Robotic Stereotactic Ablative Radiotherapy for Patients with Early-Stage Lung Cancer: Results of an Interim Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Planning
2.3. Radiobiological Considerations
2.4. Treatment and Toxicity Evaluation
2.5. Statistical Analysis
3. Results
3.1. Treatment Response and Survival
3.2. Treatment Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Mountzios, G.; Gkiozos, I.; Stratakos, G.; Pissakas, G.; Charpidou, A.; Toukfetzian, L.; Vamvakaris, I.; Syrigos, K. Lung Cancer in Greece. J. Thorac. Oncol. 2021, 16, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Lagerwaard, F.J.; Verstegen, N.E.; Haasbeek, C.J.; Slotman, B.J.; Paul, M.A.; Smit, E.F.; Senan, S. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 348–353. [Google Scholar] [CrossRef]
- Chang, J.Y.; Senan, S.; Paul, M.A.; Mehran, R.J.; Louie, A.V.; Balter, P.; Groen, H.J.; McRae, S.E.; Widder, J.; Feng, L.; et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Oncol. 2015, 16, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, N.E.; Oosterhuis, J.W.; Palma, D.A.; Rodrigues, G.; Lagerwaard, F.J.; van der Elst, A.; Mollema, R.; van Tets, W.F.; Warner, A.; Joosten, J.J.; et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): Outcomes of a propensity score-matched analysis. Ann. Oncol. 2013, 24, 1543–1548. [Google Scholar] [CrossRef]
- Bogart, J.A.; Hodgson, L.; Seagren, S.L.; Blackstock, A.W.; Wang, X.; Lenox, R.; Turrisi, A.T., 3rd; Reilly, J.; Gajra, A.; Vokes, E.E.; et al. Phase I study of accelerated conformal radiotherapy for stage I non-small-cell lung cancer in patients with pulmonary dysfunction: CALGB 39904. J. Clin. Oncol. 2010, 28, 202–206. [Google Scholar] [CrossRef]
- Cheung, P.; Faria, S.; Ahmed, S.; Chabot, P.; Greenland, J.; Kurien, E.; Mohamed, I.; Wright, J.R.; Hollenhorst, H.; de Metz, C.; et al. Phase II study of accelerated hypofractionated three-dimensional conformal radiotherapy for stage T1-3 N0 M0 non-small cell lung cancer: NCIC CTG BR.25. J. Natl. Cancer Inst. 2014, 106, dju164. [Google Scholar] [CrossRef]
- Nyman, J.; Hallqvist, A.; Lund, J.A.; Brustugun, O.T.; Bergman, B.; Bergstrom, P.; Friesland, S.; Lewensohn, R.; Holmberg, E.; Lax, I. SPACE—A randomized study of SBRT vs. conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother. Oncol. 2016, 121, 1–8. [Google Scholar] [CrossRef]
