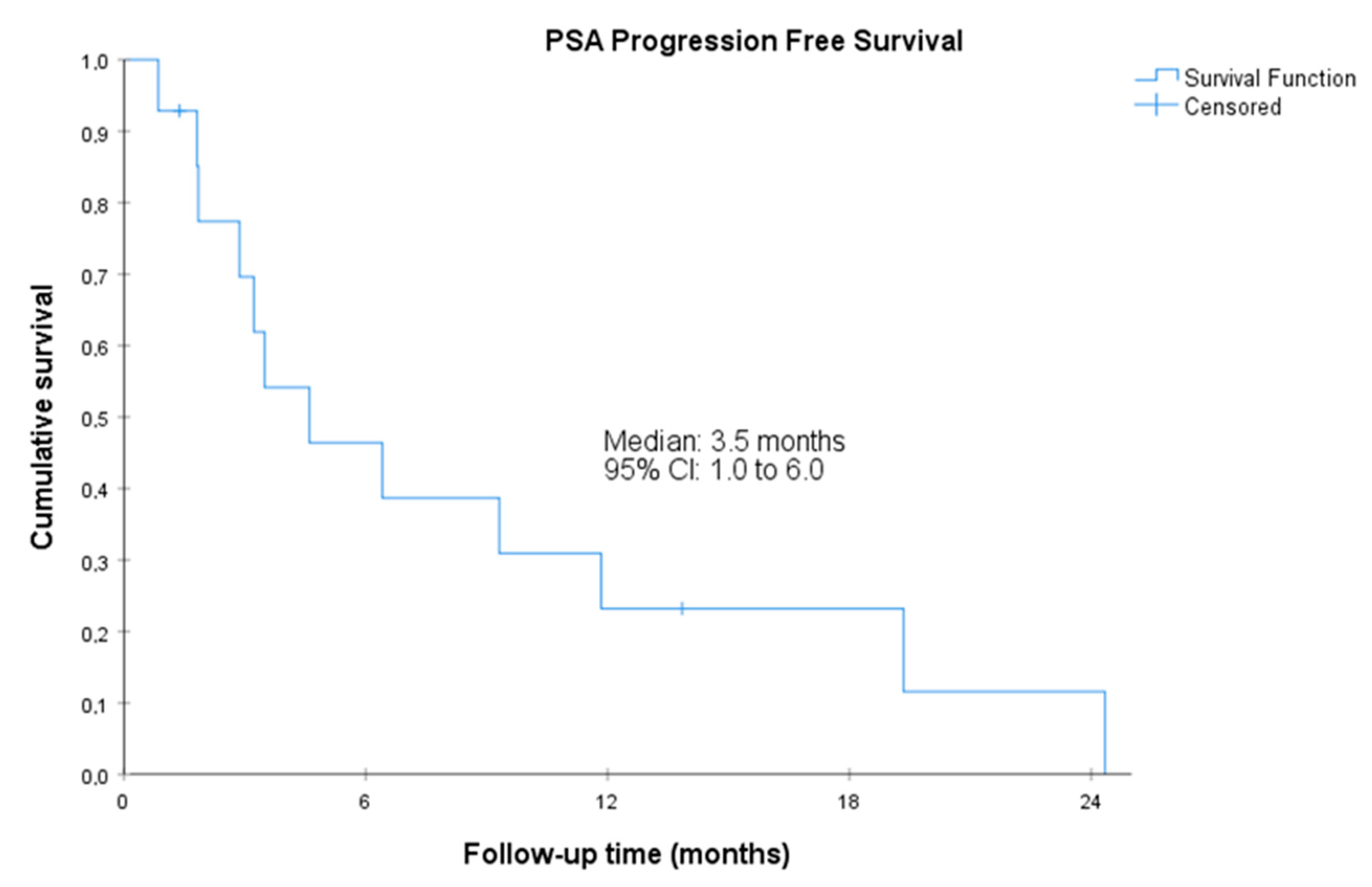
3.2. Efficacy
3.3. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S.; ESMO Guidelines Committee. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Gillessen, S.; ESMO Guidelines Committee. Updated treatment recommendations for prostate cancer from the ESMO clinical practice guideline considering treatment intensification and use of novel systemic agents. Ann. Oncol. 2023, 34, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Ozguroglu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Ozguroglu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; de Santana Gomes, A.J.P.; Given, R.; Soto, A.J.; Merseburger, A.S.; Ozguroglu, M.; Uemura, H.; et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Flechon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022, 399, 1695–1707. [Google Scholar] [CrossRef]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, A.; et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N. Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.S. Metastatic prostate cancer. N. Engl. J. Med. 2018, 378, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.G.; de Marinis, F.; Dediu, M.; Thomas, M.; Pujol, J.L.; Bidoli, P.; Molinier, O.; Sahoo, T.P.; Laack, E.; Reck, M.; et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2013, 31, 2895–2902. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.O.; Hochhauser, D.; Arnold, D.; Oh, D.Y.; et al. Maintenance olaparib for Germline BRCA-mutated metastatic pancreatic cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Donas, J.; Font, A.; Perez-Valderrama, B.; Virizuela, J.A.; Climent, M.A.; Hernando-Polo, S.; Arranz, J.A.; Del Mar Llorente, M.; Lainez, N.; Villa-Guzman, J.C.; et al. Maintenance therapy with vinflunine plus best supportive care versus best supportive care alone in patients with advanced urothelial carcinoma with a response after first-line chemotherapy (MAJA; SOGUG 2011/02): A multicentre, randomised, controlled, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 672–681a. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulovic, S.; Demey, W.; Ullen, A.; et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Parimi, S.; Eliasziw, M.; North, S.; Trudeau, M.; Winquist, E.; Chi, K.N.; Ruether, D.; Cheng, T.; Eigl, B.J. Sunitinib maintenance therapy after response to docetaxel in metastatic castration resistant prostate cancer (mCRPC). Investig. New Drugs 2016, 34, 771–776. [Google Scholar] [CrossRef]
- Cathomas, R.; Crabb, S.J.; Mark, M.; Winterhalder, R.; Rothermundt, C.; Elliott, T.; von Burg, P.; Kenner, H.; Hayoz, S.; Vilei, S.B.; et al. Orteronel switch maintenance therapy in metastatic castration resistant prostate cancer after first-line docetaxel: A multicenter, randomized, double-blind, placebo-controlled trial (SAKK 08/11). Prostate 2016, 76, 1519–1527. [Google Scholar] [CrossRef]