- Ball, D.; Mai, G.T.; Vinod, S.; Babington, S.; Ruben, J.; Kron, T.; Chesson, B.; Herschtal, A.; Vanevski, M.; Rezo, A.; et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): A phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019, 20, 494–503. [Google Scholar] [CrossRef]
- Ding, C.; Chang, C.H.; Haslam, J.; Timmerman, R.; Solberg, T. A dosimetric comparison of stereotactic body radiation therapy techniques for lung cancer: Robotic versus conventional linac-based systems. J. Appl. Clin. Med. Phys. 2010, 11, 3223. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Simone, C.B., 2nd; Allen, P.K.; Gajjar, S.R.; Shah, C.; Zhen, W.; Harkenrider, M.M.; Hallemeier, C.L.; Jabbour, S.K.; Matthiesen, C.L.; et al. Multi-Institutional Experience of Stereotactic Ablative Radiation Therapy for Stage I Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Shioyama, Y.; Onishi, H.; Takayama, K.; Matsuo, Y.; Takeda, A.; Yamashita, H.; Miyakawa, A.; Murakami, N.; Aoki, M.; Matsushita, H.; et al. Clinical Outcomes of Stereotactic Body Radiotherapy for Patients With Stage I Small-Cell Lung Cancer: Analysis of a Subset of the Japanese Radiological Society Multi-Institutional SBRT Study Group Database. Technol. Cancer Res. Treat. 2018, 17, 1533033818783904. [Google Scholar] [CrossRef]
- Chang, J.Y.; Mehran, R.J.; Feng, L.; Verma, V.; Liao, Z.; Welsh, J.W.; Lin, S.H.; O’Reilly, M.S.; Jeter, M.D.; Balter, P.A.; et al. Stereotactic ablative radiotherapy for operable stage I non-small-cell lung cancer (revised STARS): Long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol. 2021, 22, 1448–1457. [Google Scholar] [CrossRef]
- Moghanaki, D.; Karas, T.; Timmerman, R.D.; Cameron, R.B.; Ritter, T.A.; Shi, H.; Leiner, M.K.; Feng, H.; Skinner, V.L.; Robin, L.; et al. Protocol for the Veterans Affairs Cooperative Studies Program Study Number 2005: A Phase 3 Randomized Trial of Lung Cancer Surgery or Stereotactic Radiotherapy for Operable Early-Stage Non-Small Cell Lung Cancer. CHEST Pulm. 2023, 1, 100024. [Google Scholar] [CrossRef]
- Mehta, N.; King, C.R.; Agazaryan, N.; Steinberg, M.; Hua, A.; Lee, P. Stereotactic body radiation therapy and 3-dimensional conformal radiotherapy for stage I non-small cell lung cancer: A pooled analysis of biological equivalent dose and local control. Pract. Radiat. Oncol. 2012, 2, 288–295. [Google Scholar] [CrossRef]
- Song, C.W.; Kim, M.S.; Cho, L.C.; Dusenbery, K.; Sperduto, P.W. Radiobiological basis of SBRT and SRS. Int. J. Clin. Oncol. 2014, 19, 570–578. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Skoczylas, J.Z.; Bernier, J. Quantitative clinical radiobiology of early and late lung reactions. Int. J. Radiat. Biol. 2000, 76, 453–462. [Google Scholar] [CrossRef]
- Klement, R.J.; Sonke, J.J.; Allgauer, M.; Andratschke, N.; Appold, S.; Belderbos, J.; Belka, C.; Dieckmann, K.; Eich, H.T.; Flentje, M.; et al. Estimation of the alpha/beta ratio of non-small cell lung cancer treated with stereotactic body radiotherapy. Radiother. Oncol. 2020, 142, 210–216. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- O, J.H.; Lodge, M.A.; Wahl, R.L. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0, Cancer therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, NIH/National Cancer Institute. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 2 April 2024).
- Kouloulias, V.; Zygogianni, A.; Efstathopoulos, E.; Victoria, O.; Christos, A.; Pantelis, K.; Koutoulidis, V.; Kouvaris, J.; Sandilos, P.; Varela, M.; et al. Suggestion for a new grading scale for radiation induced pneumonitis based on radiological findings of computerized tomography: Correlation with clinical and radiotherapeutic parameters in lung cancer patients. Asian Pac. J. Cancer Prev. 2013, 14, 2717–2722. [Google Scholar] [CrossRef]
- Johnson, R.A.; Wichern, D.W. Applied Multivariate Statistical Analysis, 6th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
- Chen, H.; Laba, J.M.; Boldt, R.G.; Goodman, C.D.; Palma, D.A.; Senan, S.; Louie, A.V. Stereotactic Ablative Radiation Therapy Versus Surgery in Early Lung Cancer: A Meta-analysis of Propensity Score Studies. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Vlaskou Badra, E.; Baumgartl, M.; Fabiano, S.; Jongen, A.; Guckenberger, M. Stereotactic radiotherapy for early stage non-small cell lung cancer: Current standards and ongoing research. Transl. Lung Cancer Res. 2021, 10, 1930–1949. [Google Scholar] [CrossRef]