- Fizazi, K.; Ulys, A.; Sengelov, L.; Moe, M.; Ladoire, S.; Thiery-Vuillemin, A.; Flechon, A.; Guida, A.; Bellmunt, J.; Climent, M.A.; et al. A randomized, double-blind, placebo-controlled phase II study of maintenance therapy with tasquinimod in patients with metastatic castration-resistant prostate cancer responsive to or stabilized during first-line docetaxel chemotherapy. Ann. Oncol. 2017, 28, 2741–2746. [Google Scholar] [CrossRef]
- Sandhu, S.; Moore, C.M.; Chiong, E.; Beltran, H.; Bristow, R.G.; Williams, S.G. Prostate cancer. Lancet 2021, 398, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Bono, J.D.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Aprikian, A.; Finelli, A.; Fleshner, N.E.; Gleave, M.; Kapoor, A.; Niazi, T.; North, S.A.; Pouliot, F.; Rendon, R.A.; et al. 2022 Canadian Urological Association (CUA)-Canadian Uro Oncology Group (CUOG) guideline: Management of Castration-Resistant Prostate Cancer (CRPC). Can. Urol. Assoc. J. 2022, 16, E506–E515. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. NCCN guidelines(R) insights: Prostate cancer, version 1.2023. J. Natl. Compr. Cancer Netw. 2022, 20, 1288–1298. [Google Scholar] [CrossRef]
- Clarke, N.W.; Armstrong, A.J.; Thiery-Vuillemin, A.; Oya, M.; Shore, N.; Loredo, E.; Procopio, G.; de Menezes, J.; Girotto, G.; Arslan, C.; et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid. 2022, 1, EVIDoa2200043. [Google Scholar] [CrossRef]
- Chi, K.N.; Rathkopf, D.; Smith, M.R.; Efstathiou, E.; Attard, G.; Olmos, D.; Lee, J.Y.; Small, E.J.; de Santana Gomes, A.J.P.; Roubaud, G.; et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2023, 41, 3339–3351. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.; Carles, J.; Fay, A.P.; Matsubara, N.; Heinrich, D.; Szczylik, C.; De Giorgi, U.; Joung, J.Y.; Fong, P.C.C.; et al. TALAPRO-2: Phase 3 study of talazoparib (TALA) + enzalutamide (ENZA) versus placebo (PBO) + ENZA as first-line (1L) treatment in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2023, 41, LBA17. [Google Scholar] [CrossRef]
- De Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Castro, E.; Romero-Laorden, N.; Del Pozo, A.; Lozano, R.; Medina, A.; Puente, J.; Piulats, J.M.; Lorente, D.; Saez, M.I.; Morales-Barrera, R.; et al. PROREPAIR-B: A prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2019, 37, 490–503. [Google Scholar] [CrossRef]
- Gillessen, S.; Procopio, G.; Hayoz, S.; Kremer, E.; Schwitter, M.; Caffo, O.; Lorente, D.; Pedrazzini, A.; Roubaud, G.; Nenan, S.; et al. Darolutamide maintenance in patients with metastatic castration-resistant prostate cancer with nonprogressive disease after taxane treatment (SAKK 08/16). J. Clin. Oncol. 2023, 41, 3608–3615. [Google Scholar] [CrossRef]