- Khadige, M.; Salleron, J.; Marchesi, V.; Oldrini, G.; Peiffert, D.; Beckendorf, V. Cyberknife((R)) stereotactic radiation therapy for stage I lung cancer and pulmonary metastases: Evaluation of local control at 24 months. J. Thorac. Dis. 2018, 10, 4976–4984. [Google Scholar] [CrossRef]
- Kocak Uzel, E.; Bagci Kilic, M.; Morcali, H.; Uzel, O. Stereotactic body radiation therapy for stage I medically operable non-small cell lung cancer. Sci. Rep. 2023, 13, 10384. [Google Scholar] [CrossRef]
- Stanic, K.; But-Hadzic, J.; Zagar, J.; Vrankar, M. Local control and survival after stereotactic body radiation therapy of early-stage lung cancer patients in Slovenia. Radiol. Oncol. 2023, 57, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Brooks, E.D.; Komaki, R.U.; Liao, Z.; Jeter, M.D.; McAleer, M.F.; Allen, P.K.; Balter, P.A.; Welsh, J.D.; O’Reilly, M.S.; et al. 7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non-small cell lung cancer: Results of a phase 2 clinical trial. Cancer 2017, 123, 3031–3039. [Google Scholar] [CrossRef]
- Gensheimer, M.F.; Gee, H.; Shirato, H.; Taguchi, H.; Snyder, J.M.; Chin, A.L.; Vitzthum, L.K.; Maxim, P.G.; Wakelee, H.A.; Neal, J.; et al. Individualized Stereotactic Ablative Radiotherapy for Lung Tumors: The iSABR Phase 2 Nonrandomized Controlled Trial. JAMA Oncol. 2023, 9, 1525–1534. [Google Scholar] [CrossRef]
- Kang, J.; Ning, M.S.; Feng, H.; Li, H.; Bahig, H.; Brooks, E.D.; Welsh, J.W.; Ye, R.; Miao, H.; Chang, J.Y. Predicting 5-Year Progression and Survival Outcomes for Early Stage Non-small Cell Lung Cancer Treated with Stereotactic Ablative Radiation Therapy: Development and Validation of Robust Prognostic Nomograms. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 90–99. [Google Scholar] [CrossRef]
- Song, C.W.; Glatstein, E.; Marks, L.B.; Emami, B.; Grimm, J.; Sperduto, P.W.; Kim, M.S.; Hui, S.; Dusenbery, K.E.; Cho, L.C. Biological Principles of Stereotactic Body Radiation Therapy (SBRT) and Stereotactic Radiation Surgery (SRS): Indirect Cell Death. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Cases, C.; Benegas, M.; Sanchez, M.; Vollmer, I.; Casas, F.; Goma, C.; Molla, M. Biological equivalent dose is associated with radiological toxicity after lung stereotactic ablative radiation therapy. Radiother. Oncol. 2023, 183, 109552. [Google Scholar] [CrossRef] [PubMed]
- Borelli, C.; Vergara, D.; Simeone, A.; Pazienza, L.; Castorani, G.; Graziano, P.; Di Micco, C.; Quarato, C.M.I.; Sperandeo, M. CT-Guided Transthoracic Biopsy of Pulmonary Lesions: Diagnostic versus Nondiagnostic Results. Diagnostics 2022, 12, 359. [Google Scholar] [CrossRef] [PubMed]
- Dautruche, A.; Filion, E.; Mathieu, D.; Bahig, H.; Roberge, D.; Lambert, L.; Vu, T.; Campeau, M.P. To Biopsy or Not to Biopsy?: A Matched Cohort Analysis of Early-Stage Lung Cancer Treated with Stereotactic Radiation with or without Histologic Confirmation. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 88–97. [Google Scholar] [CrossRef]
- Chang, J.Y.; Lin, S.H.; Dong, W.; Liao, Z.; Gandhi, S.J.; Gay, C.M.; Zhang, J.; Chun, S.G.; Elamin, Y.Y.; Fossella, F.V.; et al. Stereotactic ablative radiotherapy with or without immunotherapy for early-stage or isolated lung parenchymal recurrent node-negative non-small-cell lung cancer: An open-label, randomised, phase 2 trial. Lancet 2023, 402, 871–881. [Google Scholar] [CrossRef]

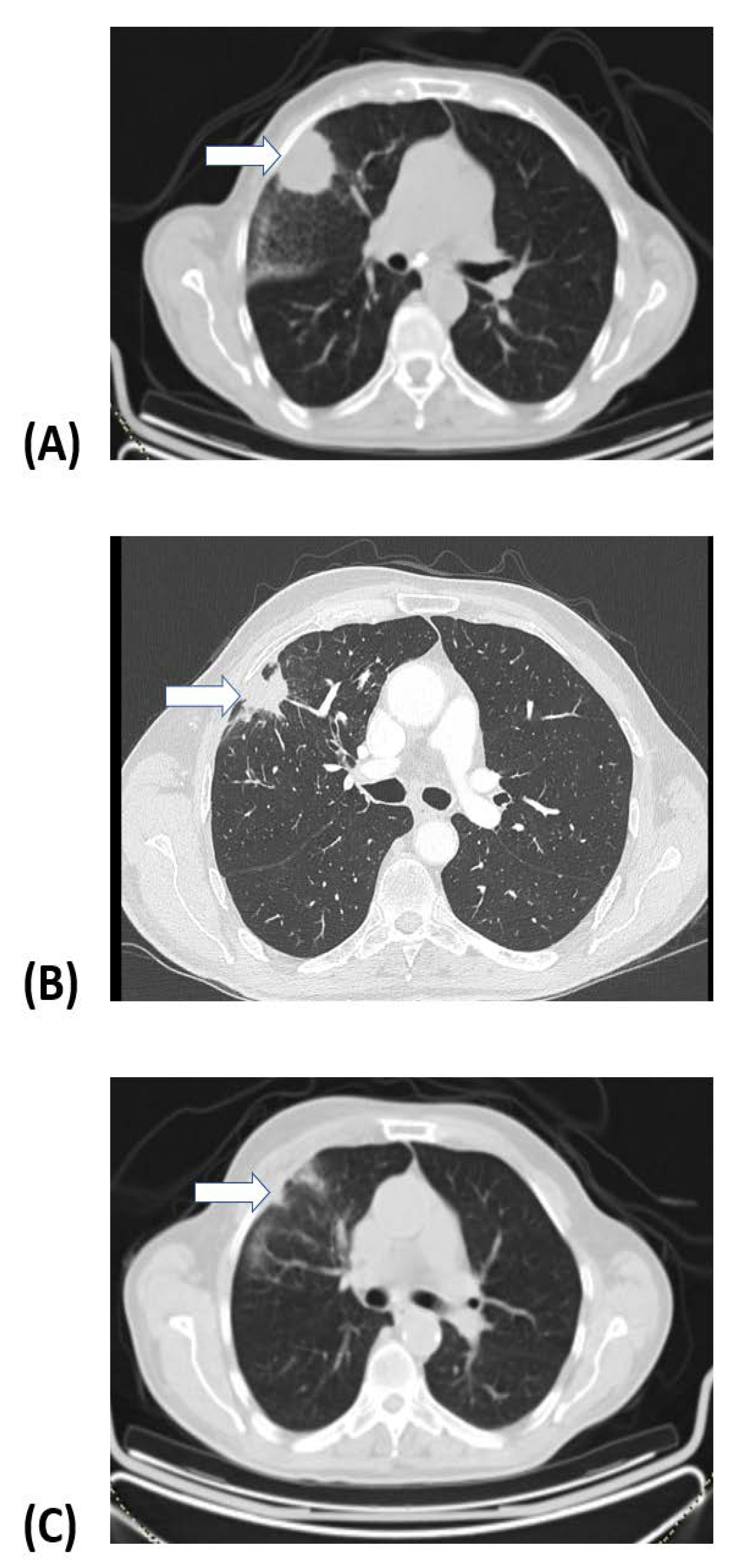

| Fractions/ Dose per Fraction | No. Pts | EQD2 (Gy) * | EQD2 (Gy) ** | BED (Gy) * | BED (Gy) ** |
|---|---|---|---|---|---|
| 3/15 Gy | 1 | 162 | 93.8 | 270 | 112.5 |
| 3/17 Gy | 2 | 204 | 114.8 | 340 | 137.7 |
| 3/18 Gy | 27 | 226.8 | 126 | 378 | 151.2 |
| 3/20 Gy | 15 | 276 | 150 | 460 | 180 |
| 4/12 Gy | 4 | 144 | 88 | 240 | 105.6 |
| 5/10 Gy | 17 | 130 | 83.3 | 216.7 | 100 |
| 5/11 Gy | 4 | 154 | 96.3 | 256.7 | 115.5 |
| 5/12 Gy | 2 | 180 | 110 | 300 | 132 |
| 6/8 Gy | 1 | 105.6 | 72 | 176 | 86.4 |
| 7/6.5 Gy | 1 | 86.5 | 62.6 | 144.1 | 75.1 |
| 8/7.5 Gy | 4 | 112 | 79.3 | 186.7 | 95.2 |
| 10/4 Gy | 1 | 56 | 46.7 | 93.3 | 56 |
| 12/5 Gy | 1 | 96 | 75 | 160 | 90 |
| 13/3.5 Gy | 1 | 59.2 | 51.2 | 98.6 | 61.5 |
| No Pts | 81 | % |
|---|---|---|
| PS | ||
| 0 | 16 | 20 |
| 1 | 53 | 65 |
| 2 | 11 | 14 |
| 3 | 1 | 1 |
| Gender | ||
| Male | 54 | 67 |