- Hussain, M.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020, 383, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Lynparza. Summary of Product Characteristics. 2023. Available online: https://www.ema.europa.eu/en/documents/product-information/lynparza-epar-product-information_en.pdf (accessed on 17 July 2023).
- VA Office of Research and Development. Carboplatin or Olaparib for BRcA Deficient Prostate Cancer (COBRA). ClinicalTrials.gov Identifier: NCT04038502. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04038502 (accessed on 17 July 2023).
- Rathkopf, D.E.; Beer, T.M.; Loriot, Y.; Higano, C.S.; Armstrong, A.J.; Sternberg, C.N.; de Bono, J.S.; Tombal, B.; Parli, T.; Bhattacharya, S.; et al. Radiographic progression-free survival as a clinically meaningful end point in metastatic castration-resistant prostate cancer: The PREVAIL randomized clinical trial. JAMA Oncol. 2018, 4, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Halabi, S.; Roy, A.; Yang, Q.; Xie, W.; Kelly, W.K.; Sweeney, C. Radiographic progression-free survival as a surrogate endpoint of overall survival in men with metastatic castrate-resistant prostate cancer. J. Clin. Oncol. 2021, 39, 5057. [Google Scholar] [CrossRef]


| Characteristic | N = 14 |
|---|---|
| Age, median (IQR) | 73.0 (72.0–75.0) |
| Time from the initial diagnosis (months), median (IQR) | 61.4 (34.21–24.4) |
| Metastatic disease at initial diagnosis, n (%) | 2 (14.3) |
| Time from diagnosis of metastatic disease (months), median (IQR) | 24.6 (12.9–37.0) |
| Location of metastases, n (%) | |
| Bone | 11 (78.6) |
| Lymph nodes | 5 (35.7) |
| Liver | 4 (28.6) |
| Prostate | 1 (7.1) |
| Lung | 1 (7.1) |
| Gleason score ≥ 8, n (%) | 6 (42.8) |
| ECOG, n (%) | |
| 0 | 10 (71.4) |
| 1 | 4 (28.6) |
| Patients with HRR gene defects, n (%) | |
| ATM | 5 (35.7) |
| CHEK2 | 2 (14.3) |
| BRCA2 | 2 (14.3) |
| BRCA1, BRCA2, BRIP1, FANCL, PALB2, RAD51C, CDK12, TUBB3 | 1 (7.1) |
| CDK12 | 1 (7.1) |
| BRCA1, CDK12 | 1 (7.1) |
| BRCA1, BRCA2 | 1 (7.1) |
| BRCA1 | 1 (7.1) |
| Previous hormonal agents, n (%) | |
| Bicalutamide | 9 (64.3) |
| Triptorelin | 7 (50.0) |
| Abiraterone | 6 (42.9) |
| Enzalutamide | 5 (35.7) |
| Goserelin | 4 (28.6) |
| Other | 5 (35.7) |
| Previous taxane use, n (%) | |
| Docetaxel | 13 (92.9) |
| Docetaxel + Carboplatin | 1 (7.1) |
| Duration of previous chemotherapy (months), median (IQR) | 4.7 (3.5–5.2) |
| Best response under taxane, n (%) | |
| Stable disease | 14 (100%) |
| Grade | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3–4 a | Total | |||||
| N | % | N | % | N | % | N | % | |
| Asthenia | 2 | 14.3 | 4 | 28.6 | 3 | 21.4 | 9 | 64.3 |