| Female | 27 | 33 |
| Age | ||
| Median | 76 | |
| Range | 47–92 | |
| Histology | ||
| Squamous | 24 | 30 |
| Adenocarcinoma | 25 | 31 |
| Adenosquamous | 1 | 1 |
| Small cell | 1 | 1 |
| No biopsy | 30 | 37 |
| Size (mm) | ||
| Median | 24 | |
| Range | 3–52 | |
| 33rd Percentile | 20 | |
| 66th Percentile | 27 | |
| T-Stage | ||
| T1 | 61 | 75 |
| T2 | 20 | 25 |
| Location | ||
| Central | 21 | 26 |
| Peripheral | 60 | 74 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| p | HR | 95% CI | p | HR | 95% CI | |
| Age | 0.41 | - | - | - | - | - |
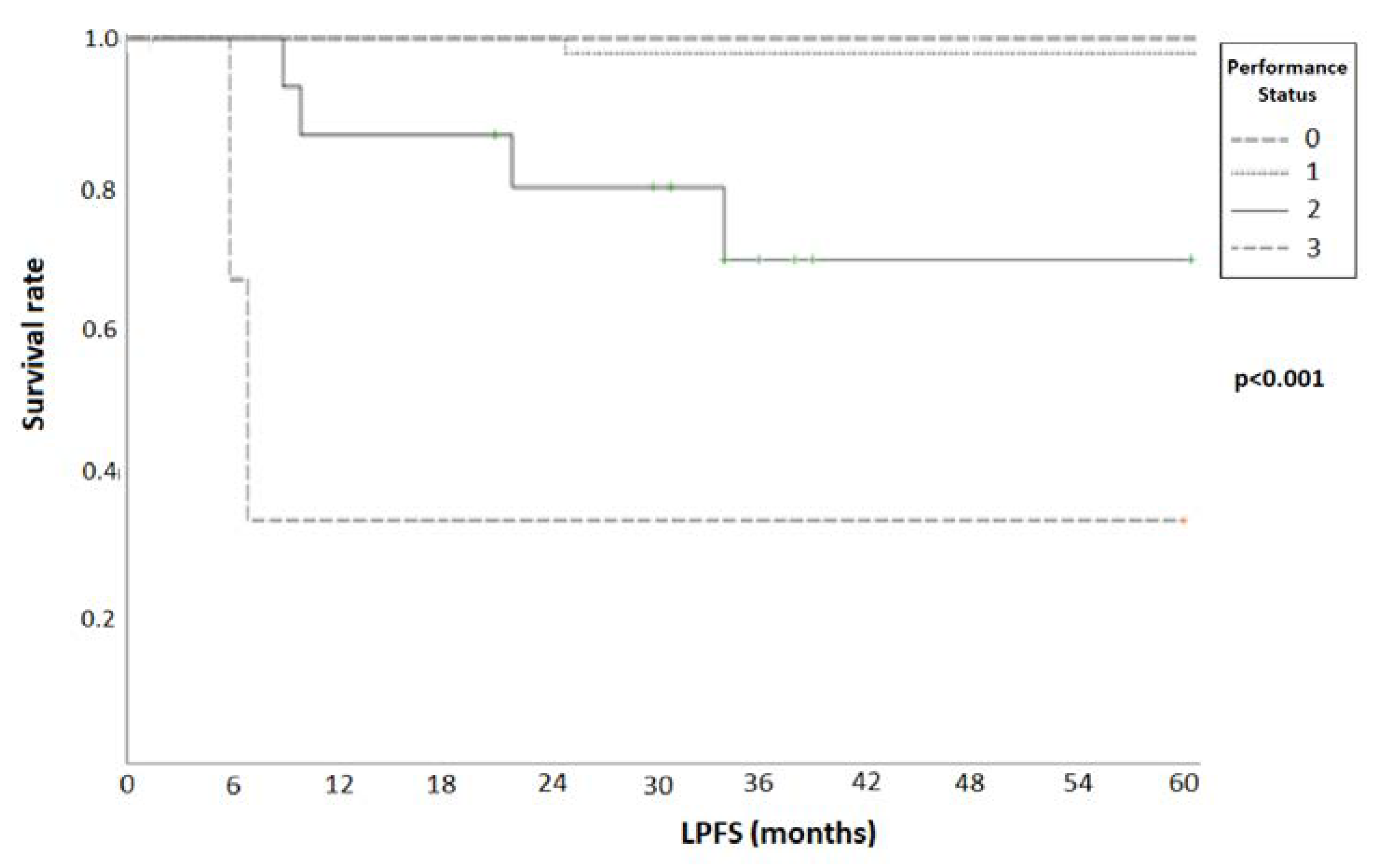
| PS | 0.014 | 0.28 | 0.07–0.49 | 0.003 | 0.34 | 0.16–0.37 |
| TVI (cc) | 0.217 | - | - | - | - | - |
| Histological status | 0.89 | - | - | - | - | - |
| BEDα/β = 10 | 0.033 | 1.061 | 1.009–1.089 | - | - | - |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| p | HR | 95% CI | p | HR | 95% CI | |
| Age | 0.44 | - | - | - | - | - |
| PS | 0.49 | - | - | - | - | - |
| TVI (cc) | <0.001 | 4.02 | 3.61–4.83 | <0.001 | 4.07 | 3.62–4.82 |
| BEDα/β = 3 | <0.001 | 5.52 | 5.12–5.81 | <0.001 | 5.62 | 5.11–5.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zygogianni, A.; Koukourakis, I.M.; Georgakopoulos, J.; Armpilia, C.; Liakouli, Z.; Desse, D.; Ntoumas, G.; Simopoulou, F.; Nikoloudi, M.; Kouloulias, V. Robotic Stereotactic Ablative Radiotherapy for Patients with Early-Stage Lung Cancer: Results of an Interim Analysis. Cancers 2024, 16, 3227. https://doi.org/10.3390/cancers16183227
Zygogianni A, Koukourakis IM, Georgakopoulos J, Armpilia C, Liakouli Z, Desse D, Ntoumas G, Simopoulou F, Nikoloudi M, Kouloulias V. Robotic Stereotactic Ablative Radiotherapy for Patients with Early-Stage Lung Cancer: Results of an Interim Analysis. Cancers. 2024; 16(18):3227. https://doi.org/10.3390/cancers16183227
Chicago/Turabian StyleZygogianni, Anna, Ioannis M. Koukourakis, John Georgakopoulos, Christina Armpilia, Zoi Liakouli, Dimitra Desse, Georgios Ntoumas, Foteini Simopoulou, Maria Nikoloudi, and Vassilis Kouloulias. 2024. "Robotic Stereotactic Ablative Radiotherapy for Patients with Early-Stage Lung Cancer: Results of an Interim Analysis" Cancers 16, no. 18: 3227. https://doi.org/10.3390/cancers16183227