| Anemia | 4 | 28.6 | 3 | 21.4 | 2 | 14.3 | 9 | 64.3 |
| Nausea | 4 | 28.6 | 4 | 28.6 | 0 | 0.0 | 8 | 57.1 |
| Neutropenia | 2 | 14.3 | 2 | 14.3 | 1 | 7.1 | 5 | 35.7 |
| Decreased appetite | 3 | 21.4 | 2 | 14.3 | 0 | 0.0 | 5 | 35.7 |
| Diarrhea | 1 | 7.1 | 3 | 21.4 | 0 | 0.0 | 4 | 28.6 |
| Edema, peripheral | 3 | 21.4 | 1 | 7.1 | 0 | 0.0 | 4 | 28.6 |
| Mucosal inflammation | 1 | 7.1 | 2 | 14.3 | 0 | 0.0 | 3 | 21.4 |
| Leukopenia | 2 | 14.3 | 1 | 7.1 | 0 | 0.0 | 3 | 21.4 |
| Vomiting | 1 | 7.1 | 1 | 7.1 | 0 | 0.0 | 2 | 14.3 |
| Abdominal pain, upper | 2 | 14.3 | 0 | 0.0 | 0 | 0.0 | 2 | 14.3 |
| Abdominal discomfort | 2 | 14.3 | 0 | 0.0 | 0 | 0.0 | 2 | 14.3 |
| Dyspnea | 2 | 14.3 | 0 | 0.0 | 0 | 0.0 | 2 | 14.3 |
| Cognitive disorder | 2 | 14.3 | 0 | 0.0 | 0 | 0.0 | 2 | 14.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juan Fita, M.J.; Anido Herranz, U.; Mendez-Vidal, M.J.; Gironés-Sarrió, R.; Muñoz-Langa, J.; Sepúlveda-Sánchez, J.; Mellado, B.; Alvarez-Fernandez, C.; Heras López, L.; López-Guerrero, J.A.; et al. Phase II Trial Evaluating Olaparib Maintenance in Patients with Metastatic Castration-Resistant Prostate Cancer Responsive or Stabilized on Docetaxel Treatment: SOGUG-IMANOL Study. Cancers 2023, 15, 5223. https://doi.org/10.3390/cancers15215223
Juan Fita MJ, Anido Herranz U, Mendez-Vidal MJ, Gironés-Sarrió R, Muñoz-Langa J, Sepúlveda-Sánchez J, Mellado B, Alvarez-Fernandez C, Heras López L, López-Guerrero JA, et al. Phase II Trial Evaluating Olaparib Maintenance in Patients with Metastatic Castration-Resistant Prostate Cancer Responsive or Stabilized on Docetaxel Treatment: SOGUG-IMANOL Study. Cancers. 2023; 15(21):5223. https://doi.org/10.3390/cancers15215223
Chicago/Turabian StyleJuan Fita, María José, Urbano Anido Herranz, María José Mendez-Vidal, Regina Gironés-Sarrió, José Muñoz-Langa, Juan Sepúlveda-Sánchez, Begoña Mellado, Carlos Alvarez-Fernandez, Lucía Heras López, José Antonio López-Guerrero, and et al. 2023. "Phase II Trial Evaluating Olaparib Maintenance in Patients with Metastatic Castration-Resistant Prostate Cancer Responsive or Stabilized on Docetaxel Treatment: SOGUG-IMANOL Study" Cancers 15, no. 21: 5223. https://doi.org/10.3390/cancers15215223
APA StyleJuan Fita, M. J., Anido Herranz, U., Mendez-Vidal, M. J., Gironés-Sarrió, R., Muñoz-Langa, J., Sepúlveda-Sánchez, J., Mellado, B., Alvarez-Fernandez, C., Heras López, L., López-Guerrero, J. A., García-Casado, Z., Calatrava, A., & Ángel Climent, M. (2023). Phase II Trial Evaluating Olaparib Maintenance in Patients with Metastatic Castration-Resistant Prostate Cancer Responsive or Stabilized on Docetaxel Treatment: SOGUG-IMANOL Study. Cancers, 15(21), 5223. https://doi.org/10.3390/cancers15215223








